Back to Journals » Journal of Pain Research » Volume 16
Single-Dose of Postoperative Ketamine for Postoperative Pain After Mastectomy: A Pilot Randomized Controlled Trial
Authors Doan LV , Li A, Brake L, Ok D, Jee HJ, Park H, Cuevas R, Calvino S, Guth A, Schnabel F, Hiotis K, Axelrod D, Wang J
Received 12 September 2022
Accepted for publication 24 December 2022
Published 14 March 2023 Volume 2023:16 Pages 881—892
DOI https://doi.org/10.2147/JPR.S389564
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Lisa V Doan,1 Anna Li,1 Lee Brake,1 Deborah Ok,1 Hyun Jung Jee,1 Hyung Park,2 Randy Cuevas,1 Steven Calvino,1 Amber Guth,3 Freya Schnabel,3 Karen Hiotis,3 Deborah Axelrod,3 Jing Wang1,4
1Department of Anesthesiology, Perioperative Care and Pain Medicine, New York University Grossman School of Medicine, New York, NY, USA; 2Department of Population Health, New York University Grossman School of Medicine, New York, NY, USA; 3Department of Surgery, New York University Grossman School of Medicine, New York, NY, USA; 4Department of Neuroscience and Physiology, New York University Grossman School of Medicine, New York, NY, USA
Correspondence: Lisa V Doan, Department of Anesthesiology, Perioperative Care and Pain Medicine, New York University Grossman School of Medicine, 240 E 38th St, 14th Floor, New York, NY, 10016, USA, Tel +1 212201-1004, Email [email protected]
Background and Objectives: Perioperative ketamine has been shown to reduce opioid consumption and pain after surgery. Ketamine is most often given as an infusion, but an alternative is single-dose ketamine. Single-dose ketamine at up to 1 mg/kg has been shown to reduce symptoms of depression, and a wide range of dosages has been used for pain in the emergency department. However, limited data exists on the tolerability and efficacy of a single-dose of ketamine at 0.6 mg/kg for pain when administered immediately after surgery. We conducted a pilot study of single-dose ketamine in patients undergoing mastectomy with reconstruction, hypothesizing that a single-dose of ketamine is well tolerated and can relieve postoperative pain and improve mood and recovery.
Methods: This is a randomized, single-blind, placebo-controlled, two-arm parallel, single-center study. Thirty adult women undergoing mastectomy with reconstruction for oncologic indication received a single-dose of ketamine (0.6mg/kg) or placebo after surgery in the post-anesthesia care unit (PACU). Patients were followed through postoperative day (POD) 7. The primary outcome was postoperative pain measured by the Brief Pain Inventory (BPI) pain subscale on POD 1 and 2. Secondary outcomes include effects on opioid use, PROMIS fatigue and sleep, mood, Quality of Recovery-15, and the Breast Cancer Pain Questionnaire.
Results: Side effects were minor and not significantly different in frequency between groups. The ketamine group reported lower scores on the BPI pain severity subscale, especially at POD 7; however, the difference was not statistically significant. There were no statistically significant differences between ketamine and placebo groups for the secondary outcomes.
Conclusion: A single-dose of ketamine at 0.6mg/kg administered postoperatively in the PACU is well tolerated in women undergoing mastectomy and may confer better pain control up to one week after surgery. Future studies with larger sample sizes are necessary to adequately characterize the effect of postoperative single-dose ketamine on pain control in this population.
Keywords: postoperative pain, non-opioid, analgesia
Introduction
Perioperative ketamine has been shown to reduce pain and opioid consumption after several types of surgery.1 Perioperative ketamine is most often administered as a continuous infusion during and/or after surgery. Continuous infusion, however, requires continuous patient monitoring. An alternative is single-dose ketamine administration which may be easier to implement across a variety of settings. Although pre-operative administration of low-dose ketamine prior to incision has shown mixed results in improving pain control, limited data exists on the feasibility and efficacy of single-dose ketamine administration for pain control immediately after surgery.2–4
Single-dose ketamine has increasingly been used in the emergency department (ED) to provide long-lasting, post-discharge pain relief while minimizing opioid use, especially in the context of the current opioid epidemic.5–7 In addition to its analgesic effects, single-dose ketamine has emerged as a powerful antidepressant and anxiolytic with effects that can last more than a week.8–10 Doses of intravenous (IV) ketamine at 0.5 mg/kg have most often been used in studies of depression, with some studies using up to 1 mg/kg. Single-dose ketamine can efficiently activate the cortical top-down system for mood and affect regulation.11–15 A complex relationship between postoperative pain and mood exists, and depression and anxiety in the perioperative setting serve as significant risk factors for severe postsurgical pain, prolonged or high-dose opioid use, and even the development of chronic postsurgical pain.16–19
Perioperative ketamine continuous infusion regimens are often limited to administration in the operating room. However, studies have indicated that some of the therapeutic analgesic effects of ketamine may be associated with its psychotomimetic side effects.20 Thus, administration of ketamine postoperatively after emergence from anesthesia may contribute to analgesic effects. In a recent randomized controlled trial (RCT) of single-dose ketamine in the postoperative care unit (PACU), it was found that ketamine reduced the affective component of pain for 7 days after bariatric surgery.21 This study utilized a ketamine dose of 0.4 mg/kg based on ideal body weight. In addition, patients had relatively low levels of baseline anxiety and depression symptoms. It is possible that a higher dose of ketamine as used in studies of depression may be more helpful for acute pain control in surgical populations with higher levels of baseline depression or anxiety, such as patients undergoing mastectomy for breast cancer.22–24 However, it is not clear how well-tolerated a single-dose of ketamine at 0.6 mg/kg is, by patients immediately after surgery in the PACU. We conducted a pilot study to examine the tolerability and efficacy of single-dose of ketamine 0.6 mg/kg IV given in the PACU after mastectomy with reconstruction. We examined the effect of ketamine on pain, mood and recovery, and opioid requirements after surgery.
Methods
We performed a randomized, single-blind, placebo-controlled, two-arm parallel, single-center study on the effect of a single postoperative dose of ketamine in women undergoing unilateral or bilateral mastectomy with reconstruction for oncologic indication from April 2021 to April 2022. This study was approved by the New York University Grossman School of Medicine Institutional Review Board and was registered at ClinicalTrials.gov (Identifier NCT04831736; first posted 05/04/2021). It was conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from all participants.
Enrollment/Randomization
Adult American Society of Anesthesiologists (ASA) physical status I, II, or III women between the ages of 18 and 80 undergoing unilateral or bilateral mastectomy with immediate reconstruction for oncologic indications were eligible for inclusion. Exclusion criteria included distant metastatic disease, American Society of Anesthesiologists (ASA) physical status greater than 3, body mass index (BMI) >35 kg/m2, cognitive impairment, past ketamine or phencyclidine misuse or abuse, schizophrenia or history of psychosis, known sensitivity or allergy to ketamine, or liver or renal insufficiency. Other exclusion criteria included a history of uncontrolled hypertension, chest pain, cardiac arrhythmia, stroke, head trauma, intracranial mass or hemorrhage or pressure, glaucoma, acute globe injury, uncontrolled thyroid disease, porphyria, or any other contraindication to ketamine. Use of lamotrigine, alfentanil, physostigmine, and 4-aminopyridine were also contraindicated.
Prospective participants were recruited, screened and consented prior to surgery. Participants were randomized to one of the two groups in a 1:1 ratio to receive either IV ketamine (0.6mg/kg) or matching equal volume of 0.9% saline placebo. Block randomization with randomly varying block sizes of two and four was used. Randomization list was generated by the statistician in R Statistical Software; randomization codes were sent only to the study physician administering study drug. Participant and assessor of outcome measures were blinded. The surgeon, anesthesiologist, and the research team members conducting the follow-up visits were all blinded to study arm assignment.
Intervention
After patients arrived at the PACU, once deemed medically stable by the study team, a single 0.6mg/kg dose of intravenous ketamine or 0.9% saline placebo was administered over approximately 45 minutes in the PACU by study physicians; this is similar to ketamine protocols for depression. Ketamine and saline are similar in appearance; both are odorless. All subjects received standard post-anesthetic monitoring care, as well as routine care after transfer out of the PACU.
There were no other alterations in anesthetic, surgical or postsurgical care. Participants were administered a general anesthetic with a combination of intravenous and inhalational anesthetics. There was no restriction on the use of other analgesics or regional blocks during the procedure or in the postoperative period. There are no standardized protocols for mastectomy care at our institution. Medications often used after mastectomy with reconstruction include acetaminophen as needed, opioid such as oxycodone as needed, and benzodiazepine such as diazepam as needed.
Outcome Measures
Tolerability was assessed with a side effects questionnaire administered 30 minutes after infusion and on postoperative day (POD) 1 as well as discontinuation rates for intervention. The side effects questionnaire assessed for symptoms of dizziness, nausea, euphoria, dysphoria, hallucinations, headache, and feeling of a dream-like state. To assess success of participant blinding, they were asked to guess their treatment assignment on POD 1. The primary efficacy endpoint was the Brief Pain Inventory-short form (BPI) pain severity subscale at 24h and 48h postoperatively. Secondary outcomes included postoperative pain at other time points, mood and anxiety, fatigue, physical function, sleep quality, opioid usage, quality of recovery, and side effects. Questionnaires used included the following: BPI, Patient-Reported Outcomes Measurement Information System (PROMIS) fatigue scale, PROMIS sleep, Patient Health Questionnaire-2 (PHQ-2), Generalized Anxiety Disorder-2 (GAD-2), Breast Cancer Pain Questionnaire (BCPQ), and Quality of Recovery (QoR-15). BPI assesses pain severity and pain interference.25 Pain is assessed as its worst, least, average, and current levels on a scale of 0 to 10. The pain severity subscale is the arithmetic mean of the four items. For acute pain, the minimal clinically important difference is 2 for a 0 to 10 scale.26 For BPI, the minimal clinically important difference in chronic pain for the average pain score and for the severity score is approximately 2.27 PROMIS fatigue and sleep were developed by the National Institute of Health (NIH) to measure fatigue and sleep, important symptoms in postoperative recovery.28 PHQ-2 and GAD-2 assess for symptoms of depression and anxiety.29,30 BCPQ assesses pain location, frequency and severity as well as sensory disturbance after breast surgery.31 QoR-15 assesses satisfaction with postoperative recovery.32 Please refer to Figure 1 for complete timeline of study interventions and data collection.
Statistical Analysis
This was a pilot study, so it was not powered to detect a hypothesized effect size for the BPI subscale. For baseline characteristics and intraoperative data, descriptive statistics were presented as mean with standard deviation, median with interquartile range, or frequency and percentage. To compare the two treatment (Ketamine vs Placebo) groups with respect to these baseline data, we used two-sample Wilcoxon’s rank-sum test for continuous data and Fisher’s exact for count and proportion data. To compare the two groups with respect to the study outcome measures, we also used two-sample Wilcoxon’s rank-sum test for the continuous outcomes and Fisher’s exact test for the binary outcomes (the side effects indicators), assessed at each available time point. As additional analyses for the BPI pain severity subscales (average pain in last 24 hours, worst, least and current pain, and the arithmetic mean of these 4 items) at POD 0, 1, 2, and 7, we also estimated: 1) a linear mixed effects model with a random intercept for each patient and fixed effects for the treatment indicator (Ketamine vs Placebo) and categorical time, and 2) the same mixed effects model but with additional treatment-by-time interaction terms (to better represent the treatment group-specific time trend) for each BPI pain severity subscale. In addition, we used Fisher’s exact test to assess the success of blinding. A p-value of less than 0.05 was considered significant. Analyses were performed using R Statistical Software (version 4.2.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Thirty one individuals were enrolled in the study and were randomized to either study intervention or placebo. One subject was withdrawn the day of procedure due to a change in surgical planning; she did not receive intervention and was not included in the final analysis (Figure 2). The two groups were similar in regards to baseline characteristics and demographics, with the exception history of anxiety (Table 1).
 |
Table 1 Baseline Characteristics |
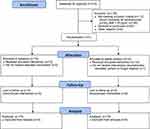 |
Figure 2 CONSORT flow-chart of the progression of participants throughout the study. |
We examined the surgical and anesthetic data (Table 2), and found that 60% of the participants randomized into the ketamine group underwent bilateral mastectomy compared to 80% of the control group. This difference, however, was not statistically significant. Rates of both sentinel lymph node dissection and axillary lymph node dissection were similar between groups. The majority of women in each group underwent tissue expander or implant-based reconstruction. Rates of regional anesthesia use was 20.0% in the ketamine group and 26.7% in the control group, but the difference was not statistically significant.
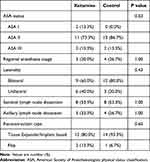 |
Table 2 Surgical and Anesthetic Data |
No patients exhibited serious adverse effects that warranted discontinuation of ketamine/saline infusion. On POD 0, thirty minutes after infusion, seven (46.7%) subjects in each group reported some form of side effect. On POD 1, eight (53.3%) subjects in the ketamine group and five (33.3%) subjects in the placebo group reported a side effect. The most common side effects were dizziness, nausea, and headache. There were no significant differences between groups in terms of side effect occurrence (Figure 3). All side effects were minor and did not require any additional medical treatment. Seven subjects in the ketamine group and five subjects in the placebo group received a benzodiazepine in the PACU. To the team’s knowledge, however, benzodiazepines were not given specifically to treat any perceived side effects. There was no statistically significant difference between groups in response to questioning regarding which group they believe they were assigned (p = 0.07).
 |
Figure 3 Side effects. |
Table 3 reports the results of Wilcoxon’s rank-sum tests comparing the ketamine and placebo groups with respect to BPI, opioid use, PROMIS fatigue and sleep disturbance, PHQ-2, GAD-2, and QoR-15. In terms of the primary endpoint of the study, there were no significant differences in the BPI pain severity subscale administered 24h and 48h postoperatively between the ketamine and placebo group. However, pain indices generally decreased over time in both groups, with the ketamine group having a statistically significant improvement in the “least pain” experienced at POD 7 when compared to placebo (p = 0.03). There were no other statistically significant differences between groups in any other pain score at any time point, although there was a trend towards a positive effect/decreased pain scores in the ketamine group for most indices.
 |
Table 3 Wilcoxon’s Rank-Sum Test for the Difference in the Outcome Between Ketamine and Placebo Groups (the Median Difference in the Distributions and the P-value) |
The estimated linear mixed effects models with random intercepts for individual patients and a fixed effect for the treatment indicator (ketamine vs placebo) adjusted for the categorical time indicate that the ketamine group has a smaller group mean than the placebo group, with respect to all the BPI pain severity subscales considered, although the difference was not statistically significant at alpha = 0.05. This effect was most notable at POD 7, which can be inferred from the estimated mean trajectories obtained from the mixed effects models with the additional treatment-by-time interaction terms. Please see Figure 4 for the group- and time-specific means and the associated 95% confidence intervals from the mixed effects models with the treatment-by-time interactions, for the BPI pain severity subscales.
With regard to responses on the BCPQ, 14 (93%) subjects in each group experienced residual pain on POD 7. In the ketamine group, the most common sites of pain were the axilla (60%) followed by the breast (53%) and chest wall (53%). In the saline group, the most common sites of pain were the breast (80%) followed by the axilla (60%). Regarding sensory disturbance, 11 (73%) subjects in the ketamine group experienced sensory disturbance of some sort on POD 7 compared to 9 (60%) in the placebo group. The breast was the most common site of disturbance in each group (10 for ketamine group, 8 for Placebo group).
There were no significant differences between the treatment groups with respect to both the PROMIS fatigue and PROMIS sleep disturbance scales postoperatively. There was also no difference between groups in respect to QoR-15 total scores on POD 2 and 7. Additionally, groups did not differ significantly in regards to postoperative opioid consumption at any time point, both as an inpatient and as an outpatient. Lastly, there were no significant differences between groups in follow-up depression and anxiety scales (PHQ-2 and GAD-2) at POD 7.
Discussion
In this pilot randomized, single-blind, placebo controlled study of single-dose ketamine after mastectomy with immediate reconstruction, a single-dose of ketamine at 0.6mg/kg was well tolerated amongst all subjects. No patients exhibited adverse cardiovascular side effects. An equal proportion of subjects in each group experienced some form of mild side effect after ketamine/placebo infusion, the most common being nausea and dizziness in each group. Importantly, these side effects are also known to be common amongst patients following normal general anesthetic and surgical care.33 Psychotomimetic side effects such as euphoria, dysphoria, and hallucinations were less common, with an overall incidence similar to previous studies utilizing ketamine at similar doses.2 Interestingly, the incidence of psychotomimetic side effects did not differ significantly in frequency between ketamine and control groups. It is possible that since our study was conducted immediately after patients have come out from surgery, residual anesthesia mitigated psychotomimetic effects, masking the effects of ketamine. All patient-reported side effects were self-limited and did not require any intervention beyond normal postoperative care. Although seven patients in the ketamine group and five patients in the control group received a benzodiazepine in the PACU, potentially blunting psychomimetic side effects, usage was similar between groups and is considered part of normal postoperative care at our institution.
One of the issues with ketamine studies is the unblinding on the part of the participant due to recognition of psychotomimetic side effects of this medication when compared to placebos such as saline. Previous studies investigating the therapeutic effects of ketamine have used “active” placebo agents such as benzodiazepines which more closely mimic ketamine in terms of behavioral side effects and thus theoretically reduce the risk of participant unblinding.34 In our study, 24 hours after surgery, all subjects were asked which group the believed they were assigned (ketamine, placebo, or cannot guess). There was not a significant difference between groups in response to this question despite the usage of saline as a placebo. 40% of the ketamine group guessed correctly their assignment to ketamine; 47% of the participants in the placebo guessed incorrectly that they received ketamine. Previous studies using ketamine at similar dosages (0.5mg/kg) but utilizing the “active placebo” midazolam have generated similar results. In one such study, 44% of the ketamine group and 42% of the midazolam placebo group were able to correctly identify their assignments.35 These results indicate an overall lack of ability to guess treatment group assignment. In our study, the relatively high level of blinding success may be due to the timing of study drug infusion, which occurred right after patients have arrived at the PACU and are feeling the effects of general anesthesia as well as receiving additional psychoactive medications like opioids and benzodiazepines, as mentioned previously.
Our results indicate that single-dose ketamine at this subanesthetic dose is well-tolerated in the postoperative setting. These findings highlight the potential utility of single-dose ketamine, because in addition to its ease of implementation, psychomimetic and cardiovascular side effects will need to be monitored for a shorter period than with continuous ketamine infusions.
In addition to serving as a pilot study to study the feasibility of administering single-dose ketamine in the PACU, this trial investigated whether this method of administration influences postoperative pain scores and opioid requirements in women undergoing mastectomy. No significant differences were noted between the two groups for pain and other secondary outcomes.
Previous studies have investigated the effectiveness of perioperative subanesthetic ketamine on postoperative pain control in women undergoing oncologic breast surgery. These studies have had mixed results. For instance, a recent randomized control trial by Nayak et al found that preoperative administration of single-dose subanesthetic ketamine was not effective in improving postoperative analgesia in women undergoing modified radical mastectomy.36 A 2009 study found that intraoperative low dose ketamine infusion was effective at reducing acute postsurgical pain in women undergoing mastectomy.37 A 2020 study found that intraoperative ketamine infusion did not improve acute pain control but reduced chronic post-mastectomy pain at 3 months.38 A recent meta-analysis and systematic review article analyzed 13 randomized control trials on the effect of ketamine on pain control in women undergoing breast surgery and found that perioperative ketamine was effective at reducing pain and reducing opioid requirements during the first 24 hours after surgery.39 Notably, in none of these studies was the ketamine administered as a single dose after surgery. Postoperative ketamine administration for non-breast-related surgical indications has been shown to be effective in reducing certain aspects of postsurgical pain and reducing morphine consumption.21,40
It is important to note that this study was limited to acute postsurgical pain with follow-up until POD 7, with ketamine’s most potent treatment effect noted on POD 7. Previous studies have found that perioperative ketamine use may also be effective in reducing the incidence and severity of chronic pain, likely due to modulations in the affective component of pain.21,41 In addition, ketamine is a well-known antidepressant with mood-elevating properties that last 7–14 days. Mastectomy patients have a particularly high incidence of chronic postsurgical pain (CPSP) with an incidence reported as high as 60%.23 Therefore, one intriguing possibility is that the long-lasting mood-elevating effects of ketamine play a role in reducing the overall experience of pain on POD 7 and possibly at later time points. Additional studies are of course warranted to further study the utility of perioperative ketamine in reducing the burden of chronic postsurgical pain in women undergoing mastectomy.
Lastly, secondary outcomes in this study included metrics of postoperative mood and recovery. We did not find any differences between groups in follow-up PHQ-2, GAD-2, PROMIS sleep, PROMIS fatigue, and QoR-15 scores. Although ketamine is known to possess potent antidepressant and anxiolytic properties, including postoperatively, we did not find any differences in PHQ-2 and GAD-2 scores on POD 7.8,9,39,42,43 Possible explanations for these findings include our small sample size as well as the brief nature of these surveys which are not fully comprehensive of the many dimensions of depression and anxiety. Meanwhile, our work is in line with some of the previous data that failed to show an effect of ketamine on improving the quality of postoperative recovery.44,45 These studies utilized the more in-depth QoR-40 survey and were administered 24 hours postoperatively, as opposed to the QoR-15 on POD 2 and 7 in our research. However, as mood, pain, and anxiety are all known factors contributing to postoperative recovery, it is reasonable for future research to continue to analyze perioperative ketamine’s effect in improving recovery, especially the potential for more long-term benefits.16–19
There are several limitations to this study. First, as mentioned above, this investigation principally served as a pilot study to determine the safety and tolerability of a single-dose ketamine in the PACU. As such, the sample size was limited not powered to evaluate differences in treatment effect. Second, there was variability in surgical and anesthetic technique. Though not a statistically significant difference, 60% of participants in the ketamine group underwent bilateral mastectomy compared to 80% in the placebo group. Additionally, anesthesiologists were not given any constraints on their provision of care beyond restricting the use of ketamine intraoperatively. Thus, some subjects received regional blocks to supplement general anesthesia while others did not. Our sample size was not large enough to control for these variables or conduct additional subgroup analyses. Future studies with larger sample sizes should address these subgroup variabilities in the responsiveness to ketamine. Lastly, the vast majority of our analyses did not control for baseline responses for repeat questionnaires, and the follow-up periods were relatively short due to our focus on tolerability and feasibility. Future longitudinal investigations with longer follow-up may provide additional insight into the potential benefits of single-dose postoperative ketamine on pain, mood, and recovery in the weeks and months after surgery.
In conclusion, we found that single subanesthetic dose ketamine (0.6mg/kg) is well tolerated amongst women undergoing mastectomy when administered immediately postoperatively in the PACU. This study showed a trend of improvement in pain reports after surgery, especially on POD 7; however, the benefits were not clinically nor statistically significant likely due to the relative small study population and heterogeneity of the patient characteristics. Future studies of larger sample sizes are needed to further investigate the role of ketamine in reducing acute and chronic postsurgical pain.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This was supported by the Interdisciplinary Pain Research Program at New York University Grossman School of Medicine.
Disclosure
Dr Freya Schnabel is a member of Scientific Advisory Board at ClearCut Medical, outside the submitted work. The authors report no conflicts of interest in this work.
References
1. Brinck EC, Tiippana E, Heesen M, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018;12:CD012033. doi:10.1002/14651858.CD012033.pub4
2. Kwok RFK, Lim J, Chan MTV, Gin T, Chiu WKY. Preoperative ketamine improves postoperative analgesia after gynecologic laparoscopic surgery. Anesth Analg. 2004;98(4):1044–1049. doi:10.1213/01.ANE.0000105911.66089.59
3. Dahl V, Ernoe PE, Steen T, Raeder JC, White PF. Does ketamine have preemptive effects in women undergoing abdominal hysterectomy procedures? Anesth Analg. 2000;90(6):1419–1422. doi:10.1097/00000539-200006000-00031
4. Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390(10091):267–275. doi:10.1016/S0140-6736(17)31467-8
5. Sheikh S, Hendry P. The expanding role of ketamine in the emergency department. Drugs. 2018;78(7):727–735. doi:10.1007/s40265-018-0904-8
6. Karlow N, Schlaepfer CH, Stoll CRT, et al. A systematic review and meta-analysis of ketamine as an alternative to opioids for acute pain in the emergency department. Acad Emerg Med. 2018;25(10):1086–1097. doi:10.1111/acem.13502
7. Pourmand A, Mazer-Amirshahi M, Royall C, Alhawas R, Shesser R. Low dose ketamine use in the emergency department, a new direction in pain management. Am J Emerg Med. 2017;35(6):918–921. doi:10.1016/j.ajem.2017.03.005
8. Zarate CA
9. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi:10.1016/S0006-3223(99)00230-9
10. Dean RL, Hurducas C, Hawton K, et al. Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder. Cochrane Database Syst Rev. 2021;9:CD011612. doi:10.1002/14651858.CD011612.pub3
11. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592–1603. doi:10.1038/s41380-018-0256-5
12. Wang J, Goffer Y, Xu D, et al. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115(4):812–821. doi:10.1097/ALN.0b013e31822f16ae
13. Zhou H, Zhang Q, Martinez E, et al. Ketamine reduces aversion in rodent pain models by suppressing hyperactivity of the anterior cingulate cortex. Nat Commun. 2018;9(1):3751. doi:10.1038/s41467-018-06295-x
14. Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi:10.1038/nature10130
15. Dale J, Zhou H, Zhang Q, et al. Scaling up cortical control inhibits pain. Cell Rep. 2018;23(5):1301–1313. doi:10.1016/j.celrep.2018.03.139
16. Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42(5):521–530. doi:10.1016/j.ijnurstu.2004.09.014
17. Nickinson RS, Board TN, Kay PR. Post-operative anxiety and depression levels in orthopaedic surgery: a study of 56 patients undergoing hip or knee arthroplasty. J Eval Clin Pract. 2009;15(2):307–310. doi:10.1111/j.1365-2753.2008.01001.x
18. Mossey JM, Mutran E, Knott K, Craik R. Determinants of recovery 12 months after hip fracture: the importance of psychosocial factors. Am J Public Health. 1989;79(3):279–286. doi:10.2105/AJPH.79.3.279
19. Fregoso G, Wang A, Tseng K, Wang J. Transition from acute to chronic pain: evaluating risk for chronic postsurgical pain. Pain Physician. 2019;22(5):479–488.
20. Olofsen E, Kamp J, Henthorn TK, et al. Ketamine psychedelic and antinociceptive effects are connected. Anesthesiology. 2022;136(5):792–801. doi:10.1097/ALN.0000000000004176
21. Wang J, Echevarria GC, Doan L, et al. Effects of a single subanaesthetic dose of ketamine on pain and mood after laparoscopic bariatric surgery: a randomised double-blind placebo controlled study. Eur J Anaesthesiol. 2019;36(1):16–24. doi:10.1097/EJA.0000000000000860
22. Dereu D, Savoldelli GL, Combescure C, Mathivon S, Rehberg B. Development of a simple preoperative risk score for persistent pain after breast cancer surgery: a prospective observational cohort study. Clin J Pain. 2018;34(6):559–565. doi:10.1097/AJP.0000000000000575
23. Wang L, Guyatt GH, Kennedy SA, et al. Predictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studies. CMAJ. 2016;188(14):E352–E361. doi:10.1503/cmaj.151276
24. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725–746. doi:10.1016/j.jpain.2010.12.005
25. Poquet N, Lin C. The Brief Pain Inventory (BPI). J Physiother. 2016;62(1):52. doi:10.1016/j.jphys.2015.07.001
26. Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. J Pain Symptom Manage. 2003;25(5):406–411. doi:10.1016/S0885-3924(03)00162-3
27. Mease PJ, Spaeth M, Clauw DJ, et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res. 2011;63(6):821–826. doi:10.1002/acr.20449
28. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi:10.1016/j.jclinepi.2010.04.011
29. Levis B, Sun Y, He C, et al. Accuracy of the PHQ-2 alone and in combination with the PHQ-9 for screening to detect major depression: systematic review and meta-analysis. JAMA. 2020;323(22):2290–2300. doi:10.1001/jama.2020.6504
30. Plummer F, Manea L, Trepel D, McMillan D. Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry. 2016;39:24–31. doi:10.1016/j.genhosppsych.2015.11.005
31. Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985–1992. doi:10.1001/jama.2009.1568
32. Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118(6):1332–1340. doi:10.1097/ALN.0b013e318289b84b
33. Johansson E, Hultin M, Myrberg T, Wallden J. Early post-operative nausea and vomiting: a retrospective observational study of 2030 patients. Acta Anaesthesiol Scand. 2021;65(9):1229–1239. doi:10.1111/aas.13936
34. Wilkinson ST, Farmer C, Ballard ED, et al. Impact of midazolam vs. saline on effect size estimates in controlled trials of ketamine as a rapid-acting antidepressant. Neuropsychopharmacology. 2019;44(7):1233–1238. doi:10.1038/s41386-019-0317-8
35. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327–335. doi:10.1176/appi.ajp.2017.17060647
36. Nayak BM, Misra S, Mitra JK, Sahoo AK. Effect of preoperative subanaesthetic ketamine on postoperative pain in women undergoing modified radical mastectomy: a randomised control trial. Eur J Anaesthesiol. 2021;38(5):556–558. doi:10.1097/EJA.0000000000001336
37. Kwon OS, Lee HJ, Yoon JY, Kim CH, Kwon JY, Kim HK. Intraoperative low dose ketamine reduce postoperative pain after combined anesthesia with propofol and remifentanil in mastectomy patients. Korean J Anesthesiol. 2009;57(5):604–609. doi:10.4097/kjae.2009.57.5.604
38. Kang C, Cho AR, Kim KH, et al. Effects of intraoperative low-dose ketamine on persistent postsurgical pain after breast cancer surgery: a prospective, randomized, controlled, double-blind study. Pain Physician. 2020;23(1):37–47.
39. Bi Y, Ye Y, Zhu Y, Ma J, Zhang X, Liu B. The effect of ketamine on acute and chronic wound pain in patients undergoing breast surgery: a meta-analysis and systematic review. Pain Pract. 2021;21(3):316–332. doi:10.1111/papr.12961
40. Zakine J, Samarcq D, Lorne E, et al. Postoperative ketamine administration decreases morphine consumption in major abdominal surgery: a prospective, randomized, double-blind, controlled study. Anesth Analg. 2008;106(6):1856–1861. doi:10.1213/ane.0b013e3181732776
41. Yang Y, Maher DP, Cohen SP. Emerging concepts on the use of ketamine for chronic pain. Expert Rev Clin Pharmacol. 2020;13(2):135–146. doi:10.1080/17512433.2020.1717947
42. Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526–1533. doi:10.1038/npp.2011.338
43. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi:10.1001/archgenpsychiatry.2010.90
44. Zhao Z, Xu Q, Chen Y, et al. The effect of low-dose ketamine on postoperative quality of recovery in patients undergoing breast cancer surgery: a randomised, placebo-controlled trial. Int J Clin Pract. 2021;75(12):e15010. doi:10.1111/ijcp.15010
45. Moro ET, Feitosa I, de Oliveira RG, et al. Ketamine does not enhance the quality of recovery following laparoscopic cholecystectomy: a randomized controlled trial. Acta Anaesthesiol Scand. 2017;61(7):740–748. doi:10.1111/aas.12919
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


