Back to Journals » Psoriasis: Targets and Therapy » Volume 7
Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis
Authors Hengge UR, Röschmann K, Candler H
Received 15 December 2016
Accepted for publication 20 April 2017
Published 15 June 2017 Volume 2017:7 Pages 41—49
DOI https://doi.org/10.2147/PTT.S130295
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Uwe Wollina
Ulrich R Hengge,1 Kristina Röschmann,2 Henning Candler3
1Skin Center, Düsseldorf, 2Department of Clinical Research, 3Department of Medical Affairs, G. Pohl‑Boskamp GmbH & Co. KG, Hohenlockstedt, Germany
Introduction: Psoriasis is a frequent inflammatory skin disease affecting ~2%–3% of the population in western countries. Scaling of the psoriatic lesions is the most impairing symptom in patients with psoriasis. In contrast to conventional keratolytic treatment concepts containing salicylic acid or urea, a dimeticone-based medical device (Loyon®) removes scales in a physical way without any pharmacological effect.
Objective: To assess the efficacy and tolerability of a dimeticone-based medical device in removal of scales in patients with psoriasis corporis/capitis under real-life conditions.
Methods: Forty patients with psoriasis capitis or corporis were included and received once-daily treatments for 7 days. Clinical assessment of the psoriasis area severity index score (psoriasis corporis) and the psoriasis scalp severity index score (psoriasis capitis) was performed and evaluated at baseline, after 3 and 7 days of treatment. Baseline scaling scores and redness scores were calculated for two target lesions of the scalp or the body on a 5-point scale each.
Results: For the primary efficacy variable scaling score, a statistically significant decrease was observed after treatment, with a relative reduction in scaling of 36.8% after 7 days of treatment within patients affected by psoriasis capitis. Treatment success was achieved in 76.8% of patients with psoriasis capitis, and time to treatment success was evaluated to be 4.14 days for these patients and 4.33 days for patients suffering from psoriasis corporis.
Conclusion: In conclusion, this trial demonstrated that the dimeticone-based medical device is a safe, well-tolerated, practicable, and efficient keratolytic compound, which can be well implemented in and recommended for standard therapy of psoriasis.
Keywords: psoriasis, keratolysis, scaling, PSSI, PASI
Introduction
Psoriasis is a frequent, inflammatory chronic skin disease with a genetic predisposition, affecting at least 2%–3% of the population in western countries.1 It is characterized by isolated or generalized, usually symmetrical, heavily infiltrated and well-demarcated, erythrosquamous plaques. Joint involvement is possible. In general, the severity of symptoms and the number and area of lesions are measured using the psoriasis area severity index (PASI) score for the assessment of psoriasis corporis or the psoriasis scalp severity index (PSSI) score for the assessment of psoriasis capitis.2,3
Currently, no cure is available for psoriasis and current therapies are aiming at minimizing the severity and the extent of the disease and its interference with quality of life. Topical treatments with corticosteroids or vitamin D3 analogs, as well as phototherapy represent the agreed first-line therapy for psoriasis.4 Current guidelines recommend keratolytic agents as basic therapy. By inducing keratolysis and subsequent descaling of affected skin areas, scaling that forms a physical barrier and inhibits penetration of pharmacological active substances is decreased.5
Most commonly used keratolytics are salicylic acid or urea, with the first being the most commonly used keratolytic compound.6 Salicylic acid is most beneficial in extremely thick or scaly psoriatic plaques.7 However, salicylic acid is not recommended in combination with phototherapy, for its photoprotective properties, and the application, especially on large-scale areas, bears the risk of chronic or acute systemic intoxication.8–10 Salicylic acid is not recommended for the use in children and patients with impaired renal function.11,12
Urea moisturizes dry and scaly skin conditions.6,13 Burning sensations (“stinging effect”) are the most common side effects.14 However, when speaking of psoriasis capitis, urea might affect physical hair condition and in part is not able to meet patient’s expectations; therefore, compliance and potentially resulting improvement in quality of life are reported to be rather low.15
A summary of the above leads to the conclusion that there is still an unmet therapeutical need for a safe, efficient, and feasible keratolytic agent that can be integrated in basic therapy of psoriasis capitis and psoriasis corporis, as reported by Jacobi et al.16
In contrast to conventional treatment concepts, the dimeticone-based medical device does not contain any corticoids, antifungal medications, or salicylic acid. Unlike with medicinal products, the formulation-induced descaling is achieved not by pharmacological or immunological means or by influencing metabolism, but physically.
Therefore, the objective of this noninterventional clinical trial was to assess the effectiveness and tolerability of the dimeticone-based medical device to facilitate the removal of scales in patients with psoriasis on the scalp or skin of the body under real-life conditions.
Methods
Study design, treatment, and clinical assessment
The trial was designed as a single-center, noninterventional clinical trial to assess the effectiveness and tolerability of a dimeticone-based medical device (Loyon, CE certified medical device, G. Pohl-Boskamp GmbH & Co. KG, Hohenlockstedt, Germany) to facilitate the removal of scaling after topical application in patients with psoriasis corporis or capitis in comparison to baseline.
The medical device represents a keratolytic preparation for scaly skin diseases and contains dicaprylyl carbonate, a fast-spreading emollient with high dermatological compatibility, and dimeticones (polydimethylsiloxane/silicones), which support the spreading properties and additionally serve as skin protectants and moisturizers. As the components are chemically inert, there are no pharmacological interactions and no risk for systemic side effects. Due to its viscosity and creeping/spreading properties, the medical device readily flows underneath and between the corneocytes and removes scaling.
The trial was performed in 40 patients with psoriasis on the scalp or skin of the body. All patients received once-daily treatments for 7 days with a final visit on day 8±1. The two target areas were psoriasis-affected skin, located on the scalp or the body. Clinical examinations of the patients were performed after the informed consent form had been signed. On the first day of the trial (baseline) two target lesions were selected and documented in the case report form, and for each patient both target lesions had to be located either on the head or on the body. For psoriasis corporis, the PASI score was calculated, and for psoriasis capitis, the PSSI score was calculated. Baseline scaling scores and redness scores were calculated for the two target lesions of the scalp or the body on a 5-point scale each. The first treatment was applied in the study center instructed by the study physician. The patients washed off the product at home using their normal skin and hair care products at the earliest 3 hours after application, but exposure overnight was encouraged. Treatments on days 2 and 3 were performed by the patients in their private setting. On day 4, patients returned to the dermatological center. Scaling scores and redness scores were calculated for the two target lesions of the scalp or of the body. For psoriasis of the body, the PASI score was calculated, and for psoriasis capitis, the PSSI was calculated. Safety variables were assessed and recorded. Treatments on days 5, 6, and 7 were performed by the patients in their private setting. On day 8, patients returned for the final visit. Scaling scores and redness scores were calculated for the two target lesions of the scalp or of the body. For psoriasis of the body, the PASI score was calculated, and for psoriasis capitis, the PSSI score was calculated. Safety variables were assessed and recorded.
Patients
Patients were included if they were male or female adults (aged ≥18 years) with psoriasis on the scalp or on the body with a scaling score of ≥1 on a 5-point scale. The trial collective involved patients who were eligible for the intended use of the product because of their degree of symptoms in the opinion of the responsible physician or dermatologist and who met the inclusion and exclusion criteria and who gave their written informed consent for voluntary participation in this trial as well as written consent according to §§4, 4a Federal Data Protection Act for collecting their data.
Before the start of the trial, the trial protocol, all documents related to informed consent, and other appropriate documents were submitted to the responsible ethics committee (Ethical Committee of the medical association Nordrhein) and a positive vote was obtained prior to inclusion of patients. The CE-certified medical device was administered in accordance with its intended use. This trial was carried out in accordance with The Medical Device Law and with the Helsinki Declaration of 1964, as revised in 2013.
Efficacy evaluation
The primary objective of this study was to gain evidence on the efficacy of the dimeticone-based product in the treatment of psoriasis as assessed by a reduction in scaling score. The scaling scores were documented within the assessment of PSSI or PASI, for each patient and target lesion. Furthermore, as secondary efficacy variables, PSSI or PASI scores, redness scores, and the time to treatment success were documented. All patients showing a treatment success were considered as responders. A treatment success was defined to be reduction in scaling of at least one grade.
Safety evaluation
The number of patients, who experienced any adverse events (AEs), and the incidence of AEs were summarized. AE characteristics such as duration, outcome, and causal relationship as assessed by the physician were documented. Safety was evaluated after 3 and 7 days of treatment. Spontaneously noted complaints that were considered to be an AE were documented in the reporting form for adverse reactions with medical devices and a copy was sent to the manufacturer.
Statistical analysis
Given the exploratory character of this study, no formal sample size calculation was performed. A total number of 40 patients was considered to be sufficient to meet the objectives of the study. All analyses of the primary and secondary endpoints were descriptive/exploratory in nature. Demographic data were summarized using descriptive statistical methods. Numerical data were summarized by mean, standard deviation (SEM = standard error of the mean), median, minimum, and maximum. Categorical demographic data were summarized by frequency tables. The clinical assessment scores of scaling are presented by visit (baseline, 3 days, and 7 days) using frequency tables and descriptive statistics. Primary objective (after 7 days of treatment) was the reduction of the scaling score. Additionally, changes from baseline in clinical assessment scores were evaluated by descriptive statistics.
Within-group comparisons were carried out by paired t-test to evaluate statistical significance of changes from baseline values to day 4 (after 3 days of treatment) and from baseline to day 8 (after 7 days of treatment). Differences were considered statistically significant at p<0.05. The calculated p-values are to be interpreted in a descriptive manner as no formal hypotheses were prespecified and no adjustment for multiplicity was applied.
Results
Demographic and baseline characteristics
In total, 40 patients were included within this study, with a mean age of 48 years. Most of the patients were females (77.5%). According to the subgroups investigated, most patients were affected by psoriasis capitis (70%). Furthermore, the vast majority of patients (92.5%) were suffering from psoriasis for ≥1 year.
Clinical assessment of scaling score
The scaling of psoriatic lesions was assessed for all subjects at baseline (day 0), after 3 and 7 days of treatment. For patients affected by psoriasis capitis or corporis, a statistically significant decrease in scaling severity was observed, resulting from treatment with the dimeticone-based medical device (Figure 1). At baseline the mean scaling score was 2.9±0.1 (mean±SEM), followed by a decrease in scaling to 2.1±0.1 (mean±SEM; p<0.05) after 3 days of the therapy, resulting in a further decrease in scaling after 7 days of treatment, with a mean scaling score of 1.9±0.1 (mean±SEM). In general, a higher decrease in scaling was identified in patients suffering from psoriasis capitis, when compared to subjects affected by psoriasis corporis (data not shown).
Analysis of responders in scaling
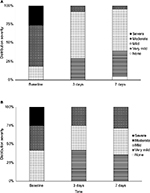
To evaluate the intensity of descaling depending on the time, we analyzed the distribution of scaling severity at baseline, after 3 and 7 days in all patients defined to be responders. The proportions of responders who displayed mild psoriasis capitis (Figure 2A) increased from 18.4% at baseline to 63.2% defined to display mild or very mild scaling after 3 days of treatment. After 7 days of treatment, in total, 95.3% of responding patients were assessed to only be affected by mild or very mild scaling. Within this group, 4.7% of patients were even assessed to be free of scaling. At baseline 26.3% of responding patients were assessed to display severe scaling. After 3 days of treatment this percentage was completely reduced to 0%. The proportion of responders in psoriasis corporis displaying moderate scaling decreased from 33.3% at baseline to 25.0% after 3 days of treatment with a moderate increase to 28.6% at the end of the therapy (Figure 2B). Within this subgroup of patients, 41.7% of responders displayed very mild scaling after 3 days of treatment, while at baseline none of these patients were assessed to display very mild scaling. The proportions of responders in psoriasis corporis suffering from severe scaling completely shifted from initially 25% of patients to 0% after 3 days of treatment.
Treatment success in scaling
The assessment of treatment success in scaling as defined by a reduction in scaling score of at least one grade revealed a treatment success within 67.6% of patients suffering from psoriasis capitis or corporis after 3 days of treatment. After 7 days of treatment, an improvement in scaling score of at least one grade was observed in 73.1% of patients (Figure 3). The overall average time to treatment success after therapy of patients affected by psoriasis capitis was 4.14 days. Individuals suffering from psoriasis corporis experienced a treatment success after 4.33 days (data not shown).
PSSI/PASI
PSSI was determined for each patient affected by psoriasis capitis at baseline (day 0), after 3 days of treatment (day 4) and 7 days of treatment (day 8), following standardized schemes. At baseline, subjects affected by psoriasis capitis showed a mean PSSI of 12.1±1.4 (mean±SEM; Figure 4). After 3 days of treatment a statistically significant reduction to a mean PSSI of 8.7±1.2 (mean±SEM) could be observed, with a further decrease of mean PSSI to 7.9±0.9 (mean±SEM) after 7 days of treatment. This reduction corresponds to a decrease in total PSSI by 33.4% after 7 days of therapy. Furthermore, 64.0% of patients affected by psoriasis capitis accomplished the PSSI25 after 3 days of treatment and 28.0% even showed a PSSI50. Comparable effects were detected when assessing the PASI of subjects suffering from psoriasis corporis over the period of the study (data not shown). At baseline patients were assessed to have a mean PASI of 2.0±0.2 (mean±SEM). After 3 days of treatment a reduction to a mean PASI of 1.8±0.2 (mean±SEM) was documented, with a further decrease of this score to 1.7±0.2 (mean±SEM) after 7 days of treatment. Here, responder analysis revealed that 54.5% of patients accomplished the PASI25 after 7 days of treatment (data not shown).
Clinical assessment of redness score and induration of psoriatic lesions
Subjects affected by psoriasis capitis showed a mean redness score of 2.3±0.1 (mean±SEM) at baseline, which corresponds to mild-to-moderate redness of the affected areas. After 3 days of treatment the mean redness score decreased to 2.1±0.1 (mean±SEM). After 7 days of treatment the mean redness score showed a further decrease to 2.0±0.1 (mean±SEM), thereby clearly shifting toward the classification “mild”. The scores assessed for erythema within the PASI remained rather unchanged throughout the course of the study (data not shown).
Besides scaling and erythema, one third of the overall PASI and PSSI scores is represented by the evaluation of the induration/infiltration of the psoriatic lesions. Patients affected by psoriasis capitis or corporis showed a mean induration score of 1.8±0.1 (mean±SEM) at baseline (Figure 5). Three days of treatment with the medical device induced a statistically significant reduction of this score to 1.1±0.1 (mean±SEM), and after 7 days of treatment a further reduction to a mean induration score of 0.9±0.1 (mean±SEM) was observed.
Discussion
Psoriasis is a common and chronic skin disease affecting ~2%–3% of the population in industrialized countries and is associated with a substantial social and psychological impact on patient’s quality of life with significant implications on physical, psychological, and social functioning.1,10 Affected children may even encounter bullying.17 The impairment in quality of life in subjects affected by psoriasis is equivalent to that from major systemic diseases such as type 2 diabetes, arthritis, or myocardial infarction.5,18,19 Especially severe scaling of the scalp appears to be burdensome and embarrassing for patients affected by psoriasis capitis, as the flaky plaques in the hair can be mistaken for dandruff.20
The aim of this noninterventional clinical trial was to assess the safety, the effectiveness, and tolerability of a dimeticone-based medical device in facilitating the removal of scaling after topical application in patients with psoriasis corporis or capitis. Unlike medicinal products, the effect the dimeticone-based medical device is achieved not by pharmacological or immunological means or by metabolism, but physically, thereby excluding the risk for systemic side effects. Scales are removed in a strictly physical manner. The physical properties of the medical device allow it to readily flow underneath the scales, removing them in a gentle way. Moreover, occlusive effects of the ingredients decrease the transepidermal water loss and can improve the epidermal barrier function. In conventional keratolytics occlusion can only be achieved by covering the affected skin with a plastic film. This commonly used technique impairs the patients’ compliance and bears the risk for an uncontrollable increase in penetration enhancement.
In total, 40 patients were included, 28 (70%) subjects affected by psoriasis capitis and 12 (30%) patients who suffered from psoriasis corporis. The high percentage of individuals affected by psoriasis capitis, who were willing to participate in the study, might at least in part reflect the unmet therapeutical need for an efficient, safe, and practicable keratolytic compound, which can be integrated in basic therapy for psoriasis. This higher frequency of psoriasis capitis we observed within the study population corresponds to recent literature, describing the scalp to be the most affected area in patients with psoriasis,21 with a total prevalence of 2% in Western Europe.3,22
Evaluation of the scaling score as a primary efficacy variable revealed a statistically significant decrease in scaling severity for patients affected by psoriasis capitis or corporis. This finding clearly underlines the potential benefit of a treatment with the dimeticone-based medical device for a difficult-to-treat area such as the human scalp. Responder analysis of patients affected by psoriasis capitis revealed a reduction of disease severity after 3 days of treatment, demonstrating the improvement induced by the therapy. After 7 days of treatment, 95.3% of patients shifted to the categories “mild” or “very mild” scaling. The described percentages correspond to the evaluation of the secondary efficacy variable “treatment success”, which shows a treatment success in 76.8% of patients with psoriasis capitis after 7 days of treatment. The overall time to treatment success was evaluated to be 4.14 days for patients affected by psoriasis capitis, indicating a fast onset of keratolysis. Responder analysis of patients affected by psoriasis corporis showed that the proportion of patients suffering from severe scaling completely shifted from initially 25% of patients to 0% already after 3 days of treatment, confirming the fast onset of keratolysis induced by treatment with the dimeticone-based medical device. This subgroup of patients experienced a treatment success after 4.33 days of therapy.
Treatment compliance in psoriasis capitis is rather poor.15 Furthermore, patients with scalp psoriasis suffer from a lower quality of life related to the highly visible site of their psoriatic lesions, irrespective of the severity of their disease as a whole.23 Therefore, a rather short duration of the treatment period, as seems to be optimal for the application of this product, might be beneficial to increase patient’s compliance and in turn quality of life.
Over the course of the treatment, the total PSSI score showed a significant reduction of 34.7% and the PASI was assessed to be reduced as well. Again, treatment efficacy was higher in patients affected by psoriasis capitis.
Interestingly, and although not primarily addressed by the product, a reduction in documented redness scores was observed after treatment. Desquamation and descaling would in general be expected to expose the underlying inflamed area of psoriatic lesions, thereby rather inducing elevated scores for redness and erythema, given the potentially positive correlation with descaling. Here, the reduction in redness scores observed throughout the course of the study might result from the physically induced desquamation and descaling that contribute to the removal of proinflammatory mediators like cytokines and chemokines present within psoriatic lesions. Thereby proinflammatory signal cascades and the resulting feedback to immune cells would be interrupted. The cytokines interleukin 17, interferon gamma, interleukin 22, and tumor necrosis factor alpha are described as inducing keratinocyte proliferation as well as chemokine, cytokine, and antimicrobial peptide production from these cells. This becomes a self-amplifying loop, where these products act back on immune cells to perpetuate the cutaneous inflammatory process.24 Therefore, removal of these mediators present within psoriatic lesions induced by the physical mode of action of the treatment might contribute to the attenuation of cutaneous inflammation.
In addition, induration of psoriatic lesions was reduced after treatment. The removal of inflammatory mediators might contribute to the reduction of infiltrating immune cells, thus inducing a decrease in induration scores.
Throughout the course of the study no serious adverse event occurred. In total, one AE was noted, which was assessed not to be product related (a common cold). No other complaints or incidences were reported and only one dropout occurred because of administrative reasons, demonstrating the safe use of the medical device. Redness score was also assessed to monitor potential skin irritation as closely as possible. As shown, the mean redness score even showed a reduction throughout the study period, demonstrating the absence of any skin-irritating potential.
This study is limited by the relatively small number of patients included (n=40) and lack of a control group. Furthermore, no assessment was performed between baseline (day 0) and day 4 (after 3 days of treatment). Therefore, potential onset of keratolysis as early as before day 4 was not monitored. Resulting from the noninterventional character of this study, safety and efficacy were not evaluated in direct comparison to an active comparator (e.g., preparations containing salicylic acid or urea). However, within a proof-of-concept pilot study, Hengge showed that the formulation is well tolerated, safe, and effective in facilitating the removal of scaling in infants and children with infantile seborrheic eczema, a common condition in infants, characterized by scaly patches on the scalp.25
Conclusion
In conclusion, this noninterventional clinical trial demonstrated that the dimeticone-based medical device is a safe, well-tolerated, practicable, and efficient keratolytic compound that can be implemented in standard psoriatic care by removing scaling that forms a physical barrier and thus inhibits penetration of pharmacological active substances. In comparison to keratolytic compounds containing salicylic acid or urea, the dimeticone-based medical device can be applied in long-term use settings, on large-scale areas in high doses/amounts, as the medicinal product does not induce any side effects, systemic reaction, or intoxication. On difficult-to-treat areas such as the scalp, this product could be shown to be practicable in daily application. Especially the early treatment response as shown in this trial could well lead to higher patient acceptance and adherence to treatment regimes, thereby improving quality of life of subjects affected by psoriasis. In line with the previous findings,25 this noninterventional clinical trial confirms that the medical device is effective and safe in removing scales independent of the underlying disease.
Taken into account the limitations of the noninterventional design, further clinical trials including appropriate controls should be performed in order to evaluate the efficiency of the medical device in direct comparison to an active comparator.
Acknowledgments
Sponsorship for this study and article processing charges were funded by G. Pohl-Boskamp GmbH & Co. KG, Hohenlockstedt, Germany. Prof Dr UR Hengge was the responsible scientific study expert conducting and overseeing the entire trial and was remunerated by the sponsor for his service provided with regard to the orderly conduct and reporting of the trial.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Prof Dr UR Hengge was responsible for the design of the trial, the evaluation of the data, and the present publication. Dr K Röschmann and Dr H Candler are employees of the sponsor and provided contributions to the design of the study, interpretation of the trial results, and reviewed the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Augustin M, Reich K, Reich C, et al. Quality of psoriasis care in Germany–results of the national study PsoHealth 2007. J Dtsch Dermatol Ges. 2008;6(8):640–645. | ||
Feldman SR, Fleischer AB Jr, Reboussin DM, et al. The self-administered psoriasis area and severity index is valid and reliable. J Invest Dermatol. 1996;106(1):183–186. | ||
Ortonne J, Chimenti S, Luger T, Puig L, Reid F, Trüeb RM. Scalp psoriasis: European consensus on grading and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23(12):1435–1444. | ||
Chiricozzi A, Chimenti S. Effective topical agents and emerging perspectives in the treatment of psoriasis. Exp Rev Dermatol. 2012;7(3):283–293. | ||
Nast A, Boehncke WH, Mrowietz U, et al. S3-Leitlinie zur Therapie der Psoriasis vulgaris Update 2011 [S3 - Guidelines on the treatment of psoriasis vulgaris (English version) Update]. J Dtsch Dermatol Ges. 2011;9: S1–S104. | ||
Fluhr JW, Cavallotti C, Berardesca E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clin Dermatol. 2008;26(4):380–386. | ||
Lebwohl M. The role of salicylic acid in the treatment of psoriasis. Int J Dermatol. 1999;38(1):16–24. | ||
Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71(1):37–40. | ||
Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2000;146(3):351–364. | ||
Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61(3):451–485. | ||
Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;61:643–659. | ||
Papp K, Berth‐Jones J, Kragballe K, Wozel G, de la Brassinne M. Scalp psoriasis: a review of current topical treatment options. J Eur Acad Dermatol Venereol. 2007;21(9):1151–1160. | ||
Hagemann I, Proksch E. Topical treatment by urea reduces epidermal hyperproliferation and induces differentiation in psoriasis. Acta Derm Venereol. 1996;76(5):353–356. | ||
Pan M, Heinecke G, Bernardo S, Tsui C, Levitt J. Urea: a comprehensive review of the clinical literature. Dermatol Online J. 2013;19(11):20392. | ||
Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26(5):448–459. | ||
Jacobi A, Mayer A, Augustin M. Keratolytics and emollients and their role in the therapy of psoriasis: a systematic review. Dermatol Ther (Heidelb). 2015;5(1):1–18. | ||
Magin P. Appearance-related bullying and skin disorders. Clin Dermatol. 2013;31(1):66–71. | ||
Cohen SN, Baron SE, Archer CB; British Association of Dermatologists and Royal College of General Practitioners. Guidance on the diagnosis and clinical management of psoriasis. Clin Exp Dermatol. 2012;37(Suppl 1):13–18. | ||
Lodén M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4(11):771–788. | ||
Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31(4):343–351. | ||
Kim TW, Shim WH1, Kim JM, et al. Clinical characteristics of pruritus in patients with scalp psoriasis and their relation with intraepidermal nerve fiber density. Ann Dermatol. 2014;26(6):727–732. | ||
van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. Diagnosis and management. Am J Clin Dermatol. 2001;2(3):159–165. | ||
Zampieron A, Buja A, Fusco M, Bortune M, Piaserico S, Baldo V. Quality of life in patients with scalp psoriasis. G Ital Dermatol Venereol. 2015;150(3):309–316. | ||
Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2013;32:227–255. | ||
Hengge UR. Topical, non-medicated LOYON® in facilitating the removal of scaling in infants and children with cradle cap: a proof-of-concept pilot study. Dermatol Ther (Heidelb). 2014;4(2):221–232. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.