Back to Journals » Infection and Drug Resistance » Volume 12
Simulating moxalactam dosage for extended-spectrum β-lactamase-producing Enterobacteriaceae using blood antimicrobial surveillance network data
Authors Huang C, Shi Q, Zheng B , Ji J , Ying C , Yu X, Wang H, Xiao Y
Received 7 November 2018
Accepted for publication 11 February 2019
Published 8 May 2019 Volume 2019:12 Pages 1199—1208
DOI https://doi.org/10.2147/IDR.S193712
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eric Nulens
Chen Huang,1,2 Qingyi Shi,1 Beiwen Zheng,1 Jinru Ji,1 Chaoqun Ying,1 Xiao Yu,1 Hui Wang,2 Yonghong Xiao1
1State Key Laboratory for Diagnosis and Treatment of Infectious Disease, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, People’s Republic of China; 2Department of Respiratory Medicine, Lihuili Hospital, Ningbo Medical Center, Ningbo, People’s Republic of China
Objectives: Monte Carlo simulation (MCS) was used to evaluate optimal dosage for cefepime (FEP), moxalactam (MOX), and cefperazone/sulbactam (CFZ/SBT) against extended-spectrum β-lactamase (ESBL) producers isolated from the Blood Bacterial Resistant Investigation Collaborative System.
Methods: Minimum inhibitory concentration (MIC) was tested by agar dilution, and ESBL producers were identified by modified Clinical and Laboratory Standards Institute tests. Pharmacokinetic parameters were derived from data on healthy individuals, and probability of target attainment (PTA) and cumulative fraction of response (CFR) %fT >MIC values were estimated by MCS.
Results: A total of 2032 Escherichia coli (875 ESBL-producing) and Klebsiella pneumoniae (157 ESBL-producing) strains, and 371 other Enterobacteriaceae strains, were isolated from patients with bloodstream infections (BSIs). MIC90 values for FEP, MOX, and CFZ/SBT against ESBL-producing E. coli and K. pneumoniae were 64/64 mg/L, 2/32 mg/L, and 64/128 mg/L, respectively. Conventional MOX and CFZ/SBT doses failed to reach 90% PTA against isolates with MICs ≥8 mg/L and ≥4 mg/L, respectively. Against ESBL producers, neither FEP nor CFZ/SBT achieved ≥90% CFR, while CFRs for MOX (1 g iv q6h, 2 g iv q12h, and 2 g iv q8h) exceeded 90% against ESBL-producing E. coli. Simulated CFRs for FEP and MOX were similar (>90%) against non-ESBL-producing Enterobacteriaceae, and higher than CFRs for CFZ/SBT.
Conclusion: ESBL producers from BSIs were highly susceptible to MOX, and PTA values were generally higher for MOX than FEP or CFZ/SBT for conventional dosing regimens. This large MCS analysis shows that MOX but not FEP or CFZ/SBT can be used empirically to treat BSIs caused by ESBL-producing E. coli strains.
Keywords: Monte Carlo simulation, Enterobacteriaceae, extended-spectrum β-lactamase, moxalactam, cefperazone/sulbactam, cefepime
Introduction
Pharmacokinetics (PK), expressed in the form of mathematical formulae, are used to describe the absorption, distribution, and elimination of drug compounds, whereas pharmacodynamics (PD) indicate the relationship between drug concentration and bactericidal outcome.1 PK/PD modeling can clarify the interactions of drugs, hosts, and pathogens for antibiotics and other active agents. The introduction of PK/PD theory and population probability theory into Monte Carlo simulation (MCS) integrates population-PK parameters and population-minimum inhibitory concentration (MIC) pathogen data to calculate the likelihood of achieving a certain target.1 MCS can be applied to optimize the therapeutic approach, maximize the desired effects, and re-evaluate reasonable clinical breakpoints.2
Third-generation cephalosporins are empirically used for the treatment of infections caused by Enterobacteriaceae. However, such drugs are often ineffective and result in poor outcomes in China and elsewhere due to the prevalence of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, which can be as high as 30–60% for Escherichia coli.3 The mortality rate of ESBL-producing bacterial bacteremia ranges from 20% to 40% and is significantly higher than that of non-ESBL-producing isolates.4,5 Goodman et al (2016) designed a user-friendly decision tree (history of ESBL colonization/infection, chronic indwelling vascular hardware, age ≥43 years, recent hospitalization in an ESBL high-burden region, and ≥6 days of antibiotic exposure in the prior 6 months) to predict ESBL producers in bacteremia, achieving positive and negative predictive values of 90.8% and 91.9%.6 Third-generation cephalosporins should be avoided in such high-risk patients. Thus, accurately predicting ESBL producers and appropriate regimens in a timely manner is crucial to improve outcomes for patients with bloodstream infections (BSIs).7
Although carbapenems are regarded as the first-line drug in the treatment of ESBL-producing isolates, associated with lower mortality and higher clinical cure rates than non-carbapenems, the increasing prevalence of carbapenem-resistant Enterobacteriaceae has forced us to decrease the consumption of carbapenems.8–11 Therefore, optimizing the regimens of non-carbapenems is essential for treating such infections. Although previous studies showed that non-carbapenems, including cefperazone/sulbactam (CFZ/SBT), cefepime (FEP), and moxalactam (MOX), can be used to treat ESBL-producing bacterial bacteremia, their efficacy, especially in empirical treatment against ESBL producers, remains controversial.12–14 MCS of these drugs in the treatment of ESBL producers has been performed, but early tests were insufficient, the number of isolates was limited, and the focus was not on BSIs.15–22
Therefore, the primary aim of the present study was to optimize the dosage of FEP, MOX, and CFZ/SBT against ESBL-producing Enterobacteriaceae isolated from the Blood Bacterial Resistant Investigation Collaborative System (BRICS) using MCS. The results will inform the prescription of these three agents, for which PK/PD studies in the treatment of ESBL-producing bacterial bacteremia are scarce, especially for MOX and CFZ/SBT.
Materials and methods
Bacterial isolates
Clinical Enterobacteriaceae, including E. coli, Klebsiella pneumoniae, and others such as Proteus mirabilis, Enterobacter cloacae, Enterobacter aerogenes, Enterobacter agglomerans, Morganella morganii, Salmonella sp., Citrobacter freundii, Klebsiella oxytoca, Klebsiella ozaenae, and Serratia marcescens, isolated from BRICS were collected between March 2014 and December 2015 from 31 tertiary and secondary hospitals in China. Pathogens were isolated and identified in accordance with clinical microbiological methods using the API20 system (bioMérieux, Durham, NC, USA). After pure isolates were shipped to our laboratory, pathogens were re-identified by matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF, Bruker) mass spectrometry (MS).23
Antimicrobial susceptibility testing
MICs for FEP (#MB1760, Dalian Meilun Biotechnology), MOX (#1609014001; Whiteson Pharma), and CFZ/SBT (2:1, #J19928; Pfizer) were determined by the agar dilution method according to the Clinical and Laboratory Standards Institute (CLSI).24 The results were interpreted based on the following: FEP ≤2 mg/L = susceptible, 4–8 mg/L = susceptible-dose-dependent, ≥16 mg/L = resistant; MOX ≤8 mg/L = susceptible, 16–32 mg/L = intermediate, ≥64 mg/L = resistant (as stipulated in the CLSI criteria). The breakpoint of CFZ/SBT was consistent with the breakpoint stipulated by the CLSI for CFZ alone.25 E. coli American Type Culture Collection (ATCC) 25922 was used as a quality control.
ESBL phenotype confirmation test
All E. coli and K. pneumoniae isolates from BRICS were tested against ceftazidime (30 μg) and cefotaxime (30 μg) with and without clavulanic acid (10 μg) as previously described.26 Isolates showing an increase in zone diameter of ≥5 mm between single and combination disks for any of the antibiotics were considered ESBL producers. K. pneumoniae ATCC 700603 was used as the control strain.
Pharmacokinetics (PK)
PK data for FEM, MOX, and CFZ/SBT were obtained from previously published studies on healthy volunteers.16,27–30 All studies included at least 10 healthy volunteers, described the assay used to determine drug concentrations, used clinically relevant dosing regimens, and performed an adequate PK analysis with appropriate parameters, including the volume of distribution in liters at steady state (Vd), the total body clearance in liters per hour (CLT), and the fraction of unbound drug (fu), as summarized in Table 1. The parameters obtained from previous studies were modeled using a two-compartment open model. However, lack of PK parameters (k12, k21, α, β) limited the use of two-compartment equations and a one-compartment intravenous (i.v.) bolus simulation was used to calculate %fT >MIC for β-lactam drugs as previously described sing the following equation:31,32
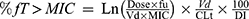
 | Table 1 The pharmacokinetic parameters used in the Monte Carlo simulation |
where Ln is the natural logarithm, Dose is the intermittent dose in mg, MIC is the minimum inhibitory concentration in mg/L, and DI is the dosing interval in hours.
Monte Carlo simulation (MCS)
FEP, MOX, and CFZ/SBT, like other cephalosporins, display time-dependent bactericidal effects against Enterobacteriaceae, and %fT >MIC is the PD index most closely linked to the efficacy. Of note, CFZ is regarded as the main agent exerting bactericidal effects against Enterobacteriaceae in CFZ/SBT combinations. Therefore, parameters of CFZ were used for MCS. %fT >MIC of 50% was defined as the bactericidal PD target for comparative purposes.16
The dosage regimens of FEP modeled by MCS were 1 g every 12 h (q12h), 1 g every 8 h (q8h), 2 g q12h, and 2 g q8h; for MOX, these were 1 g q12h, 1 g q8h, 1 g q6h, 2 g q12h, and 2 g q8h; for CFZ/SBT, these were 3 g q12h, 3 g q8h, 6 g q12h, 6 g q8h, and 6 g q6h. A 10,000 patient MCS was conducted to calculate the cumulative fraction of response (CFR) of each dosage regimen against bacterial population using Crystal Ball software (version 11.1.2.4; Oracle) to evaluate their efficacy. An optimal regimen was defined as achieving >90% CFR against a population of organisms.33 Probability of target attainment (PTA) was also calculated to evaluate the MIC breakpoint of each dosage regimen. During simulations, CLT and Vd obeyed a log-normal distribution, fu obeyed a uniform distribution, and MIC obeyed a discrete distribution.33
Results
Microbiological data
A total of 1512 E. coli, 520 K. pneumoniae, and 371 other Enterobacteriaceae bloodstream isolates were collected from the BRICS program between March 2014 and December 2015. Of these, 875 and 157 ESBL-producing E. coli and K. pneumoniae were confirmed by ESBL phenotype tests. ESBL rates in BSIs caused by E. coli and K. pneumoniae were 57.8% and 30.1%, respectively. The in vitro activities of FEP, MOX, and CFZ/SBT against these isolates are summarized in Table 2 and Figures 1 and S1. Compared with the poor antibacterial activity of FEP and CFZ/SBT against ESBL producers, the antibacterial potency of MOX was evident by the high rates of inhibition. When CLSI breakpoints for susceptibility of Enterobacteriaceae were used, 94.5% and 87.9% of ESBL-producing E. coli and K. pneumoniae remained susceptible to MOX, while only 50–60% of ESBL producers were susceptible to FEP or CFZ/SBT. MIC50 and MIC90 values for MOX against ESBL-producing E. coli and K. pneumoniae were 0.5 and 2 mg/L, and 0.5 and 32 mg/L, respectively. Of note, the rate of MOX-susceptible ESBL-producing K. pneumoniae accounted for 87.9%, which was close to the MIC90. However, the MICs of FEP against ESBL producers were widely distributed from 4 to 32 mg/L. Furthermore, MICs for CFZ/SBT against CFZ/SBT-susceptible ESBL-producing isolates were mostly between 8 and 16 mg/L. Thus, FEP, MOX, and CFZ/SBT displayed promising efficacies against non-ESBL-producing isolates and the other Enterobacteriaceae.
 | Table 2 The in vitro activities of FEP, MOX and CFZ/SBT against Enterobacteriaceae isolated from BRICS |
Monte Carlo simulation (MCS)
In this study, a 10,000 subject MCS was performed to calculate PTA and CFR values based on PK data for FEP, MOX, and CFZ/SBT from different regimens. Relationships between MIC and PTA for different dosage regimens are presented in Figure 2. PTA was close to 100% at low MIC values, and decreased rapidly to 0 at high MICs. Of note, increasing the dosing frequency achieved a higher PTA at relatively high MICs of FEP, MOX, and CFZ/SBT (1 g iv q8h and 2 g iv q12h). The target attainment rates for simulated FEP regimens (1 g iv q12h, 1 g iv q8h, 2 g iv q12h, and 2 g iv q8h) against isolates with MICs ≤2, ≤8, ≤4, and ≤16 mg/L, respectively, exceeded 90%. The cut-off for achieving >90% PTA was lowered to ≤4 and ≤2 mg/L for MOX (2 g iv q8h) and CFZ/SBT (3 g iv q6h), respectively.
As shown in Table 3 and Figure S2, MOX displayed the highest CFR, with >90% probabilities against E. coli and Enterobacteriaceae, and CFRs of 86–89.7% against K. pneumoniae. For MOX regimens (1 g iv q6h, 2 g iv q12h, and 2 g iv q8h), the probability was >90% for reaching 50% fT >MIC against ESBL-producing E. coli, compared with <80% for FEP and <15% for CFZ/SBT. Similar results were also observed for the simulated regimens of FEP and CFZ/SBT against ESBL-producing K. pneumoniae, while the CFRs of MOX were slightly lower (80–86%) against ESBL-producing K. pneumoniae. MOX and FEP performed comparably against non-ESBL-producing isolates and the other Enterobacteriaceae, depending on the dosage simulated (85–95%). The CFZ/SBT regimen (3 g iv q6h) displayed the greatest CFR (81.87%) against non-ESBL-producing K. pneumoniae, followed by 74.84% against non-ESBL-producing E. coli.
 | Table 3 The CFRs for different dosage regimens of FEP, MOX and CFZ/SBT against Enterobacteriaceae isolated from BRICS |
Discussion
The abundance of ESBL-producing isolates among Enterobacteriaceae has emerged as a global public health concern. Previous studies demonstrated that inappropriate antibiotic therapy for patients with BSIs is associated with longer hospital stays, greater hospital costs, and higher hospital mortality.3,34 Thus, finding effective antimicrobials and optimizing the use of agents in the clinic is crucial in the treatment of BSIs, both to improve the therapeutic effects, and avoid exacerbating the spreading of ESBL- and carbapenem-producing isolates. In the present study, MCS was performed using different dosage regimens of FEP, MOX, and CFZ/SBT against ESBL-producing Enterobacteriaceae isolated from BRICS to evaluate PTAs. The number of pathogens included in the present work (2032 Enterobacteriaceae strains) is significantly larger than in previous studies, and unlike previous studies, the focus was on the treatment of BSIs.16,20,32,35 With this type of data and population-PK parameters, clinicians can optimize national treatment plans that acknowledge changing resistance patterns. To the best of our knowledge, the BRICS program is the largest blood antimicrobial surveillance network in China.
MCS is an advanced statistical modeling approach that expands sample size based on inter-individual variation of PK and microbiological susceptibility information to predict the likelihood of success for therapeutic targets using different simulated regimens. Two different estimations of clinical outcome, PTA and CFR, were calculated to explore the optimal breakpoint for different simulated dosage regimens, and to identify appropriate dosage regimens in empirical antimicrobial treatments that display the best likelihood of success for treating pathogens, respectively.
Our results revealed promising in vitro antibacterial activity for MOX against Enterobacteriaceae, especially ESBL producers. Resistance to MOX was observed in 2.7% of ESBL-producing E. coli and 9.6% of ESBL-producing K. pneumoniae. Furthermore, most ESBL-producing isolates were highly susceptible to MOX (≤2 mg/L). Similar results were also found in a previous epidemiology antimicrobial surveillance study in China, demonstrating the in vitro stability of the antibacterial activity against ESBL producers.36 However, MICs for CFZ/SBT were much higher than for MOX against ESBL-producing E. coli and K. pneumoniae, with resistance rates of 17.7% and 29.3%, respectively. Meanwhile, MIC50 and MIC90 values were 16 and 64 mg/L, and 16 and 128 mg/L, respectively, indicating that only 50% of clinical ESBL producers were susceptible to CFZ/SBT. It should be noted that, even though ESBL-producing isolates were susceptible to CFZ/SBT, most of the MICs were relatively high (8–16 mg/L). Against isolates with high MICs (4, 8, and 16 mg/L), MCS of CFZ/SBT simulated regimens failed to reach 90% PTA. Moreover, our previous in vitro PK/PD study demonstrated that regimens of CFZ/SBT (2 g iv q8h) against ESBL producers with high MICs failed to maintain effective killing, and allowed significant regrowth.37 FEP was relatively stable against ESBL compared with third-generation cephalosporins. The sensitivity rates of ESBL-producing E. coli and K. pneumoniae to FEP were 58.5% and 61.8%, respectively, which was much higher than those of ESBL producers (<10% of isolates mainly isolated from urinary tract infections and intra-abdominal infections) in China.38 The distribution of MICs for FEP against ESBL producers (MICs of most isolates were 4 and 8 mg/L in the range of susceptibility) was similar to that of those for CFZ/SBT. Therefore, high-dose and multiple-dose regimens should be used in empirical therapies when ESBL-producing isolates are suspected. However, in a previous propensity score-matched study, two dosage regimens of FEP (2 g iv q8h and 1 g iv q12h) were used to empirically treat FEP-susceptible ESBL bacteremia (MIC =4–8 mg/L, 76.5%, 13/17), and a trend toward increased mortality in the FEP group was observed compared with carbapenems (HR, 2.87; 95% confidence interval, 0.88–9.41).39 Furthermore, Andes et al (2005) found that cephalosporin monotherapy in the treatment of ESBL bacteremia resulted in a stepwise reduction in treatment success, falling from 81% for MIC ≤1 mg/L to 11% for MIC =8 mg/L.40 These findings indicate that treating ESBL producers with a relatively high MIC, but still in the range of susceptibility, should be attempted cautiously or avoided due to lower PTA and worse clinical outcome.
Due to the low recommended dose for prescribing CFZ/SBT and unfavorable PK properties, we found that %fT >MIC for CFZ/SBT (3 g iv q6h) reached 50% against isolates with MIC ≤2 mg/L. In addition, the dosage regimen of MOX (2 g iv q8h) failed to reach 90% PTA against isolates with MIC =8 mg/L. According to the guidelines of CLSI, the susceptible breakpoint for MOX is ≤8 mg/L, and the breakpoint for susceptibility of CFZ/SBT was in accordance with that of CFZ (≤16 mg/L).25 For strains with high MICs, conventional dosing regimens do not always achieve adequate PK/PD targets, especially for CFZ/SBT. The probability of achieving 50% fT >MIC for CFZ/SBT against isolates with MIC =8–16 mg/L was <1% in our previous study, resulting in the failure to maintain effective killing.37 Therefore, the susceptible breakpoint for CFZ/SBT against Enterobacteriaceae should be reconsidered.
The current study found that the simulated regimens of FEP against non-ESBL-producing E. coli and K. pneumoniae isolated from BSIs exceeded 90% CFR. However, the CFRs of FEP were lower against ESBL producers (<80%), unlike the MCS results for FEP (1 g iv q12h) against ESBL-producing E. coli and K. pneumoniae reported by Ambrose et al (CFR >95%).35 The main reason for this discrepancy may be that the earlier collections of strains were highly susceptible to FEP, since the MIC90 was 4 mg/L. Therefore, reevaluating the dosing regimens of antibiotics is imperative to optimize empirical therapies due to dynamic changes in resistance patterns. CFRs for CFZ/SBT (2 g iv q6h) against non-ESBL-producing isolates were less than 80%, and values dropped to <20% against ESBL producers. CFZ/SBT accounts for a large proportion of antibiotic consumption in China, and this combination is often used to treat Gram-negative bacterial infections.41 Expert opinion in China states that mild-to-moderate infections caused by ESBL producers can be treated by CFZ/SBT, including BSIs.19 However, our results indicate that CFZ/SBT should be avoided in empirical treatments when ESBL bacteremia is suspected due to the weak inhibition of CFZ/SBT against ESBL producers (MICs mostly range from 8 to 64 mg/L) and unfavorable PK characteristics (shorter t1/2 and lower fu values), resulting in inadequate PK/PD ratios associated with worse outcome. This regimen can only be used in definitive therapy when pathogens are highly susceptible to CFZ/SBT (MIC ≤2 mg/L). Moreover, multiple-dose regimens are needed when treating ESBL producers.37 Due to the favorable PK properties of MOX and its marked stability in vitro against ESBL-producing E. coli, the CFRs of MOX (1 g iv q8h or q6h, and 2 g iv q12h or q8h) exceeded 90%, indicating that these regimens can be used in the empirical treatment of ESBL bacteremia. Against ESBL-producing K. pneumoniae, none of the treatments achieved >90% CFR. The MIC results showed that isolates were highly susceptible to MOX (≤2 mg/L); hence MOX regimens should be adjusted to reach adequate %T >MIC values according to clinical responses and MIC results. However, the CLSI is reluctant to recommend the use of MOX for treating ESBL-producing bacterial infections because it has limited availability in many countries, and there exists a lack of clinical experience. Our previous in vitro PK/PD study confirmed the bactericidal effects of MOX against ESBL-producing E. coli and K. pneumoniae.37 Meanwhile, other retrospective clinical studies also showed that the efficacy of oxacephems against ESBL bacteremia was similar to that of carbapenem groups.14,42 Thus, its clinical value as a carbapenem-sparing option for treating ESBL-producing bacterial bacteremia is worthy of exploration.
PK data for MOX, FEP, and CFZ/SBT were derived from healthy individuals, and may differ from those of severe patients. The three drugs were excreted largely through the glomerulus. Reitberg et al (1988) found that PK data for CFZ were not statistically different between normal individuals and patients with decreased renal function.43 For FEP and MOX, CL and t1/2 values will be prolonged in the case of renal dysfunction, possibly resulting in higher %T >MIC values. It is noteworthy that the dosing interval should be extended to avoid excessive drug accumulation for such patients.44,45 In addition, a one-compartment i.v. bolus model was employed to calculate %T >MIC values for these drugs, whereas they are often administrated over 15–30 min in the clinic. Of note, a 15–30 min infusion had little effect on total exposure, and other investigators have also used the same model to evaluate %T >MIC.46,47 Meanwhile, the short elimination half-life for CFZ (t1/2=1 h) does not induce drug accumulation in vivo following multiple dosing.43 Although the t1/2 of FEP and MOX is longer,28,48 FEP (2 g iv q8h) and MOX (1 g intramuscular q8 or q12h) underwent little or no accumulation.48,49 Finally, our MIC distributions against Enterobacteriaceae were derived from BSIs in China, which may be different from those in other large surveillance databases.
MCS can be used to calculate PTA values for monotherapies using established mathematical models. However, the conditions of patients are commonly complicated, and combination antibiotic therapies are prescribed in clinical practice. MCS is unfortunately helpless in such situations at present. Furthermore, this study used 50% fT >MIC as the target attainment for the bactericidal PD target. However, some previous studies found that β-lactamase antibiotics only achieved limited killing against isolates, even when %fT >MIC exceeded 50%.37,50 Therefore, further studies, including dynamic PK/PD simulations, animal models, and clinical trials, are urgently needed to evaluate the efficacy of FEP, MOX, and CFZ/SBT monotherapies and combinations against ESBL producers to alleviate the selective pressure on carbapenem-resistant germs.
Conclusion
To our knowledge, the present work utilized the largest numbers of strains in a MCS analysis in China. ESBL producers isolated from BSIs were highly susceptible to MOX. FEP and CFZ/SBT are not suitable to empirically treat ESBL-producing bacterial bacteremia. By contrast, MOX can be used empirically to treat BSIs caused by ESBL-producing E. coli, and definitively for isolates with MIC ≤4 mg/L, due to its marked in vitro stability and favorable PD properties.
Acknowledgment
This work was supported by Key Research and Development Program of Zhejiang Province (No. 2015C03032) and the National Natural Science Foundation of China (No. 81711530049).
Disclosure
The authors reported no conflicts of interest in this work.
References
1. Nielsen EI, Friberg LE. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Pharmacol Rev. 2013;65(3):1053–1090. doi:10.1124/pr.111.005769
2. Rodriguez-Tudela JL, Almirante B, Rodriguez-Pardo D, et al. Correlation of the MIC and dose/MIC ratio of fluconazole to the therapeutic response of patients with mucosal candidiasis and candidemia. Antimicrob Agents Chemother. 2007;51(10):3599–3604. doi:10.1128/AAC.00296-07
3. De Rosa FG, Pagani N, Fossati L, et al. The effect of inappropriate therapy on bacteremia by ESBL-producing bacteria. Infection. 2011;39(6):555–561. doi:10.1007/s15010-011-0201-x
4. Scheuerman O, Schechner V, Carmeli Y, et al. Comparison of predictors and mortality between bloodstream infections caused by ESBL-producing Escherichia coli and ESBL-producing Klebsiella pneumoniae. Infect Cont Hosp Ep. 2018;39(6):660–667. doi:10.1017/ice.2018.63
5. Tumbarello M, Spanu T, Sanguinetti M, et al. Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Chemother. 2006;50(2):498–504. doi:10.1128/AAC.50.2.498-504.2006
6. Goodman KE, Lessler J, Cosgrove SE, et al. A clinical decision tree to predict whether a bacteremic patient is infected with an extended-spectrum beta-lactamase-producing organism. Clin Infect Dis. 2016;63(7):896–903. doi:10.1093/cid/ciw425
7. Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: A distinct entity? Insights from a large U.S. database. Crit Care Med. 2006;34(10):2588–2595. doi:10.1097/01.CCM.0000239121.09533.09
8. Ofer-Friedman H, Shefler C, Sharma S, et al. Carbapenems versus piperacillin-tazobactam for bloodstream infections of nonurinary source caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Infect Control Hosp Epidemiol. 2015;36(8):981–985. doi:10.1017/ice.2015.101
9. Yang CC, Li SH, Chuang FR, et al. Discrepancy between effects of carbapenems and flomoxef in treating nosocomial hemodialysis access-related bacteremia secondary to extended spectrum beta-lactamase producing Klebsiella pneumoniae in patients on maintenance hemodialysis. BMC Infect Dis. 2012;12:206. doi:10.1186/1471-2334-12-166
10. Falcone M, Vena A, Mezzatesta ML, et al. Role of empirical and targeted therapy in hospitalized patients with bloodstream infections caused by ESBL-producing Enterobacteriaceae. Ann Ig. 2014;26(4):293–304. doi:10.7416/ai.2014.1989
11. Hu F, Guo Y, Zhu D, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance. Chin J Infect Chemother. 2017;17(1):93–99.
12. Harris PN, Tambyah PA, Paterson DL. beta-lactam and beta-lactamase inhibitor combinations in the treatment of extended-spectrum beta-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic options? Lancet Infect Dis. 2015;15(4):475–485. doi:10.1016/S1473-3099(14)70950-8
13. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and beta-lactam/beta-lactamase inhibitors in the treatment of infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69(4):871–880. doi:10.1093/jac/dkt450
14. Lee CH, Su LH, Tang YF, Liu JW. Treatment of ESBL-producing Klebsiella pneumoniae bacteraemia with carbapenems or flomoxef: a retrospective study and laboratory analysis of the isolates. J Antimicrob Chemother. 2006;58(5):1074–1077. doi:10.1093/jac/dkl381
15. Ito A, Tatsumi Y, Wajima T, Nakamura R, Tsuji M. Potent antibacterial activities of latamoxef (moxalactam) against ESBL producing Enterobacteriaceae analyzed by Monte Carlo simulation. Jpn J Antibiot. 2014;67(2):109–122.
16. Wang H, Zhang B, Ni Y, et al. Pharmacodynamic target attainment of seven antimicrobials against Gram-negative bacteria collected from China in 2003 and 2004. Int J Antimicrob Agents. 2007;30(5):452–457. doi:10.1016/j.ijantimicag.2007.06.005
17. Yu G, Chang T, Sun W, et al. Optimization of β-lactam treatment regimens for extended-spectrum β-lactamases-producing strains infections based on pharmacokinetics/pharmacodynamics. Chin J Nosocomiology. 2009;19(22):3108–3110.
18. Yu G, Chen G, Gao C, et al. Evaluation of β-lactam treatment regimens for gram-negative infections using Monte Carlo simulation. COMPUT Appl Chem. 2010;27(11):001553–001556.
19. Zhou H, Li G, Chen B, et al. Chinese experts concensus on the treatment of infections caused by ESBL-producing Enterobacteriaceae. Natl Med J China. 2014;94(24):1847–1856.
20. Xiao Y, Hu Y. The reliability of using impenem, meropenem, cefoperazone-sulbactam and piperacillin-tazobactam to treat nosocomial gram-negative bacterial infections with Monte Carlo simulation. Chin J Intern Med. 2017;56(8):595–600.
21. Cai T, Ye L. Pharmacodynamics of prolonged and continuous infusion regimens of three β-lactam antimicrobial agents against extended-spectrum β-lactamases producing bacteria. Chin J Nosocomiology. 2010;20(14):2110–2113.
22. Chen S, Fan J. Optimization of latamoxef regimens for ESBLs-producing Enterobacteriaceae infection by Monte-Carlo simulation method. China Pharm. 2013;6:509–511.
23. Wattal C, Oberoi JK, Goel N, Raveendran R, Khanna S. Matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) for rapid identification of micro-organisms in the routine clinical microbiology laboratory. Eur J Clin Microbiol. 2017;36(5):807–812. doi:10.1007/s10096-016-2864-9
24.
25. Jones RN, Barry AL, Packer RR, Gregory WW, Thornsberry C. In vitro antimicrobial spectrum, occurrence of synergy, and recommendations for dilution susceptibility testing concentrations of the cefoperazone-sulbactam combination. J Clin Microbiol. 1987;25(9):1725–1729.
26. Hansen DS, Schumacher H, Hansen F, et al. Extended-spectrum beta-lactamase (ESBL) in Danish clinical isolates of Escherichia coli and Klebsiella pneumoniae: prevalence, beta-lactamase distribution, phylogroups, and co-resistance. Scand J Infect Dis. 2012;44(3):174–181. doi:10.3109/00365548.2011.632642
27. Friden M, Ljungqvist H, Middleton B, Bredberg U, Hammarlund-Udenaes M. Improved measurement of drug exposure in the brain using drug-specific correction for residual blood. J Cereb Blood Flow Metab. 2010;30(1):150–161. doi:10.1038/jcbfm.2009.200
28. Israel KS, Black HR, Brier GL, Wolny JD, DeSante KA. Single- and multiple-dose pharmacokinetics of moxalactam in normal subjects. Antimicrob Agents Chemother. 1982;22(1):94–102.
29. Wei M, Zhao C, Qi H, et al. Pharmacokinetics of sulbactam/cefoperazon (1:1) in healthy adult and old people. Chin J Clin Pharmacol. 2007;23(1):28–32.
30. Nye KJ, Shi YG, Andrews JM, Wise R. Pharmacokinetics and tissue penetration of cefepime. J Antimicrob Chemother. 1989;24(1):23–28.
31. DeRyke CA, Kuti JL, Nicolau DP. Pharmacodynamic target attainment of six beta-lactams and two fluoroquinolones against Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, and Klebsiella species collected from United States intensive care units in 2004. Pharmacotherapy. 2007;27(3):333–342. doi:10.1592/phco.27.3.333
32. Kuti JL, Nightingale CH, Nicolau DP. Optimizing pharmacodynamic target attainment using the MYSTIC antibiogram: data collected in North America in 2002. Antimicrob Agents Chemother. 2004;48(7):2464–2470. doi:10.1128/AAC.48.7.2464-2470.2004
33. Li C, Sun J, Miao J, et al. Using Monte Carlo simulation to determine optimal dosing regimen for cefetamet sodium for injection. J Chemother. 2016;28(3):172–179. doi:10.1179/1973947814Y.0000000214
34. Micek S, Johnson MT, Reichley R, Kollef MH. An institutional perspective on the impact of recent antibiotic exposure on length of stay and hospital costs for patients with gram-negative sepsis. BMC Infect Dis. 2012;12:56. doi:10.1186/1471-2334-12-166
35. Ambrose PG, Bhavnani SM, Jones RN. Pharmacokinetics-pharmacodynamics of cefepime and piperacillin-tazobactam against Escherichia coli and Klebsiella pneumoniae strains producing extended-spectrum-lactamases: report from the ARREST program. Antimicrob Agents Chemother. 2003;47(5):1643–1646.
36. Quan JJ, Wang Y, Ji JS, et al. The activity of moxalactam against Enterobacteriaceae and anaerobia in vitro. Zhonghua Yi Xue Za Zhi. 2016;96(18):1459–1464. doi:10.3760/cma.j.issn.0376-2491.2016.18.015
37. Huang C, Zheng B, Yu W, et al. Antibacterial effect evaluation of moxalactam against extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae with in vitro pharmacokinetics/pharmacodynamics simulation. Infect Drug Resist. 2018;11:103–112. doi:10.2147/IDR.S150431
38. Yang Q, Zhang H, Cheng J, et al. In vitro activity of flomoxef and comparators against Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis producing extended-spectrum beta-lactamases in China. Int J Antimicrob Agents. 2015;45(5):485–490. doi:10.1016/j.ijantimicag.2014.11.012
39. Wang R, Cosgrove SE, Tschudin-Sutter S, et al. Cefepime therapy for cefepime-susceptible extended-spectrum beta-lactamase-producing Enterobacteriaceae bacteremia. Open Forum Infect Dis. 2016;3(3):ofw132. doi:10.1093/ofid/ofw132
40. Andes D, Craig WA. Treatment of infections with ESBL-producing organisms: pharmacokinetic and pharmacodynamic considerations. Clin Microbiol Infect. 2005;11(Suppl 6):10–17. doi:10.1111/j.1469-0691.2005.01265.x
41. Guo H, Qin J, Xiang J. Surveillance for and susceptibility of Acinetobacter baumannii in a large hospital and burn center in Shanghai, China, 2007–2013. Am J Infect Control. 2016;44(12):1718–1719. doi:10.1016/j.ajic.2016.06.014
42. Matsumura Y, Yamamoto M, Nagao M, et al. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-beta-lactamase-producing Escherichia coli bacteremia. Antimicrob Agents Chemother. 2015;59(9):5107–5113. doi:10.1128/AAC.00701-15
43. Reitberg DP, Marble DA, Schultz RW, Whall TJ, Schentag JJ. Pharmacokinetics of cefoperazone (2.0 G) and sulbactam (1.0 G) coadministered to subjects with normal renal-function, patients with decreased renal-function, and patients with end-stage renal-disease on hemodialysis. Antimicrob Agents Chemother. 1988;32(4):503–509.
44. Carmine AA, Brogden RN, Heel RC, Romankiewicz JA, Speight TM, Avery GS. Moxalactam (latamoxef). A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1983;26(4):279–333. doi:10.2165/00003495-198326040-00001
45. Wynd MA, Paladino JA. Cefepime: a fourth-generation parenteral cephalosporin. Ann Pharmacother. 1996;30(12):1414–1424. doi:10.1177/106002809603001211
46. Kim MK, Capitano B, Mattoes HM, et al. Pharmacokinetic and pharmacodynamic evaluation of two dosing regimens for piperacillin-tazobactam. Pharmacotherapy. 2002;22(5):569–577.
47. Kuti JL, Florea NR, Nightingale CH, Nicolau DP. Pharmacodynamics of meropenem and imipenem against Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa. Pharmacotherapy. 2004;24(1):8–15.
48. Barbhaiya RH, Forgue ST, Gleason CR, et al. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother. 1992;36(3):552–557.
49. Meyers BR, Hirschman SZ, Giron J, Srulevitch ES. Pharmacokinetic studies of single and multiple doses of moxalactam (moxam) in normal volunteers. Clin Trials J. 1982;19(2):63–73.
50. McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31(4):345–351. doi:10.1016/j.ijantimicag.2007.12.009
Supplementary materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




