Back to Journals » OncoTargets and Therapy » Volume 12
Silencing of CARMA3 inhibits bladder cancer cell migration and invasion via deactivating β-catenin signaling pathway
Authors Man X, Liu T, Jiang Y, Zhang Z, Zhu Y, Li Z, Kong C, He J
Received 19 October 2018
Accepted for publication 2 July 2019
Published 9 August 2019 Volume 2019:12 Pages 6309—6322
DOI https://doi.org/10.2147/OTT.S191502
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Xiaojun Man,1,2 Tao Liu,1,2 Yuanjun Jiang,1,2 Zhe Zhang,1,2 Yuyan Zhu,1,2 Zhenhua Li,1,2 Chuize Kong,1,2 Jiani He3
1Department of Urology, The First Hospital of China Medical University, Shenyang 110001, People’s Republic of China; 2Institute of Urology, Department of Urology, China Medical University, Shenyang 110001, People’s Republic of China; 3Breast Division, Department of Surgical Oncology, The First Hospital of China Medical University, Shenyang 110001, People’s Republic of China
Background: Bladder cancer (BC) is the ninth most common cancer and the fourteenth leading death worldwide. CARD-containing MAGUK 3 (CARMA3) protein is a novel scaffold protein known to activate NF-κB pathway and is overexpressed in BC tissues.
Purpose: The objective of this study was to identify how CARMA3 affects the metastasis of BC cells via the β-catenin signaling pathway.
Materials and methods: In the present study, 5637 and T24 BC cells with stable low expression of CARMA3 were established, and their migratory and invasive capabilities were further evaluated by wound-healing and transwell assay. The activity and expression of β-catenin were determined by Luciferase assay and immunofluoresence staining. The mRNA and protein expression levels of CARMA3, matrix metallopeptidase (MMP) 9 and MMP2 were detected by quantitative real-time PCR (qRT-PCR) and Western blot analysis. The nude mouse tumor xenograft model was established for in vivo study.
Results: By comparison to the control cells, CARMA3-silenced cells acquired a less aggressive phenotype: decreased migration and invasion. More importantly, we confirmed that CARM3 knockdown could inhibit β-catenin mRNA and protein expression and activity, and reduce the expression and/or activity of matrix metallopeptidase (MMP) 9, MMP2 and C-myc. Also, CARM3 silencing increased E-cadherin expression and attenuated the expression of β-catenin. Moreover, we demonstrated that β-catenin overexpression reversed the inhibiting effect of CARMA3 silencing on cell invasion and migration. Furthermore, our study illustrated that knockdown of CARMA3 suppressed BC cells xenograft tumor growth in nude mice.
Conclusion: We demonstrated that CARMA3 contributes to the malignant phenotype of BC cells at least by activating β-catenin signaling pathway, and it may serve as a therapeutic target for clinic treatment in BC.
Keywords: CARMA3, β-catenin signaling pathway, bladder cancer, migration, invasion
Introduction
Metastasis results in approximately 90% of cancer-associated mortality, though the high grade risk of why people die from cancers is still unclear.1 Bladder cancer (BC), as one of the most prevalent cancers among men, is the ninth common cancer and the fourteenth leading death worldwide. This cancer may be caused by some known risk factors, such as cigarette smoking, urinary tract infection, and occupational hazards.2 It had been diagnosed 430,000 new cases of BC in 2012, and only less than 15% patients can survive for 5 years from metastatic BC.3 The management of BC depends mainly on pathological and radiological diagnosis. The main challenge faced by many researchers is the poor understanding of molecular parameters and available therapeutic targets in BC. Therefore, elucidating the mechanisms of genes involved in tumor invasion and migration is urgently needed.
CARD-Containing MAGUK Protein family (CARMA) proteins are scaffold proteins, which have three members: CARMA1, CARMA2, CARMA3.4–7 Increasing evidence suggests that CARMA family of proteins play an important role in tumor progression. CARMA3, also known as Caspase Recruitment Domain Family Member 10 (CARD10), has been reported to promote the growth and metastasis of cancer cells, such as lung cancer cells, colorectal carcinoma cells, glioma cancer cells, breast and skin cancer cells.8–11 Our prior work demonstrated that CARMA3 was upregulated in BC tissues, and such elevation contributed to the proliferation of BC cells.12 Further, a study from Zhang et al indicated that CARMA3 re-expression reversed the anti-tumor effects of microRNA-24 in BC cells, which indirectly suggested an involvement of CARMA3 in BC carcinogenesis.13 It should be noted that most of the previous studies have linked CARMA3 to nuclear factor kappa B (NF-κB) pathway.14,15 Whether there are pathways other than NF-κB involved in CARMA3-regulating tumor metastasis remains unclear.
Wnt/β-catenin signal pathway plays a vital role in cell development and maintenance, whereas its aberrant activation triggers tumorigenesis.16,17 Active β-catenin clusters in the cell nucleus to activate the transcription of target genes.18 It has been reported that β-catenin activation can synergize with Phosphatase and Tensin Homolog (PTEN) deficiency to induce BC formation.19 Furthermore, inactivation of β-catenin induced by either microRNA20 or pharmaceutical drug21 leads to a less malignant behavior of BC cells. Therefore, given the important role of β-catenin pathway in BC carcinogenesis, we assessed whether CARMA3 can regulate this pathway in BC cells.
In the present study, we first constructed BC cells with stable low expression of CARMA3, and evaluated their mobility. We found that CARMA3-silenced cells acquired a less migratory and invasive phenotype, and the β-catenin pathway was deactivated in these cells. Additionally, forced re-expression of active β-catenin partly reversed the alterations induced by CARMA3 shRNA in BC cells.
Materials and methods
Cell culture and transfection
The 5637 and T24 cells were obtained from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in PRMI-1640 (R8458, Sigma, USA) containing 10% fetal calf serum (FBS; F8067, Sigma, USA). All cells were grown at 37 °C under 5% CO2.
Two shRNAs exclusively targeting CARMA3 gene and negative control shRNA were inserted into pRNAH1.1 plasmid (Sangon Biotech, Shanghai, China) to form sh-1-CARMA3, sh-2-CARMA3 and NC plasmids. For transfection, 5637 and T24 cells were plated into 6-well plate at a density of 4×105 cells/well, and 24 hrs later, these cells were transfected with NC, sh1-CARMA3 or sh2-CARMA3 vector. The transfection was mediated by Lipofectamine 2000 (11,668,019, Invitrogen, USA) according to the manufacturer’s instructions. After transfection, 5637 cells were screened by 400 g/mL G418, and T24 cells were screened by 300 g/mL (11,811,023, Invitrogen, USA) for about two weeks. Then cells resistant to G418 were used in the following study.
After transfection, the 5637 and T24 cells were treated with 100 μg/mL cycloheximide (CHX) for 6h, then the β-catenin expression levels of these cells were detected by Western blot.
For co-transfection, 5637 and T24 cells were cultured for 24 hrs, and co-transfected with NC, sh1-CARMA3 or sh2-CARMA3 vector and pcDNA3 or pcDNA3-CARMA3 vector for further study. The transfection was mediated by Lipofectamine 2000 (11,668,019, Invitrogen, USA) according to the manufacturer’s instructions.
Gelatinase-zymography
The proteins were extracted from 5637 and T24 cells, and analyzed for gelatin degradation activity through electrophoresis. After SDS-PAGE, the gels were washed in Buffer A containing 2.5% (v/v) Triton® X-100 (694, Amersco, Canada), 50 mmol/L Tris-HCl, 5 mmol/L CaCl2, 1 μmol/L ZnCl2 for 40 mins at room temperature (RT), equilibrated for 20 mins at RT in Buffer B containing 50 mmol/L Tris-HCl, 5 mmol/L CaCl2, 1 μmol/L ZnCl2, and followed by overnight incubation with developing buffer containing 50 mmol/L Tris-HCl, 5 mmol/L CaCl2, 1 μmol/L ZnCl2, 0.02%Brij, 0.2 mol/L NaCl at 37 °C. Then, the zymogram gels were stained with the Commassie Blue Staining Solution (0427, Amersco, Canada) for 3 hrs at RT with gentle shaking. After being washed by Coomassie Blue destaining solution, the zymogram gel was scanned and the band intensities were measured.
Immunofluoresence staining
The 5637 and T24 cell slides were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton-X-100 in PBS. Cells were then blocked for 60 mins and incubated overnight with anit-β-Catenin antibody (1:50, #8480, Cell Signaling Technology, USA) or E-cadherin (20874-1-AP, proteintech, China), followed by FITC-labeled goat anti-rabbit IgG (H + L) (1:200, A0562, Beyotime, China) or Cy3-labeled goat anti-rabbit IgG (H + L) (1:50, A0516, Beyotime, China) secondary antibody for 30 mins. After DAPI staining, images were captured under a fluorescent microscope.
Cell migration assay
Wound-healing assay was performed to detect to changes in cell migration. Cells were seeded in 6-well plates, and grew to confluent, and then a scratch wound was made with a 200-μl pipette tip in a straight line. After rinsing with serum free RPMI-1640 medium (SFM), cells were incubated in a condition of 37 °C under 5% CO2 for 24 hrs. Cell images were captured under a microscope at 0 hr and 24 hrs post-wounding.
Cell invasion assay
Cells were counted and diluted into cell suspension of 1×105 cells/ml. In the lower chamber of transwell inserts with 8.0-μm pore size membrane filters (#3422, corning life science, USA), 800 μL of 30% FBS was added. In the upper chamber of transwell inserts, 2×104 cells in 200 μL SFM were loaded. Twenty-four hours later, the transwell inserts were washed twice with PBS. The membrane were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet dye.
Western blotting
Cells were lysed with RIPA Lysis Buffer (P0013B, Beyotime, China) or Nuclear and Cytoplasmic Protein Extraction Kit (P0027, Beyotime, China). Then protein concentrations were quantified with BCA Protein Assay Kit (P0011, Beyotime, China). Twenty microlitre of proteins were separated by SDS-PAGE and then transferred onto PVDF membranes. After blocking, the membranes were incubated with the one of following antibodies: anti-CARMA3 antibody (1:1000, bs-7081R, Bioss, China), anti-MMP2 antibody (bs-0412R, Bioss), anti-MMP9 antibody (1:1000, bs-4593R, Bioss, China), anti-β-catenin antibody (1:1000, #8480, Cell Signaling Technology, USA), anti-Survivin antibody (1:1000, #2808, Cell Signaling Technology, USA), anti-C-myc antibody (1:500, sc-40, Santa Cruz Biotechnology, USA), anti-beta-Actin (1:5000, bsm-33036 M, Bioss, China), or anti-Histone H3.1 (1:500, bs-17422R, Bioss, China) at 4 °C overnight. Then the membranes were incubated in HRP-labeled Goat Anti-Rabbit IgG (H + L) (1:5000, A0208, Beyotime, China) or HRP-labeled Goat Anti-Mouse IgG (H + L) (1:5000, A0216, Beyotime, China) at 37 °C for 45 mins. After incubating ECL reagent for 5 mins, the proteins were detected by ECL Select Western Blotting Detection System (Beyotime, China).
Real-time quantitative RT-PCR (qRT-PCR) analysis
qRT-PCR was performed using SYBR Green PCR Master Mix (SY1020, Solarbio, China) in a total volume of 20 μl on ExicyclerTM 96 Real-Time PCR System (Bionner, Korea). The relative gene expression levels were represented as ΔCt = Ct gene−Ct reference, and the change of relative mRNA level was calculated by the 2−ΔΔCt method. We chose β-actin as a reference gene, and experiments were repeated in quadruplicate. The sequences of primer pairs were as follows: β-actin-F, 5′- CTTAGTTGCGTTACACCCTTTCTTG −3′; β-actin-R, 5′- CTGTCACCTTCACCGTTCCAGTTT −3′; CARMA3-F, 5′-GGTGCCGAGCCCTTCTACATT-3′; and CARMA3-R, 5′-TCCAGGTCCCGCAGAGTGAG-3′.
Mouse tumor xenograft model
The animal experiments were based on the Guide for the Care and Use of Laboratory Animals and conducted in accordance with the protocol approved by the China Medical University Application for Laboratory Animal Welfare and Ethical Committee. The experiments were performed using 6-week-old male mice (n=24) (BALB/c-nu mice; Beijing HFK Bioscience, China). Mice were kept throughout the experiment in pleasant conditions (temperature, 24–26 °C; humidity, 45–55%) and were able to access food and water freely. After a week of adjustable feeding, 3×106 cells were injected into nude mice armpits per group. Tumor size was measured and recorded every three days since formation. After 21 days of injection, mice were killed by injecting of 200 mg/kg sodium pentobarbital. The tumor nodules were subsequently removed for following analysis.
Immunohistochemistry
The tumor nodules from mice were subsequently removed and fixed into paraffin sections. The fixed tissue paraffin sections were first incubated in 3% hydrogen peroxide for 15 mins at RT. After blocking, they were incubated with anti-CARD10 (1:200, bs-7081R, Bioss, China) or anti-β-Catenin (1:100, #8480, Cell Signaling Technology, USA) at 4 °C overnight, and then incubated in Biotin-labeled Goat Anti-Rabbit IgG (H + L) (1:200, A0277, Beyotime, China) at 37 °C for 30 mins. Thereafter, the protein signals were magnified with HRP-labeled Streptavidin (1:200, A0303, Beyotime, China), and visualized with DAB (DA1010, Solarbio, China). Cell nuclei were counterstained with hematoxylin (H8070, Solarbio, Beijing, China). Images were captured under a microscope.
Luciferase assay
After tansfected with NC, sh1-CARMA3 or sh2-CARMA3, the activity of β-catenin was detected by Dual-Luciferase Reporter Assay System (E1910, Promega, USA) and M50 Super 8×TOPFlash (12,456, Addgene, USA).
Co-immunoprecipitation assay
The Co-IP analysis was used for examination of the change in association between β-catenin and E-cadherin in the sh-CARMA3 cells. The protein was extracted from sh-CARMA3 and NC cells by Protein Extraction Kit (WLA019, Wanleibio, China), precipitated by E-cadherin antibody (20874-1-AP, Proteintech, China), and then the precipitated protein was evaluated by Western blotting using β-catenin antibody (1:2000; #2698, CST, USA).
Analysis of data
All data were analyzed with GraphPad Prism 7.0 (GraphPad Software Inc., USA), and present as means ± standard deviation. Comparisons between groups were conducted by un-paired student’s t test or one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test. Statistically significant was written as P<0.05.
Moral statement
The animal experiments in the present study were based on the Guide for the Care and Use of Laboratory Animals and conducted in accordance with the protocol approved by the China Medical University Application for Laboratory Animal Welfare and Ethical Committee (KT2018050).
Results
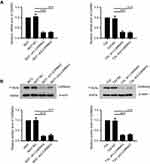
CARMA3 was silenced efficiently in 5637 and T24 cells
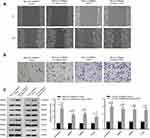
To explore the effect of CARMA3 on tumor progression of BC, the endogenous CARMA3 was stably knocked down in 5637 and T24 BC cell lines with two shRNAs. The expression levels of CARMA3 in BC cells were analyzed by Western blot and real-time PCR (Figure 1). The results showed that cells transfected with sh-1/-2-CARMA3 plasmids had much lower mRNA and protein expression of CARMA3 than cells transfected with NC shRNA (****p<0.0001) (Figure 1A and B). These results suggested that CARMA3 was silenced efficiently in 5637 and T24 cells.
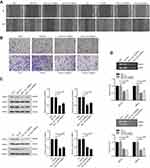
Knockdown of CARMA3 decreases the cell migration, invasion and the expression of MMP2 and MMP9 in 5637 and T24 cells
We next investigated whether the CARMA3 silencing could influence the migration and invasion of 5637 and T24 cells. The obtained data showed that 5637 and T24 cells transfected with sh-CARMA3 exhibited weaker migratory and invasive ability than the control cells (Figure 2A and B). Since matrix metalloproteinases participate in cell metastasis and invasion, we also examined whether CARMA3 knockdown affected the expression of MMP2 and MMP9 (Figure 2C). Results of Western blotting indicated that the expression levels of MMP2 and MMP9 were dramatically reduced when CARMA3 was knocked down (**p<0.01, ***p<0.001). Moreover, we measured the activities of MMP-2 and MMP9 by gelatinase-Zymography, and found that their activities were reduced in CARMA3-silenced cells (**p<0.01, ***p<0.001) (Figure 2D). These data suggested that CARMA3 contributed to BC cell migration and invasion.
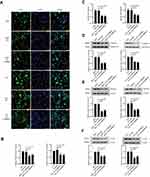
Silencing of CARMA3 deactivates β-catenin signaling pathway
We next explored how CARMA3 silencing affected the transduction of β-catenin signals in 5637 and T24 cells. Immunofluorescence images indicated that nuclear β-catenin presented in normal BC cells, and decreased in cells transfected with CARMA3 shRNA (Figure 3A). The mRNA and protein levels and activity of β-catenin were decreased in CARMA3-silenced 5637 and T24 cells (Figure 3B–D). Data from Western blot analysis confirmed the immunofluorescence results of β-catenin (Figure 3B; **p<0.01, ***p<0.001). Interestingly, the significant decreasing of mRNA and expression levels of β-catenin were observed. To investigate the mechanism by which sh-CARMA3 decreased β-catenin expression, we examined the expression of β-catenin after 100 μg/mL Cycloheximide (CHX) treatment. CHX was a translation blocker and could inhibit protein synthesis22 After the β-catenin protein synthesis was inhibited by treatment with CHX in transfected with NC or sh-CARMA3 5637 and T24 cells, the expression of β-catenin in sh-CARMA3 cells was still significantly decreased compared to NC cells (Figure S1). This finding could provide evidence for that the regulation of β-catenin expression by knockdown of CARMA3 depends on both transcriptional and post-translational mechanisms. Furthermore, the expression levels of survivin and C-myc, two downstream targets of β-catenin pathway, were detected with Western blot. As shown in Figure 3E and F, the expression levels of survivin and C-myc were significantly reduced in sh-CARMA3 transfected cells (**p<0.01, ***p<0.001). The above evidence revealed that CARMA3 inhibition significantly blocked β-catenin pathway activation.
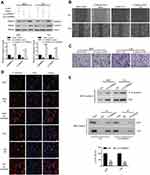
Knockdown of CARMA3 inhibited the activity of adherens junction between the β-catenin and e-cadherin
Furthermore, we designed a new siRNA against CARMA3 (si-CARMA3) that targeted the 3ʹ-untranslated non-coding region of the CARMA3 mRNA. Our result showed that overexpression of CARMA3 attenuated the inhibiting effect of si-CARMA3 on β-catenin (Figure 4A). The function of β-Catenin-S33Y (constitutively active β-catenin) on migration and invasion ability of BC cells was studied by wound healing and transwell assays. As shown in Figure 4B and C, overexpression of β-catenin could increase the migration and invasion ability of 5637 and T24 cells. Moreover, knockdown of CARMA3 increased the expression of E-cadherin, which was detected by IF (Figure 4D). The interaction between b-catenin and E-cadherin was evaluated by co-IP experiment and the co-IP efficiency was calculated as Li et al described23 Surprisingly, the results showed that downregulation of CARMA3 inhibited the interaction between b-catenin and E-cadherin (Figure 4E). Moreover, the Wnt signaling pathway was activated by over-expression of CARMA3 (Figure S1). Collectively, these data suggested that β-catenin besides being a major component of the E-cadherin adhesion complex, is also a central mediator in the canonical Wnt pathway while E-cadherin acts as a negative mediator of this pathway.
Overexpression of β-catenin reversed the inhibition of CARM3 silencing on cell migration and invasion
We asked whether β-catenin pathway activation was important for CARMA3’s regulation on the mobility of BC cells and explored further. The reactivation of β-catenin was then introduced in CARMA3-silenced 5637 and T24 BC cells. We found that CARMA3-silenced cells transfected with β-catenin-S33Y plasmids regained aggressive behaviors: enhanced migration and invasion (Figure 5A and B). Moreover, silencing of CARMA3-induced reduction in MMP9, MMP2 and C-myc were counteracted by β-catenin-S33Y expression (Figure 5C; **p<0.01, ***p<0.001). These data suggested that alterations induced by CARMA3 knockdown in BC cells were rescued by forced overexpression of β-catenin.
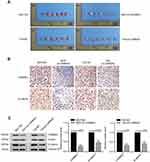
Silencing of CARMA3 impairs tumor progression in vivo
In vivo, BC cells stably transfected by NC shRNA or CARMA3 shRNA were subcutaneously injected into nude mice. We found that CARMA3 silencing did not affect the formation rate of 5637 and T24 cell lines, but restrained their growth in vivo (Figure 6A). Like in vitro, we also observed a reduction of β-catenin in CARMA3-silenced tumors (Figure 6B and C).
Discussion
In the present study, we confirm a direct link between CARMA3 and β-catenin signaling pathway: knockdown of CARMA3 can inhibit β-catenin signaling pathway. Moreover, excessive activation of β-catenin signaling pathway recovers cell invasion and migration capability in CARMA3-silenced BC cells.
In ovarian cancer cells, CARMA3 depletion inhibited the proliferation and blocked cell cycle progression in A2780 and HO8910 cancer cells.24 In lung cancer cells, CARMA3 overexpression promoted cell motility and enhanced cancer cell stemness.8 These previous studies suggest a role of CARMA3 in promoting cancer cell growth. We prior proved that knockdown of CARMA3 suppressed the growth of BC cells.12 In the present study, we further demonstrated that CARMA3 contributed to BC cell migration and invasion by performing loss-of-function experiments of CARMA3. Such findings also suggest that CARMA3 expression is required for bladder carcinogenesis.
Wnt/β-catenin pathway plays a key role in carcinogenesis. Wnt stimulation induces β-catenin stabilization and nuclear translocation, thereby resulting in gene transcription.25 In BC tissues, enhanced nuclear accumulation of β-catenin has been observed.26 β-catenin can form a complex with androgen receptor and T-cell factor (TCF) to accelerate androgen-induced metastasis of BC cells.26,27 Notably, the activated Wnt/β-catenin signaling is capable of initiating the NF-κB-dependent transcription in cancer cells.28,29 We previously found that, in CARMA3-silenced BC cells, the DNA binding activity of NF-κB was reduced.12 Given the fact that the activated β-catenin regulates NF-κB signaling transduction, we here assume that CARMA3 silencing also affects the signaling delivery of wnt/β-catenin pathway. In the present study, we found that over-expression of CARMA3 could up-regulate Wnt1 and Wnt3a expression (two canonical Wnt signalling ligands) (Fig.Supplement C). CARMA3 shRNA hindered the nuclear accumulation of β-catenin, and inhibited the expression of its downstream targets, survivin and c-myc,30,31 in two BC cell lines. Moreover, co-IP assay showed that there was no direct interaction between CARMA3 and beta-catenin (Fig.Supplement B). However, CARMA3 expression in bladder cancer shows a weak positive correlation with β-catenin expression by Gene Expression Profiling Interactive Analysis.42
β-catenin as a component of adherens junctions plays an additional role in several diseases through binding with various cadherins such as E-cadherin. The E-cadherin-β-catenin complex relates to actin filaments and forms a link with the actin cytoskeleton32 In other studies, Inhibitor of β-catenin and T-cell factor (ICAT) could disrupt the E-cadherin/β-catenin complex and reduce the expression of E-cadherin, and thus inhibiting Wnt/β-catenin signaling pathway.33–35 Considering that overexpression of CARMA3 induced the expression of β-catenin, we examined and found that silence of CARMA3 could decrease the interaction between b-catenin and E-cadherin. Nevertheless, the E-cadherin expression was increased in sh-CARMA3 cells. Given that over-expression of CARMA3 could up-regulate Wnt1 and Wnt3a expression, we could speculate that the CARMA3 knockdown mainly increased E-cadherin expression and then down-regulated β-catenin expression, although the interaction between b-catenin and E-cadherin was attenuated. These findings indicated that, in this study, β-catenin acted mainly as a central mediator in the canonical Wnt pathway while E-cadherin acts as a negative mediator of this pathway36 Interestingly, tumor xenograft model was further established by injecting control or CARMA3-silenced BC cells in nude mice. We found that, like in vitro, nuclear expression of β-catenin was also reduced in xenograft tumors formed by CARMA3-silenced BC cells.
MMP2 and MMP9 are traditional prognostic variables in BC, and their expression elevation has been proved to contribute to BC cell metastasis.37,38 Herein, we found that the expression and activity of both MMP2 and MMP9 were decreased in CARMA3-silenced BC cells. A previous study showing CARMA3 was required for the upregulation of MMPs in breast cancer cells supported our findings.39 Translocation to the cell nucleus of β-catenin and sequential formation of the TCF/β-catenin complex induce expression of MMP2 and MMP9 in cancer cells.40,41 Thus, we next evaluated whether CARMA3 regulated MMP2 and MMP9 expression through β-catenin pathway. We further found that the forced overexpression of active β-catenin induced upregulation of MMP2 and MMP9 in CARMA3-silenced BC cells. Such alterations were accompanied by enhanced cell migration and invasion. These results demonstrate that CARMA3 participates in extracellular matrix remodeling via modulating β-catenin-associated MMPs.
Conclusion
In conclusion, our study demonstrates that silencing of CARMA3 inhibits cell migration and invasion of BC cells by inducing deactivation of β-catenin. These results further suggest CARMA3 as a candidate target in treating patients of metastatic BC.
Acknowledgments
This study was supported by grants from the Shenyang Science and Technology Project (No. 17-230-9-38), the Natural Science Foundation Guide Project of Liaoning Province (No. 201602830), and the Natural Science Foundation of Liaoning Province (No. 2015020519).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi:10.1126/science.1203543
2. Hartge P, Harvey EB, Linehan WM, et al. Unexplained excess risk of bladder cancer in men. J Natl Cancer Inst. 1990;82:1636–1640. doi:10.1093/jnci/82.20.1636
3. Bellmunt J, Guix M. New agents for bladder cancer. Ann Oncol. 2010;21 Suppl 7:vii56–vii58. doi:10.1093/annonc/mdq367
4. Blonska M, Lin X. NF-kappaB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55–70. doi:10.1038/cr.2010.182
5. Gaide O, Martinon F, Micheau O, Bonnet D, Thome M, Tschopp J. Carma1, a CARD-containing binding partner of Bcl10, induces Bcl10 phosphorylation and NF-kappaB activation. FEBS Lett. 2001;496:121–127. doi:10.1016/s0014-5793(01)02414-0
6. McAllister-Lucas LM, Inohara N, Lucas PC, et al. Bimp1, a MAGUK family member linking protein kinase C activation to Bcl10-mediated NF-kappaB induction. J Biol Chem. 2001;276:30589–30597. doi:10.1074/jbc.M103824200
7. Wang L, Guo Y, Huang WJ, et al. Card10 is a novel caspase recruitment domain/membrane-associated guanylate kinase family member that interacts with BCL10 and activates NF-kappa B. J Biol Chem. 2001;276:21405–21409. doi:10.1074/jbc.M102488200
8. Chang YW, Chiu CF, Lee KY, et al. CARMA3 represses metastasis suppressor NME2 to promote lung cancer stemness and metastasis. Am J Respir Crit Care Med. 2015;192:64–75. doi:10.1164/rccm.201411-1957OC
9. Miao Z, Zhao T, Wang Z, et al. CARMA3 is overexpressed in colon cancer and regulates NF-kappaB activity and cyclin D1 expression. Biochem Biophys Res Commun. 2012;425:781–787. doi:10.1016/j.bbrc.2012.07.152
10. Feng X, Miao G, Han Y, Xu Y. CARMA3 is overexpressed in human glioma and promotes cell invasion through MMP9 regulation in A172 cell line. Tumour Biol. 2014;35:149–154. doi:10.1007/s13277-013-1018-2
11. Jiang T, Grabiner B, Zhu Y, et al. CARMA3 is crucial for EGFR-Induced activation of NF-kappaB and tumor progression. Cancer Res. 2011;71:2183–2192. doi:10.1158/0008-5472.CAN-10-3626
12. Man X, He J, Kong C, Zhu Y, Zhang Z. Clinical significance and biological roles of CARMA3 in human bladder carcinoma. Tumour Biol. 2014;35:4131–4136. doi:10.1007/s13277-013-1540-2
13. Zhang S, Zhang C, Liu W, et al. MicroRNA-24 upregulation inhibits proliferation, metastasis and induces apoptosis in bladder cancer cells by targeting CARMA3. Int J Oncol. 2015;47(4):1351–1360. doi:10.3892/ijo.2015.3117
14. Zhao T, Miao Z, Wang Z, et al. CARMA3 overexpression accelerates cell proliferation and inhibits paclitaxel-induced apoptosis through NF-kappaB regulation in breast cancer cells. Tumour Biol. 2013;34:3041–3047. doi:10.1007/s13277-013-0869-x
15. Li Z, Qu L, Dong Q, et al. Overexpression of CARMA3 in non-small-cell lung cancer is linked for tumor progression. PLoS One. 2012;7:e36903. doi:10.1371/journal.pone.0036903
16. Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi:10.1016/s0304-419x(03)00005-2
17. Weeraratna AT. A Wnt-er wonderland–the complexity of Wnt signaling in melanoma. Cancer Metastasis Rev. 2005;24:237–250. doi:10.1007/s10555-005-1574-z
18. Jamieson C, Mills KM, Lui C, et al. Characterization of a beta-catenin nuclear localization defect in MCF-7 breast cancer cells. Exp Cell Res. 2016;341:196–206. doi:10.1016/j.yexcr.2016.01.020
19. Ahmad I, Morton JP, Singh LB, et al. beta-Catenin activation synergizes with PTEN loss to cause bladder cancer formation. Oncogene. 2011;30:178–189. doi:10.1038/onc.2010.399
20. Wu CL, Ho JY, Chou SC, et al. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget. 2016;7:26593–26603. doi:10.18632/oncotarget.8557
21. Wu K, Ning Z, Zeng J, et al. Silibinin inhibits beta-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial-mesenchymal transition and stemness. Cell Signal. 2013;25:2625–2633. doi:10.1016/j.cellsig.2013.08.028
22. Goulart-Silva F, Serrano-Nascimento C, Texeira SS, Nunes MT. Triiodothyronine (T3) induces proinsulin gene expression by activating PI3K: possible roles for GSK-3beta and the transcriptional factor PDX-1. Exp Clin Endocrinol Diabetes. 2013;121:14–19. doi:10.1055/s-0032-1327598
23. Li M, Zhang Z, Koh H, et al. The beta1-subunit of the MaxiK channel associates with the thromboxane A2 receptor and reduces thromboxane A2 functional effects. J Biol Chem. 2013;288:3668–3677. doi:10.1074/jbc.M112.426585
24. Xie C, Han Y, Fu L, Li Q, Qiu X, Wang E. Overexpression of CARMA3 is associated with advanced tumor stage, cell cycle progression, and cisplatin resistance in human epithelial ovarian cancer. Tumour Biol. 2014;35:7957–7964. doi:10.1007/s13277-014-2070-2
25. Montagner A, Le Cam L, Guillou H. β-catenin oncogenic activation rewires fatty acid catabolism to fuel hepatocellular carcinoma. Gut. 2019;68(2):183–185. doi:10.1136/gutjnl-2018-316557
26. Jing Y, Cui D, Guo W, et al. Activated androgen receptor promotes bladder cancer metastasis via Slug mediated epithelial-mesenchymal transition. Cancer Lett. 2014;348:135–145. doi:10.1016/j.canlet.2014.03.018
27. Li Y, Zheng Y, Izumi K, et al. Androgen activates beta-catenin signaling in bladder cancer cells. Endocr Relat Cancer. 2013;20:293–304. doi:10.1530/ERC-12-0328
28. Elcheva I, Tarapore RS, Bhatia N, Spiegelman VS. Overexpression of mRNA-binding protein CRD-BP in malignant melanomas. Oncogene. 2008;27:5069–5074. doi:10.1038/onc.2008.141
29. Grabiner BC, Blonska M, Lin PC, et al. CARMA3 deficiency abrogates G protein-coupled receptor-induced NF-{kappa}B activation. Genes Dev. 2007;21:984–996. doi:10.1101/gad.1502507
30. Fernandez JG, Rodriguez DA, Valenzuela M, et al. Survivin expression promotes VEGF-induced tumor angiogenesis via PI3K/Akt enhanced beta-catenin/Tcf-Lef dependent transcription. Mol Cancer. 2014;13:209. doi:10.1186/1476-4598-13-209
31. Varol N, Konac E, Onen IH, et al. The epigenetically regulated effects of Wnt antagonists on the expression of genes in the apoptosis pathway in human bladder cancer cell line (T24). DNA Cell Biol. 2014;33:408–417. doi:10.1089/dna.2013.2285
32. Guo Y, Sun L, Xiao L, et al. Aberrant Wnt/beta-catenin pathway activation in dialysate-induced peritoneal fibrosis. Front Pharmacol. 2017;8:774. doi:10.3389/fphar.2017.00774
33. Zhang K, Zhu S, Liu Y, et al. ICAT inhibits glioblastoma cell proliferation by suppressing Wnt/beta-catenin activity. Cancer Lett. 2015;357:404–411. doi:10.1016/j.canlet.2014.11.047
34. Jiang Y, Ren W, Wang W, et al. Inhibitor of beta-catenin and TCF (ICAT) promotes cervical cancer growth and metastasis by disrupting E-cadherin/beta-catenin complex. Oncol Rep. 2017;38:2597–2606. doi:10.3892/or.2017.5962
35. Shi Z, Qian X, Li L, et al. Nuclear translocation of beta-catenin is essential for glioma cell survival. J Neuroimmune Pharmacol. 2012;7:892–903. doi:10.1007/s11481-012-9354-3
36. Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24:73–76.
37. Gontero P, Banisadr S, Frea B, Brausi M. Metastasis markers in bladder cancer: a review of the literature and clinical considerations. Eur Urol. 2004;46:296–311. doi:10.1016/j.eururo.2004.04.001
38. Gao Y, Guan Z, Chen J, et al. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int J Oncol. 2015;47:690–700. doi:10.3892/ijo.2015.3041
39. Pan D, Zhu Y, Zhou Z, et al. The CBM COMPLEX UNDERWRITES NF-kappaB activation to promote HER2-associated tumor malignancy. Mol Cancer Res. 2016;14:93–102. doi:10.1158/1541-7786.MCR-15-0229-T
40. Perez-Yepez EA, Ayala-Sumuano JT, Lezama R, et al. A novel beta-catenin signaling pathway activated by IL-1beta leads to the onset of epithelial-mesenchymal transition in breast cancer cells. Cancer Lett. 2014;354:164–171. doi:10.1016/j.canlet.2014.08.015
41. Tsai CY, Wang CS, Tsai MM, et al. Interleukin-32 increases human gastric cancer cell invasion associated with tumor progression and metastasis. Clin Cancer Res. 2014;20:2276–2288. doi:10.1158/1078-0432.CCR-13-1221
42. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247
Supplementary material
 |
Figure S1 (A) After transfected with NC or sh-CARMA3 into 5637 and T24 cells, the cells were treated with 100 μg/mL cycloheximide (CHX) for 6h. Then the expression of β-catenin was detected by Western blot. (B) The interaction between β-catenin and E-cadherin in sh-CARMA3 cells or control cells was detected by Co-IP assay. (C) The expression of Wnt1 and Wnt3a in OV-CARMA3 or Vector cells were evaluated by Western blot. (D) The correlation between CARMA3 and β-catenin expression in bladder cancer was analyzed by Gene Expression Profiling Interactive Analysis, Tang, Z. et al. (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res, 10.1093/nar/gkx2471 |
Reference
1. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.