Back to Journals » Cancer Management and Research » Volume 12
Silencing lncRNA AFAP1-AS1 Inhibits the Progression of Esophageal Squamous Cell Carcinoma Cells via Regulating the miR-498/VEGFA Axis
Authors Shen W, Yu L, Cong A, Yang S, Wang P, Han G, Gu B, Zhang W
Received 17 March 2020
Accepted for publication 11 July 2020
Published 29 July 2020 Volume 2020:12 Pages 6397—6409
DOI https://doi.org/10.2147/CMAR.S254302
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Kenan Onel
Wenhao Shen,1,2,* Lei Yu,1,2,* Aihua Cong,1,2 Song Yang,1,2 Peng Wang,1,2 Gaohua Han,1,2 Bin Gu,2,3 Wei Zhang2,4
1Department of Oncology, Taizhou People’s Hospital, Taizhou, Jiangsu, People’s Republic of China; 2Medical School of Nantong University, Nantong, Jiangsu, People’s Republic of China; 3Department of Emergency, Taizhou People’s Hospital, Taizhou, Jiangsu, People’s Republic of China; 4Department of Infectious Disease, Taizhou People’s Hospital, Taizhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Zhang Department of Infectious Disease, No. 399, South Hailing Road, Taizhou 225300, Jiangsu, People’s Republic of China
Tel +86-523-86606739
Email [email protected]
Bin Gu Department of Emergency, No. 399, South Hailing Road, Taizhou 225300, Jiangsu, People’s Republic of China
Tel +86-523-86606739
Email [email protected]
Purpose: In view of the continuous increase of the mortality rate, esophageal squamous cell carcinoma (ESCC) develops into a major health concern. In this study, we aimed to investigate the underlying mechanism of long noncoding RNA (lncRNA) actin filament-associated protein 1 antisense RNA (AFAP1-AS1)/microRNA-498 (miR-498)/vascular endothelial growth factor A (VEGFA) in ESCC cells.
Methods: The expression levels of AFAP1-AS1, miR-498 and VEGFA in ESCC tissues and cells were detected using quantitative real-time polymerase chain reaction (qRT-PCR). The effects of AFAP1-AS1 on ESCC cells proliferation and apoptosis were measured by methyl thiazolyl tetrazolium (MTT) and flow cytometry, respectively. Transwell assay was carried out to determine cell migration. In addition, VEGFA and cell behaviors-related proteins were determined by Western blot analysis. The targeted relationships of AFAP1-AS1 were verified by dual-luciferase reporter and RNA pull-down assays.
Results: The expression levels of lncRNA AFAP1-AS1 and VEGFA mRNA were upregulated, but miR-498 was downregulated in ESCC tissues and cells. Moreover, miR-498 was directly targeted by AFAP1-AS1 and there was a negative correlation between miR-498 and AFAP1-AS1. Functionally, AFAP1-AS1 silencing inhibited the proliferation and migration and induced apoptosis of ESCC cells. Interestingly, miR-498 inhibition rescued the effects of AFAP1-AS1 knockdown on cell proliferation, apoptosis and migration and restored the expression levels of tumor-developing marker proteins of AFAP1-AS1 silencing in Eca109 and KYSE-30 cells. Furthermore, VEGFA was verified as a direct target of miR-498 and reversed the effects of miR-498 overexpression on cell behaviors of ESCC in vitro.
Conclusion: Downregulation of AFAP1-AS1 impeded the proliferation and migration and induced apoptosis of ESCC cells by regulating miR-498/VEGFA axis, which might serve as a novel biomarker for the diagnosis and treatment of ESCC.
Keywords: lncRNA AFAP1-AS1, miR-498, VEGFA, ESCC
Introduction
Esophageal cancer is one of the most aggressive cancers, characterized as the highest incidence in Eastern Asia.1 According to statistics, China alone accounted for 53.8% of cases and 51.9% of deaths from esophageal cancer in 2008.2 Based on the histological types, esophageal cancer is classified into squamous cell carcinoma and adenocarcinoma. Particularly, esophageal squamous cell carcinoma (ESCC) is the most common histological subtype in less-developed regions of the world, including Eastern Asia.3 Although the treatments of ESCC have been continuously developed, such as surgery, chemotherapy and radiotherapy, patients with ESCC still have a dismal prognosis.4,5 Thus, it is important to uncover several helpful therapeutic strategies for the treatment of ESCC.
Nowadays, long non-coding RNAs (lncRNAs) have received widespread attention from researchers. By definition, lncRNAs are composed of 200 nucleotides in length and do not have functional protein-coding capacity. Numerous evidence has shown that lncRNAs participate in extensive biological processes and play oncogene or tumor suppressor roles in several diseases.6,7 In ESCC, several lncRNAs with aberrant expression have been reported to function as potential oncogenes, such as taurine up-regulated 1 (TUG1),8 SPRY4 intronic transcript (SPRY4-IT1),9 colon cancer-associated transcript 2 (CCAT2)10 and Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1).11 Actin filament-associated protein 1 antisense RNA (AFAP1-AS1) is a lncRNA located on chromosome 4p16.1 with 6810 base pair (bp) in length has been reported to play a vital role in the molecular mechanism of human cancers.12 AFAP1-AS1 is found to be markedly upregulated in non-small-cell lung cancer (NSCLC) tissues, and the ectopic expression can facilitate the proliferation of NSCLC cells.13 According to previous findings, disordered expression of AFAP1-AS1 is identified to be associated with prognosis of cholangio carcinoma,14 gastric cancer,15 hepatocellular carcinoma (HCC),16 nasopharyngeal carcinoma (NPC),17 pancreatic ductal adenocarcinoma (PDAC),18 colorectal cancer19 and gallbladder cancer.20 Importantly, AFAP1-AS1 has also been demonstrated to be upregulated in esophageal adenocarcinoma (EAC) tissues and cells.21 Furthermore, Luo et al revealed that AFAP1-AS1 overexpression promoted ESCC cell proliferation and inhibited cell apoptosis.22 However, in progression of ESCC, the deeper molecular function of AFAP1-AS1 is still not well elucidated.
MicroRNA (miRNA) is a shorter noncoding RNA that regulating gene expression by binding to the 3ʹ-untranslated region (3ʹUTR) of target mRNAs. Recent studies have demonstrated the dysregulation of miRNAs is closely associated with cancer progression.23 In both ESCC tissues and cell lines, miRNA-146a is downregulated and its unusual expression level affects cell growth and metabolism through regulating IRS2 mRNA expression.24 Previous study showed that miR-31 is decreased in esophageal cancer cells, and overexpression of miR-31 can hinder esophageal cancer cell growth, migration and invasion.25 In ESCC, miR-130b as an oncogenic gene regulates the expression of (phosphatase and tensin homolog deleted on chromosome ten) PTEN.26 Downregulated miR-126 is connected with poor prognosis of ESCC.27 Thus, miRNA-based treatments will be recommended as the emerging strategies for drug designs. Interestingly, miR-498 is reported to play important roles in mediating the pathogenesis of ESCC and restrain ESCC cell activities like cell proliferation and barrier penetration,28 which is the opposite of the effects of AFAP1-AS1 in ESCC. However, whether AFAP1-AS1 can regulate miR-498 and its roles in ESCC progression remain unclear.
Vascular endothelial growth factor (VEGF) was an oncogene in multiple all known cancers,29–31 including ESCC.32 Previous studies showed that VEGFA could activate PI3K-Akt/PKB,33 Akt/mTOR34 signaling pathway and play a critical role in cell proliferation, migration and angiogenesis in ESCC.35 All evidences revealed that VEGF might participate in ESCC progression, while the mechanism of VEGF in ESCC and the interaction among AFAP1-AS1, miR-498 and VEGFA need to be further elucidated.
Based on the previous researches, the present research aimed to investigate the potential functional mechanisms of AFAP1-AS1 and miR-498 on cell growth and metastasis in ESCC.
Methods
Patients and Tissue Samples
A total of 42 ESCC patients with no selection bias were selected to participate in this study at Taizhou People’s Hospital. The patients did not receive radiotherapy or chemotherapy before surgery. The main inclusion criteria contain age, gender, differentiation, depth of invasion (pT), lymph node metastasis (pN), clinical stage and tumor location. The detailed information of these ESCC patients was shown in Tables S1–S3. While surgical resection of ESCC was undertaken, 42 ESCC tissues and 35 adjacent normal specimens were obtained, and samples were promptly frozen until use. All experiments were performed in accordance with the Guidelines “Declaration of Helsinki”. Experiments were approved by the ethics committee at “Taizhou People’s Hospital”. Informed consents were obtained from human participants of this study.
Cell Lines and Cell Culture
The human esophageal epithelial cell line (HET-1A) was provided by the American Type Culture Collection (ATCC, Manassas, VA, USA). While human ESCC cell lines (Eca109 and KYSE-30) were obtained from Biological Sciences Institute (Shanghai, China). All cells grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco), 100 U/mL penicillin and 100 μg/mL streptomycin (Hyclone, Logan, UT, USA) with 5% CO2 atmospheres at 37°C.
Cell Transfection
The ESCC cell lines were transfected with three specific small interfering (siRNA) oligonucleotides (si-AFAP1-AS1#1, #2, and #3) or negative control siRNA (si-NC), respectively. The cDNA sequences of vascular endothelial growth factor A (VEGFA) and AFAP1-AS1 were introduced into pcDNA3.1 expression vector (Invitrogen, Carlsbad, CA, USA). Lipofectamine 3000 reagent (Invitrogen) was applied to transfect oligonucleotides or plasmids into cells. The miR-498 inhibitor, miR-498 mimics and negative control (NC mimics/NC inhibitor) were synthesized in GenePharma (Shanghai, China). The sequences of siRNAs oligonucleotides were as followed: si-AFAP1-AS1#1, GCCAUGUCAUCUGACUGGCUCUGAA; si-AFAP1-AS1#2, AUUUGAUGCCAGUUCAGUAGAGCCG; si-AFAP1-AS1#3, CAACACCUGCCUUCCCUCCUCUAAA. Finally, cells were harvested for following analyses.
RNA Extraction and qRT-PCR
Total RNA was isolated from tissues or cells using Trizol reagent (Takara, Dalian, China). RNA was transcribed into complementary DNA (cDNA) using PrimeScript RT Reagent Kit with gDNA Eraser (Takara). Subsequently, SYBR Premix Ex Taq (Takara) was adopted for qRT-PCR reactions with the corresponding cDNA. The PCR amplification was carried out on an ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The relative expression levels of genes were calculated using 2−ΔΔCt method. The GAPDH and U6 were chosen as endogenous controls in analyzing the data from qRT-PCR assay. The sequences of the primers are summarized as follows: AFAP1-AS1-F, 5ʹ-TCGCTCAATGGAGTGACGGCA-3ʹ and AFAP1-AS1-R, 5ʹ-CGGCTGAGACCGCTGAGAACTT-3ʹ; miR-498-F, 5-TTTCAAGCCAGGGGGCGTTTTTC-3 and miR-498-R, 5-GCTTCAAGCTCTGGAGGTGCTTTTC-3; VEGFA-F, 5ʹ-TTCAAGCCATCCTGTGC-3ʹ and VEGFA-R, 5ʹ-TGCTCTATCTTTCTTTGGTCTGC-3ʹ; GAPDH-F, 5ʹ-CGGAGTCAACGGA TTTGGTCGTAT-3ʹ and GAPDH-R, 5-AGCCTTCTCCATGGTGGTGAAGAC-3ʹ; U6-F, 5ʹ-CTCGCTTCGGCAGCACA-3ʹ and U6-R, 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ.
Dual-Luciferase Reporter Assay
Firstly, online software has validated the putative binding sites between miR-498 and AFAP1-AS1 or VEGFA using LncBase Predicted v.2.0 (http://carolina.imis.athena-innovation.gr/) and starBase v2.0 (http://starbase.sysu.edu.cn/mirCircRNA.php). In order to further confirm the relationships among them, we used Dual-luciferase reporter assay. Wild type AFAP1-AS1 fragments (AFAP1-AS1 WT) containing miR-498 binding sites or AFAP1-AS1 mutant sequence (AFAP1-AS1 MUT) without miR-498 binding sites was inserted into the pGL3 dual-luciferase reporter vector (Promega, Madison, WI, USA). After that, the dual-luciferase reporter plasmid of AFAP1-AS1 WT or AFAP1-AS1 MUT was co-transfected into Eca109 and KYSE-30 cells with NC mimics or miR-498 mimic by using Lipofectamine 3000 (Invitrogen). After transfection for 24 hrs, the relative luciferase activity was measured with the use of a dual-luciferase reporter assay system (Promega, Madison, WI, USA). Similarly, wild or mutant VEGFA 3ʹUTR sequence containing miR-498 binding sites or not was cloned into the pGL3 dual-luciferase reporter vector to form the reporter vectors VEGFA WT and VEGFA MUT.
RNA Pull-Down Assay
Biotinylated AFAP1-AS1 was transfected into ESCC cells using lipofectamine 3000 (Invitrogen). After transfection for 2 hrs, RNA-protein pull-down kit (Millipore, Billerica, MA, USA) was serviced for RNA pull-down assay, and then cells were lysed and incubated with streptavidin-coupled beads. Next, RNA was eluted and the enrichment of miR-498 was identified by qRT-PCR assay.
3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
Cell viability was determined using MTT assay. ESCC cells were cultured in 96-well plates with three replicate wells. Cell MTT Reagent Kit (Roche, Basel, Switzerland) was employed in this assay. Each well was added with MTT solution at 24 hrs, 48 hrs and 72 hrs. After incubation for 4 hrs, cells were washed and supplemented with Dimethyl Sulfoxide (DMSO; Roche) into each well. Optical density (OD) values of cell lysates were detected with a microplate reader (Bio-Rad, Richmond, CA, USA).
Cell Apoptosis Assay
ESCC cells were collected after transfection with miR-498 mimics/inhibitor or pcDNA VEGFA. The FITC-AnnexinV Apoptosis Detection Kit (BD Pharmingen, San Diego, CA, USA) was used according to the manufacturer’s instructions. After stained with the fluorescein isothiocyanate (FITC)-AnnexinV and propidium iodide (PI), the apoptotic cells were recognized with a flow cytometry system (Bio-Rad) and analyzed via Cell Quest software.
Cell Migration Assay
Approximate 2×104 cells were harvested and added into the upper chamber of a well without Matrigel with 100 μL serum-free medium. Normal medium containing 10% fetal bovine serum (FBS; Hyclone) was added in the bottom wells as a stimulation of migration. The cells were then incubated for 24 hrs at 37°C, and the cells on the bottom were fixed with 20% methanol for 30 mins and stained with 0.1% crystal violet for 15 mins. Lastly, the stained cells, which had migrated through the transwell plate, were photographed and counted using a light microscope (Nikon, Tokyo, Japan).
Localization of Nucleus and Cytoplasm
To further understand the function of lncRNA AFAP1-AS1 in ESCC cells. The NE-PER™ Nuclear and Cytoplasmic Extraction Reagents Kit (Thermo Scientific, Waltham, MA, USA) was utilized to detect the distribution of AFAP1-AS1. According to the manufacturers’ instructions, the cytoplasm and nuclear components of ESCC cells were separated and collected. Then, the qRT-PCR was conducted to examine the expression of AFAP1-AS1 in cell cytoplasm and nucleus, respectively. GAPDH is cytoplasm positioning control, U6 is the nucleus positioning control.
Western Blot Assay
Transfected cells were collected and digested with trypsin (Beyotime, Beijing, China). Cell lysis buffer was utilized for cell lysis. Protein was extracted and equal quantified proteins were separated on Sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bradford, MA, USA). The membranes were blocked with 10% skim milk at 37°C for 1 h, and then the immunoblots incubated overnight at 4°C with antibody against proliferating cell nuclear antigen (PCNA) (PCNA; 1:500, Abcam, Cambridge, UK), Cleaved-caspase-3 (c-caspase 3; 1:150, Cell Signaling Technology, Beverly, MA, USA), matrix metalloproteinase (MMP-9; 1:100, Santa CruzBiotechnology, Santa Cruz, UA, USA), GAPDH (1:1000, Santa Cruz Biotechnology) and VEGFA (1:2000, Abcam). After a combination with the HRP-conjugated secondary antibodies, quantitation of signal intensities was determined by densitometry on a scanner using ImageJ software (NIH, Bethesda, MD, USA).
Statistical Analysis
The SPSS software 20.0 was used for statistical analysis, and then results were shown as mean ± standard deviation (SD). GraphPad5.0 software was employed to display the data in graphs. Significance was tested using Student’s t-test between groups. P < 0.05 was considered to be statistically significant.
Results
AFAP1-AS1 and VEGFA Were Upregulated, Whereas miR-498 Was Downregulated in ESCC Tissues and Cell Lines
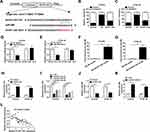
To expose the biological functional roles of AFAP1-AS1, miR-498, and VEGFA in ESCC, qRT-PCR was performed to detect the expression levels of them. The results showed that the relative levels of AFAP1-AS1 and VEGFA were significantly upregulated, while miR-498 was markedly downregulated in ESCC tissues compared with normal tissues (Figure 1A–C). According to the median of AFAP1-AS1, miR-498 and VEGFA expression in ESCC tissues, patients were divided into two groups: Low expression (n=21) and high expression (n=21). The statistical analysis presented that AFAP1-AS1, miR-498 and VEGFA were correlated with the malignancy of ESCC (Tables S1–S3). Moreover, similar alterations were observed of the expression levels of AFAP1-AS1 and VEGFA in ESCC cells (Eca109 and KYSE-30) compared with that in HET-1A cell (Figure 1D–F). From these data, we speculated that AFAP1-AS1, miR-498 and VEGFA might be involved in the development of ESCC.
AFAP1-AS1 Downregulated miR-498 Expression by Competitively Binding to miR-498
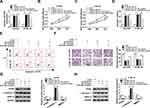
It is reported that lncRNA functions as a molecular sponge of miRNA to liberate mRNA transcript targeted by miRNA, thereby affecting cancer progression.36 Based on the contrary expression of miR-498 and AFAP1-AS1 in ESCC tissues and cell lines, the binding sites between them were predicted by LncBase Predicted v.2.0 and shown in Figure 2A. Next, we detected the location of AFAP1-AS1 in ESCC cells, the results showed that AFAP1-AS1 was mostly distributed in the cytoplasm in Eca109 and KYSE-30 cells (Figure 2B and C). Dual-luciferase reporter analysis showed that luciferase activity in Eca109 and KYSE-30 cells co-transfected with AFAP1-AS1 WT and miR-498 mimics was obviously declined compared with that in cells co-transfected with AFAP1-AS1 WT and NC mimics, and there was no distinct change in the cells transfected with AFAP1-AS1 MUT (Figure 2D and E). At the same time, RNA pull-down further confirmed the interaction between miR-498 and AFAP1-AS1 in Eca109 and KYSE-30 cell (Figure 2F and G). Subsequently, we analyzed the efficiency of AFAP1-AS1 overexpression and interference. The data showed that AFAP1-AS1 level was significantly increased after transfection with AFAP1-AS1 overexpression vector in Eca109 and KYSE-30 cells (Figure 2H). Moreover, after transfection with si-AFAP1-AS1#1, si-AFAP1-AS1#2 and si-AFAP1-AS1#3, the expression level of AFAP1-AS1 was significantly downregulated and si-AFAP1-AS1#1 played the most obvious role in Eca109 and KYSE-30 cells (Figure 2I). So si-AFAP1-AS1#1 was chosen for further experiments. Interestingly, miR-498 level was negatively regulated by AFAP1-AS1, when AFAP1-AS1 overexpression vectors were transfected, miR-498 expression level was significantly downregulated in Eca109 and KYSE-30 cells (Figure 2J). Simultaneously, AFAP1-AS1 knockdown could steeply augment the expression of miR-498 in the two ESCC cells (Figure 2K). In addition, we found miR-498 expression was negatively correlated with AFAP1-AS1 expression in ESCC tissues (R2= 0.548, P < 0.0001) (Figure 2L). In sum, these data suggested that miR-498 was directly targeted by AFAP1-AS1 in ESCC cells.
AFAP1-AS1 Silencing Inhibited ESCC Cells Proliferation and Migration and Led to Apoptosis via Regulating miR-498 Expression
Given the molecular mechanism between miR-498 and AFAP1-AS1, we researched the functional regulation in ESCC cells between them. Firstly, si-AFAP1-AS1#1 alone or along with miR-498 inhibitor was introduced into ESCC cells. As described in Figure 3A, the downregulated expression level of miR-498 in Eca109 and KYSE-30 cells caused by si-AFAP1-AS1 deletion could be recovered via co-transfection with si-AFAP1-AS1 and miR-498 inhibitor. What’s more, the results of the restoration experiments suggested that AFAP1-AS1 silencing could restrain the proliferation of Eca109 and KYSE-30 cells, such the results were abrogated via co-introduction of miR-498 inhibitor (Figure 3B and C). Meanwhile, the promoting role of AFAP1-AS1 deficiency in cell apoptosis was relieved after simultaneous transfection with miR-498 inhibitor in Eca109 and KYSE-30 cells (Figure 3D and E). At the same time, reintroduction of miR-498 inhibitor could regain the repressive impact of si-AFAP1-AS1 on cell migration in vitro (Figure 3F). In addition, Western blot analysis showed that AFAP1-AS1 silencing generated a significant reduction in the protein levels of PCNA and MMP-9, and dramatically promoted protein level of c-caspase 3 in Eca109 and KYSE-30 cell. And the changes of AFAP1-AS1 knockdown on related protein expression level were rescued by silencing miR-498 in ESCC cells (Figure 3G and H). Taken together, silencing of AFAP1-AS1 inhibited ESCC cells proliferation and migration, and promoted cells apoptosis via regulating miR-498 expression.
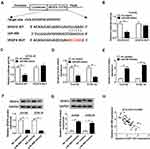
VEGFA Was Directly Targeted by miR-498 in ESCC Cells
In order to illuminate the mechanism of VEGFA involved in regulating ESCC progression, bioinformatics software starBase v2.0 revealed putative binding sites between VEGFA 3ʹUTR and miR-498 (Figure 4A). The dual-luciferase reporter assay further proved that VEGFA was the target mRNA of miR-498, which miR-498 mimics significantly decreased the luciferase activity of VEGFA WT but not VEGFA MUT in Eca109 and KYSE-30 cells (Figure 4B and C). In addition, the mRNA and protein levels of VEGFA in Eca109 and KYSE-30 cells were significantly reduced by miR-498 mimics, while miR-498 inhibitor observably elevated the mRNA and protein levels of VEGFA (Figure 4D–G). Moreover, there was a negative interlink between the expression of miR-498 and VEGFA (Figure 4H). These results suggested that miR-498 bound to 3ʹUTR of VEGFA mRNA and regulated VEGFA expression in ESCC cell lines.
VEGFA Reversed the Inhibitory Effect of miR-498 on ESCC Cell Progression
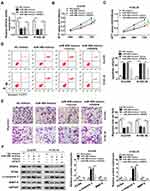
Considering the negative regulatory effect of VEGFA on the expression of miR-498 in ESCC, we further explored the functional effects of VEGFA in ESCC cell lines in vitro, As presented in Figure 5A, the declined expression level of VEGFA mRNA in Eca109 and KYSE-30 cells caused by miR-498 mimics could be improved by transfecting with VEGFA relative to that cells transfected with vector. Subsequently, MTT assay reflected a phenomenon that weakened cells vitality triggered by miR-498 overexpression was restored via VEGFA overexpression (Figure 5B and C). Apoptotic assay indicated that reinforced expression of miR-498 significantly induced ESCC cell apoptosis, whereas ectopic expression of VEGFA abolished this effect (Figure 5D). Transwell assay demonstrated that miR-498 overexpression significantly suppressed ESCC cell migration, and this effect was changed after transfection with VEGFA in vitro (Figure 5E). Furthermore, miR-498 overexpression significantly decreased the protein levels of PCNA and MMP-9 and up-regulated c-caspase 3 protein expression in Eca109 and KYSE-30 cells, whereas overexpression of VEGFA reversed the effects of miR-498 overexpression on these proteins (Figure 5F–I). All results indicated that VEGFA reversed the inhibitory effect of miR-498 on ESCC cell progression.
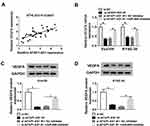
Silencing AFAP1-AS1 Downregulated VEGFA Expression by Sponging miR-498 in ESCC Cells
Next, we aimed to explore whether AFAP1-AS1 could regulate VEGFA expression through miR-498 in ESCC. The positive correlation between AFAP1-AS1 and VEGFA was analyzed and exhibited in Figure 6A. Besides, qRT-PCR analysis indicated that VEGFA mRNA expression was inhibited in Eca109 and KYSE-30 cells transfected with si-AFAP1-AS1#1, whereas miR-498 inhibitor evidently improved expression of VEGFA (Figure 6B). As well as VEGFA protein expression level, reduced by AFAP1-AS1, was markedly promoted following inhibition of miR-498 in Eca109 and KYSE-30 (Figure 6C and D) cell. From the above, these data suggested that AFAP1-AS1 silencing downregulated VEGFA expression by sponging miR-498 in ESCC cells.
Discussion
During the past years, numerous dysregulated lncRNAs have been reported to be implicated in the carcinogenesis and progression of different malignancies.37 Recently, study discovered that AFAP1-AS1 was aberrantly upregulated in esophageal adenocarcinoma (EAC) tissues and cells and could dysregulate biologic functions of EAC cells.21 Moreover, Luo et al showed that AFAP1-AS1 was also upregulated in ESCC tissues and cell lines, and promoted ESCC cell growth and inhibited cell apoptosis,22 which provide a clue that there may be a potential correlation between AFAP1-AS1 and the progression of ESCC. In this study, we found that AFAP1-AS1 was significantly upregulated in ESCC tissues compared with normal tissues, and this result was also confirmed in ESCC cell lines. Functionally, silencing of AFAP1-AS1 restrained ESCC cell proliferation and migration, and induced cell apoptosis in the two ESCC cell lines. More importantly, we found miR-498 was significantly downregulated and VEGFA was significantly upregulated in ESCC tissues and cells. Accordingly, the data suggested that AFAP1-AS1, miR-498 and VEGFA might play critical roles in ESCC.
It has been the most common regulation patterns that lncRNAs acted as miRNA molecular sponges to regulate miRNA expression over the last few years.38 For example, lncRNA AFAP1-AS1 promoted nasopharyngeal carcinoma cell migration by sponging miR-423-5p.39 In addition, AFAP1-AS1 acted as a competing endogenous RNA (ceRNA) of miR-384 to regulate tumorigenicity of pancreatic cancer cells.40 Furthermore, through targeting miR-103a-3p, AFAP1-AS1 facilitated cell proliferation and viability of Pituitary Adenoma.41 Among this research, miR-498 was identified to be directly targeted by AFAP1-AS1 in ESCC cells. On the basis of previous researches, miR-498 was discovered to be located in 19q13.41 and downregulated in colorectal adenocarcinoma tissues.42 miR-498 could affect telomerase expression43 and was correlated with tumor progression in non-small cell lung cancer.44 Moreover, miR-498 acted as a tumor suppressor and inhibited the proliferation of ovarian cancer cell45 and ESCC cell.46 In our study, dual-luciferase assay, RNA pull-down assay and gain or loss-of-function assay were performed, and our data supported that AFAP1-AS1 downregulated the expression of miR-498 by competitively binding to miR-498. Additionally, interference with miR-498 could restore the inhibition effect of si-AFAP1-AS1 transfection on cell proliferation and metastasis, and recover the promotion influence of si-AFAP1-AS1 in cell apoptosis in vitro. These evidences also revealed that AFAP1-AS1 functioned as an endogenous decoy for miR-498 to inhibit the growth and metastasis of ESCC cells.
Subsequently, we analyzed the target genes of miR-498 and found that VEGFA could be targeted by miR-498. VEGFA was initially discovered to be a regulator of angiogenesis.47 Recently, increasing evidence has shown that VEGFA was involved in multiple biological processes, such as embryogenesis tumor survival,48,49 proliferation and migration.50 More than that, we found that interference with AFAP1-AS1 retarded the mRNA and protein levels of VEGFA, and the inhibitory impact of AFAP1-AS1 knockdown on the expression of VEGFA was attenuated via miR-498 inhibitor. These results revealed that there may be a directly competitive correlation between AFAP1-AS1 and VEGFA for binding miR-498.
Conclusion
To sum up, our study clarified that AFAP1-AS1 was upregulated in ESCC tissues and cell lines, and silencing of AFAP1-AS1 suppressed ESCC cell proliferation and migration, and promoted apoptosis. Furthermore, AFAP1-AS1 could act as a sponge of miR-498 to regulate VEGFA expression. Thus, our findings provided a theoretical basis for the diagnosis and treatment of ESCC.
Acknowledgments
The authors would like to thank the participants in this study.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):
2. Di Pardo BJ, Bronson NW, Diggs BS, Thomas CR
3. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):
4. Zhang Q, Gan H, Song W, Chai D, Wu S. MicroRNA-145 promotes esophageal cancer cells proliferation and metastasis by targeting SMAD5. Scand J Gastroenterol. 2018;53(7):
5. Codipilly DC, Qin Y, Dawsey SM, et al. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc. 2018;88(3):
6. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):
7. Chen S, Wu DD, Sang XB, et al. The lncRNA HULC functions as an oncogene by targeting ATG7 and ITGB1 in epithelial ovarian carcinoma. Cell Death Dis. 2017;8(10):e3118. doi:10.1038/cddis.2017.486
8. Zhang Z, Xiong R, Li C, Xu M, Guo M. LncRNA TUG1 promotes cisplatin resistance in esophageal squamous cell carcinoma cells by regulating Nrf2. Acta Biochim Biophys Sin (Shanghai). 2019;51(8):
9. Cui F, Wu D, He X, Wang W, Xi J, Wang M. Long noncoding RNA SPRY4-IT1 promotes esophageal squamous cell carcinoma cell proliferation, invasion, and epithelial-mesenchymal transition. Tumour Biol. 2016;37(8):
10. Zhang X, Xu Y, He C, et al. Elevated expression of CCAT2 is associated with poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2015;111(7):834–839. doi:10.1002/jso.23888
11. Yao Q, Yang J, Liu T, Zhang J, Zheng Y. Long noncoding RNA MALAT1 promotes the stemness of esophageal squamous cell carcinoma by enhancing YAP transcriptional activity. FEBS Open Bio. 2019;9(8):
12. Zhang F, Li J, Xiao H, et al. AFAP1-AS1: a novel oncogenic long non-coding RNA in human cancers. Cell Prolif. 2018;51(1):e12397. doi:10.1111/cpr.12397
13. Yu S, Yang D, Ye Y, et al. Long noncoding RNA actin filament-associated protein 1 antisense RNA 1 promotes malignant phenotype through binding with lysine-specific demethylase 1 and repressing HMG box-containing protein 1 in non-small-cell lung cancer. Cancer Sci. 2019;110(7):2211. doi:10.1111/cas.14039
14. Shi X, Zhang H, Wang M, et al. LncRNA AFAP1-AS1 promotes growth and metastasis of cholangiocarcinoma cells. Oncotarget. 2017;8(35):58394. doi:10.18632/oncotarget.16880
15. Feng Y, Zhang Q, Wang J, et al. Increased lncRNA AFAP1-AS1 expression predicts poor prognosis and promotes malignant phenotypes in gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(17):3842–3849.
16. Lu X, Zhou C, Li R, et al. Critical role for the long non-coding RNA AFAP1-AS1 in the proliferation and metastasis of hepatocellular carcinoma. Tumor Biol. 2016;37(7):9699–9707. doi:10.1007/s13277-016-4858-8
17. Bo H, Gong Z, Zhang W, et al. Upregulated long non-coding RNA AFAP1-AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget. 2015;6(24):20404. doi:10.18632/oncotarget.4057
18. Ye Y, Chen J, Zhou Y, et al. High expression of AFAP1-AS1 is associated with poor survival and short-term recurrence in pancreatic ductal adenocarcinoma. J Transl Med. 2015;13(1):137. doi:10.1186/s12967-015-0490-4
19. Wang F, Ni H, Sun F, et al. Overexpression of lncRNA AFAP1-AS1 correlates with poor prognosis and promotes tumorigenesis in colorectal cancer. Biomed Pharmacother. 2016;81:152–159. doi:10.1016/j.biopha.2016.04.009
20. Ma F, Wang S-H, Cai Q, et al. Overexpression of LncRNA AFAP1-AS1 predicts poor prognosis and promotes cells proliferation and invasion in gallbladder cancer. Biomed Pharmacother. 2016;84:1249–1255. doi:10.1016/j.biopha.2016.10.064
21. Tang J, Zhong G, Wu J, Chen H, Jia Y. Long noncoding RNA AFAP1-AS1 facilitates tumor growth through enhancer of zeste homolog 2 in colorectal cancer. Am J Cancer Res. 2018;8(5):
22. Luo HL, Huang MD, Guo JN, et al. AFAP1-AS1 is upregulated and promotes esophageal squamous cell carcinoma cell proliferation and inhibits cell apoptosis. Cancer Med. 2016;5(10):2879–2885. doi:10.1002/cam4.848
23. Wu W, Bhagat TD, Yang X, et al. Hypomethylation of noncoding DNA regions and overexpression of the long noncoding RNA, AFAP1-AS1, in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology. 2013;144(5):
24. Liu H, Ren G, Zhu L, et al. The upregulation of miRNA-146a inhibited biological behaviors of ESCC through inhibition of IRS2. Tumor Biol. 2016;37(4):4641–4647. doi:10.1007/s13277-015-4274-5
25. Koumangoye RB, Andl T, Taubenslag KJ, et al. SOX4 interacts with EZH2 and HDAC3 to suppress microRNA-31 in invasive esophageal cancer cells. Mol Cancer. 2015;14(1):24. doi:10.1186/s12943-014-0284-y
26. Yu T, Cao R, Li S, et al. MiR-130b plays an oncogenic role by repressing PTEN expression in esophageal squamous cell carcinoma cells. BMC Cancer. 2015;15(1):29. doi:10.1186/s12885-015-1031-5
27. Liu R, Gu J, Jiang P, et al. DNMT1-microRNA126 epigenetic circuit contributes to esophageal squamous cell carcinoma growth via ADAM9-EGFR-AKT signaling. Clin Cancer Res. 2015;21(4):854–863. doi:10.1158/1078-0432.CCR-14-1740
28. Islam F, Gopalan V, Law S, et al. MiR-498 in oesophageal squamous cell carcinoma: clinicopathological impacts and functional interactions. Hum Pathol. 2017;62:141–151. doi:10.1016/j.humpath.2017.01.014
29. Chen X, Xu X, Pan B, et al. miR-150-5p suppresses tumor progression by targeting VEGFA in colorectal cancer. Aging (Albany NY). 2018;10(11):
30. Cao W, Zhao Y, Wang L, Huang X. Circ0001429 regulates progression of bladder cancer through binding miR-205-5p and promoting VEGFA expression. Cancer Biomark. 2019;25(1):
31. Guo J, Chen M, Ai G, Mao W, Li H, Zhou J. Hsa_circ_0023404 enhances cervical cancer metastasis and chemoresistance through VEGFA and autophagy signaling by sponging miR-5047. Biomed Pharmacother. 2019;115:108957. doi:10.1016/j.biopha.2019.108957
32. Cancer Genome Atlas Research Network. Analysis working group: Asan university; BC cancer agency; integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):
33. An G, Liang S, Sheng C, Liu Y, Yao W. Upregulation of microRNA-205 suppresses vascular endothelial growth factor expression-mediated PI3K/Akt signaling transduction in human keloid fibroblasts. Exp Biol Med (Maywood). 2017;242(3):
34. Huang J, Wang X, Wen G, Ren Y. miRNA‑205‑5p functions as a tumor suppressor by negatively regulating VEGFA and PI3K/Akt/mTOR signaling in renal carcinoma cells. Oncol Rep. 2019;42(5):
35. Chen Y, Wang D, Peng H, et al. Epigenetically upregulated oncoprotein PLCE1 drives esophageal carcinoma angiogenesis and proliferation via activating the PI-PLCε-NF-κB signaling pathway and VEGF-C/Bcl-2 expression. Mol Cancer. 2019;18(1):1. doi:10.1186/s12943-018-0930-x
36. Pilyugin M, Irminger-Finger I. Long non-coding RNA and microRNAs might act in regulating the expression of BARD1 mRNAs. Int J Biochem Cell Biol. 2014;54:356–367. doi:10.1016/j.biocel.2014.06.018
37. Saus E, Brunet-Vega A, Iraola-Guzmán S, et al. Long non-coding RNAs as potential novel prognostic biomarkers in colorectal cancer. Front Genet. 2016;7:54. doi:10.3389/fgene.2016.00054
38. Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi:10.1038/nature11993
39. Lian Y, Xiong F, Yang L, et al. Long noncoding RNA AFAP1-AS1 acts as a competing endogenous RNA of miR-423-5p to facilitate nasopharyngeal carcinoma metastasis through regulating the Rho/Rac pathway. J Exp Clin Cancer Res. 2018;37(1):253. doi:10.1186/s13046-018-0918-9
40. Wu XB, Feng X, Chang QM, et al. Cross-talk among AFAP1-AS1, ACVR1 and microRNA-384 regulates the stemness of pancreatic cancer cells and tumorigenicity in nude mice. J Exp Clin Cancer Res. 2019;38(1):107. doi:10.1186/s13046-019-1051-0
41. Tang H, Zhu D, Zhang G, Luo X, Xie W. AFAP1-AS1 promotes proliferation of pituitary adenoma cells through miR-103a-3p to activate PI3K/AKT signaling pathway. World Neurosurg. 2019;130:
42. Gopalan V, Smith RA, Lam AK-Y. Downregulation of microRNA-498 in colorectal cancers and its cellular effects. Exp Cell Res. 2015;330(2):423–428. doi:10.1016/j.yexcr.2014.08.006
43. Kasiappan R, Shen Z, Anfernee K, et al. 1, 25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J Biol Chem. 2012;287(49):41297–41309. doi:10.1074/jbc.M112.407189
44. Wang M, Zhang Q, Wang J, et al. MicroRNA-498 is downregulated in non-small cell lung cancer and correlates with tumor progression. J Cancer Res Ther. 2015;11(Suppl 1):C107. doi:10.4103/0973-1482.163859
45. Liu R, Liu F, Li L, et al. MiR-498 regulated FOXO3 expression and inhibited the proliferation of human ovarian cancer cells. Biomed Pharmacother. 2015;72:52–57. doi:10.1016/j.biopha.2015.04.005
46. Wang Z, Liu J, Wang R, Wang Q, Liang R, Tang J. Long non-coding RNA taurine upregulated gene 1 (TUG1) downregulation constrains cell proliferation and invasion through regulating cell division cycle 42 (CDC42) expression via MiR-498 in esophageal squamous cell carcinoma cells. Med Sci Monit. 2020;26:e919714. doi:10.12659/MSM.919714
47. Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273(2):
48. Leng K, Xu Y, Kang P, et al. Akirin2 is modulated by miR-490-3p and facilitates angiogenesis in cholangiocarcinoma through the IL-6/STAT3/VEGFA signaling pathway. Cell Death Dis. 2019;10(4):262. doi:10.1038/s41419-019-1506-4
49. Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871–882. doi:10.1038/nrc3627
50. Han KY, Chang JH, Azar DT. MMP14-containing exosomes cleave VEGFR1 and promote VEGFA-induced migration and proliferation of vascular endothelial cells. Invest Ophthalmol Vis Sci. 2019;60(6):
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.