Back to Journals » OncoTargets and Therapy » Volume 10
Significant association of the EXO1 rs851797 polymorphism with clinical outcome of ovarian cancer
Authors Shi T , Jiang R, Wang P, Xu Y, Yin S, Cheng X, Zang R
Received 12 May 2017
Accepted for publication 6 August 2017
Published 3 October 2017 Volume 2017:10 Pages 4841—4851
DOI https://doi.org/10.2147/OTT.S141668
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Tingyan Shi,1,2 Rong Jiang,1 Pan Wang,1 Yuan Xu,2 Sheng Yin,1 Xi Cheng,3 Rongyu Zang1,3
1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Zhongshan Hospital, Fudan University, 2Cancer Institute, 3Gynecologic Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
Background: Exonuclease 1 (EXO1), one of DNA mismatch repair pathway genes, functions in maintaining genomic stability and affects tumor progression. We hypothesized that genetic variations in EXO1 may predict clinical outcomes in epithelial ovarian cancer (EOC).
Methods: In this cohort study with 1,030 consecutive EOC patients, we genotyped four potentially functional polymorphisms in EXO1 by the Taqman assay and evaluated their associations with patients’ survival.
Results: Using multivariate Cox proportional hazards regression models, we found that rs851797AG/GG genotypes were significantly associated with recurrence and cancer death (HR =1.30 and 1.38, 95% CI =1.11–1.52 and 1.02–1.88, respectively). Kaplan–Meier survival estimates showed that patients who carried rs851797AG/GG genotypes had poorer progression-free survival and poorer overall survival, compared with rs851797AA genotype carriers (log-rank test, P=0.002 and 0.025, respectively). Moreover, patients with older age at menophania, advanced stage tumor, or being received incomplete cytoreduction were more likely to be recurrent and dead.
Conclusion: EXO1 rs851797 polymorphism can predict the clinical outcomes in EOC patients. In addition, age at menophania, FIGO stage, and complete cytoreduction might be independently prognostic factors of ovarian cancer. Large studies with functional experiments are warranted to validate these findings.
Keywords: EXO1, ovarian cancer, polymorphism, prognosis
Introduction
Ovarian cancer is the third most commonly diagnosed gynecologic cancer and the first leading cause of death from gynecologic malignancies, up to 238,700 new cases and 151,900 cancer deaths worldwide in 2012.1 In China, there were 52,100 new ovarian cancer cases and 22,500 related deaths in 2015.2 More than 90% of these cases are epithelial ovarian cancer (EOC), among which 70% are diagnosed with bulky intra-abdominal disease or distant metastases.3 Despite improvements in surgical techniques and chemotherapeutic options, most of advanced-stage patients will relapse within 18 months, and 5-year overall survival still remains at ~46% in the United States.4 Recently, genetic variations have been highly strengthened along with the development of molecular subtyping and targeting therapy in ovarian cancer and related research. Considerable efforts on prognostic genetic variations have been focused on germline or somatic mutations, such as BRCA1/2 and other DNA repair pathway genes.5 However, few reports were performed on the predictive value of single nucleotide polymorphisms (SNPs), especially in Chinese Han ethnics.
Exonuclease 1 (EXO1) is a member of the RAD2 nuclease family with evolutionarily conserved domains,6 and exhibits both 5′ to 3′ exonuclease activity and 5′ flap structure-specific endonuclease activity.7 A large number of studies have demonstrated that EXO1 can function in DNA replication, repair, and recombination by participating in various DNA repair pathways, such as mismatch repair (MMR), DNA double-strand break repair, and error-free DNA damage tolerance pathway, and thus may play a critical role in genome maintenance and tumor suppression.8,9 Recently, three meta-analysis publications reported the significant associations of EXO1 SNPs with cancer susceptibility.10–12 Moreover, several investigations focused on the prognostic role of EXO1 polymorphisms in human cancers. For example, EXO1 N279S13 and R354H14 could predict the overall survival in pancreatic cancer patients. EXO1 K589E (rs1047840) might be a prognostic biomarker for relapse-free survival in head and neck squamous cell carcinoma.15 Recently, EXO1 rs9350 was reported to be associated with poor survival of non-small cell lung cancer patients who were treated by platinum-based chemotherapy.16 To date, only a pooled genome-wide association study showed the EXO1 polymorphism region (1q43) to be associated with EOC susceptibility.17 No investigations were reported on the association of EXO1 polymorphisms with EOC survival, let alone the mechanism of EXO1 polymorphisms in regulating gene and protein expression.
In this study, we hypothesized that potentially functional genetic variations in EXO1 may affect the clinical outcome in EOC patients. We also conducted a relatively large-scale cohort study to identify four SNPs in the functional region of EXO1 and their associations with EOC prognosis in Chinese Han women.
Materials and methods
Study subjects
The study population consisted of 1,165 consecutive EOC patients between March 2009 and August 2012 from Shanghai Ovarian Cancer Study as described previously in the Chinese EOC genome-wide association study,18 mainly from Fudan University Shanghai Cancer Center (FUSCC). Among all the 1,165 patients, 135 cases were lost to follow-up. Thus, 1,030 EOC patients were involved in the final survival analysis. All cases were genetically unrelated ethnic Han Chinese, who were mainly from Eastern China where they lived, according to the records of in-patient registration and cancer registration system. The tumors were histopathologically confirmed independently as primary epithelial ovarian carcinoma based on World Health Organization Classification criteria, including serous, endometrioid, clear cell, and so on, by two gynecologic pathologists as routine diagnosis.19 Patients with borderline ovarian tumors were not included. Age at menarche was defined as the age at the first menstruation. We defined post-menopause as the absence of menstrual periods for ≥12 months since the last period, or pre-menopausal hysterectomy. Cancer family history was defined when first-, second-, or more-degree relatives had cancer history. We defined female cancer family history as breast, ovarian, cervical, endometrial cancers in first-, second-, or more-degree relative women.
The detailed clinicopathological information was extracted from the patients’ electronic database, including FIGO stage (International Federation of Gynecology and Obstetrics, 2013), histopathology, tumor grade, tumor type according to the dualistic model of carcinogenesis (categorized as type I tumor [low-grade serous carcinomas, low-grade endometrioid, clear cell, and mucinous carcinomas] and type II tumor [high-grade serous carcinoma, high-grade endometrioid carcinoma, malignant mixed mesodermal tumors and undifferentiated carcinomas]),20 pelvic lymph node metastasis, the expression of estrogen receptor and progesterone receptor (dichotomized into positive [+] if >10% of cells stained positive and negative [−] if ≤10% stained positive),21 neoadjuvant chemotherapy, residual disease after primary cytoreduction (categorized as 0 [no grossly visible tumor], 1 [0.1–0.5 cm], 2 [0.5–1.0 cm], and 3 [>1.0 cm]), tumor recurrence, and death. The residual disease was reviewed in the pelvis, middle abdomen, and upper abdomen. Complete cytoreduction was defined as no grossly visible tumor overall after surgical procedure. Optimal cytoreduction was defined as no more than 1 cm of residual tumor overall after surgical procedure. After surgery, all patients received adjuvant chemotherapy with platinum and paclitaxel for six to eight cycles. Unfortunately, in our data set, there were no enough information about patient’s response to platinum.
SNP selection and genotyping
By searching the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP) and the International HapMap Project database (http://hapmap.ncbi.nlm.nih.gov/), we found that there were 1064 SNPs in EXO1, including 602, 22, and 43 SNPs located in the coding region, 5′-UTR, and 3′-UTR, respectively. Among them, four SNPs were finally selected, based on the following criteria: 1) minor allele frequency of at least 5% in Chinese populations, 2) with low linkage disequilibrium by using an r2 threshold of <0.8 for each other, 3) predicted to be a potentially functional SNP by the SNP function prediction platform (http://snpinfo.niehs.nih.gov/snpfunc.htm), 4) not included in the published genome-wide association studies, and 5) meet the Hardy–Weinberg equilibrium criteria. They are rs1047840G>A (NM_130398.3:c.1765G>A, Glu589Lys, exon 10); rs9350C>T (NM_130398.3:c.2270C>T, Pro757Leu, exon 12); rs851797A>G [NM_130398.3:c.*140A>G, 3′-untranslated region (UTR)]; and rs3754093A>G (NM_130398.3:c.-1959A>G, 5′-flanking). The RNAfold online tool (http://rna.tbi.univie.ac.at/) was used to estimate the RNA secondary structure based on minimum free energy (MFE) values for the potentially functional SNP.
DNA extraction and genotyping
Genomic DNA was obtained from the whole blood, and the Taqman method by 384-fomate was conducted for genotyping, as described previously.22 As a result, the discrepancy rate in all positive controls (ie, duplicated samples, overlapping samples from previous studies, and samples randomly selected to be sequenced) was <0.1%.
Statistical analysis
Progression-free survival (PFS) and overall survival (OS) times were calculated from the date of first treatment to the date of disease recurrence and to the date of death, respectively. Patients without progression, lost to follow-up, or died from other causes were censored at their last date of record. Kaplan–Meier survival estimate and log-rank test were calculated to evaluate PFS and OS. We performed univariate and multivariate Cox proportional hazards regression analyses to evaluate the effects of EXO1 genotypes on the cumulative probability of survival in EOC patients. Multivariate analyses were adjusted by those variables that were independently associated with survival in the univariate model. All statistical analyses were performed with SAS 9.1 software (SAS Institute, Cary, NC, USA), unless stated otherwise. All P-values were two-sided with a significance level of P<0.05.
Ethics approval and consent to participate
The research was approved by the Institutional Review Board of FUSCC. Each patient signed a written informed consent.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Results
Population characteristics
Among the 1,165 consecutive EOC patients, 135 cases were lost to follow-up. Thus, 1,030 EOC patients were involved in the final analysis (Tables 1 and 2). The patients’ median age at diagnosis was 54.5 years (range, 18–85 years). Totally, there were 32 (3.11%), 55 (5.34%), 492 (47.77%), and 74 (7.18%) patients diagnosed with stage I, II, III, and IV, respectively. The rates of complete and optimal cytoreduction were 33.40% and 70.68%, respectively. The median follow-up time was 37.7 months, and there were 752 (73.01%) recurrences and 207 (20.10%) cancer deaths during the follow-up period.
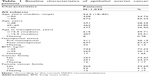  | Table 1 Baseline characteristics of epithelial ovarian cancer patients |
Association between clinicopathological characteristics and survival
As shown in Table 3, age at menophania, FIGO stage, and complete cytoreduction were independently associated with tumor recurrence and death by multivariate Cox proportional hazards regression models. Specifically, patients with age at menophania above 15.5 years or with advanced stage tumor (III–IV) were more likely of poor survival (for recurrence: adjusted HR =1.81 and 1.67, 95% CI =1.31–2.50 and 1.02–2.75; for cancer death: adjusted HR =1.50 and 6.94, 95% CI =1.07–2.08 and 2.14–22.48; respectively). Complete cytoreduction was significantly associated with better survival (adjusted HR =0.46 and 0.40, 95% CI =0.31–0.68 and 0.25–0.63 for recurrence and death, respectively).
EXO1 genotypes predict clinical outcomes
Using multivariate Cox proportional hazards regression models, we found that rs851797AG/GG genotypes were significantly associated with recurrence and cancer death (Table 4, adjusted HR =1.30 and 1.38, 95% CI =1.11–1.52 and 1.02–1.88, respectively). Kaplan–Meier survival estimates showed that patients who carried rs851797AG/GG genotypes had poorer PFS and OS, compared with rs851797AA genotype carriers (log-rank test, P=0.002 and 0.025, respectively; Figure 1A and B). However, in the subgroup of type II tumor, the prognostic value of rs851797 was only observed in tumor recurrence (adjusted HR =1.44, 95% CI =1.01–2.07; Table S1). More interestingly, when combining all four EXO1 SNPs, we found that patients who carried more than one risk genotype had a poor PFS than 0–1 risk genotype carriers (adjusted HR =1.30, 95% CI =1.02–1.65; Table 4).
The mRNA secondary structure is critical for mRNA–miRNA interactions. Thus, we explored whether the EXO1 rs851797 SNP in the 3′-UTR of EXO1 could alter the local secondary structure of the EXO1 mRNA based on the MFE value. Using the RNAfold online tool and inputting 201-nt long DNA sequence of the EXO1 3′-UTR containing the rs851797 locus, we found that the MFE changed from −31.2 kcal/mol to −34.0 kcal/mol, when the rs851797 allele changed from A to G (Figure 2).
  | Figure 2 In silico analysis of potential functional rs851797 variant. The predicted secondary structure of the EXO1 mRNA. The secondary structures of the EXO1 3′-UTR were predicted by inputting two 201-nt long DNA sequences centering rs851797 into RNAfold, with either the A (left) or G (right) allele. The figures and the values of minimum free energy were generated by RNAfold (http://rna.tbi.univie.ac.at). |
Discussion
To the best of our knowledge, this is the first study that investigates associations between potentially functional SNPs in EXO1 and clinical outcomes in EOC patients. In the present study with a total of 1,030 EOC cases, we found that patients who carried rs851797AG/GG genotypes had poorer PFS and OS, compared with rs851797AA genotype carriers. Further in silico analysis indicated that rs851797 might be a functional SNP by affecting mRNA secondary structure of EXO1, thus contribute to tumor progression.
EXO1 polymorphisms have previously been reported to be associated with the development of many other types of human cancer. Meta-analysis showed that EXO1 rs851797 was conferred an increased overall susceptibility to cancer in an allelic model.11 Recently, a pooled genome-wide association study reported that the EXO1 polymorphism region (1q43) was associated with the risk of EOC.17 However, in our unpublished case–control study with a total of 1,320 EOC patients and 1,383 normal female controls, there were no significant associations between EXO1 rs851797 genotype and EOC susceptibility in Chinese Han women (unpublished data). Based on the HapMap database, the frequency of rs851797 AG/GG genotype varies among ethnics, with 100%, 82.6%, and 70% in European, African–American, and Asian, respectively. On the other hand, the risk factor and mechanisms of genetic susceptibility might be different from that of tumor progression. It could be necessary to further evaluate the prognostic role of EOX1 polymorphisms. We here reported a potentially functional variant in EXO1 (rs851797) that involved in the process of ovarian cancer progression and prognosis. Unlike the findings from head and neck squamous cell carcinoma15 and non-small cell lung cancer,16 we did not observe predictive values of rs1047840 and rs9350 polymorphisms in EOC survival. It might be caused by the heterogeneity among various types of human cancer.
EXO1, which is located at chromosome 1q42–1q43, contains one untranslated exon followed by 13 coding exons, encodes a protein with 846 amino acid, and acts as a double-stranded DNA exonuclease.6,7 Accumulated data have demonstrated that EXO1 participates in the process of DNA damage repair, replication, and the maintenance of genomic stability through its exonulease activity to correct overhanging flap structures.6,7 EXO1-mutant cells showed increased microsatellite instability and incomplete MMR capability.23 In addition, higher mutation rates were accompanied with higher susceptibility to lymphomas.23 EXO1 has also been implicated in hereditary nonpolyposis colorectal cancer due to its role in DNA MMR.24
SNPs are the most common type of genetic variations. At least 14,304 SNPs have been identified in the EXO1 gene (http://www.ncbi.nlm.nih.gov/projects/SNP). The majority of SNPs are silent or have limited influences on the function and expression of genes. Only a small fraction of SNPs have been identified to be involved in the process of tumor progression as potentially functional variants.25 It is well in accordance with the theory of the driver and passenger somatic mutations in human cancer genome.26 Rs851797, located at 3′-UTR of EXO1 gene, was found to be associated with risk of several human cancers.11 In silico analysis by using the RNAfold online tool showed that rs851797 could alter the local secondary structure of the EXO1 mRNA based on the MFE value, thus contribute to tumor progression and prognosis. Given that the mRNA secondary structure is critical for mRNA–miRNA interactions, it was reasonable to suspect the rs851797 variant as a functional SNP. Moreover, the intrinsic mechanism might be explained by that the 3′-UTR could contain sequence motifs crucial for the regulation of transcription, mRNA stability, and cellular location of the mRNA or the binding of microRNA.27 Further functional studies are warranted to validate the association data.
Several limitations in the present study need to be addressed. First, there are selection bias and information bias by the study design, which may have been minimized by the adjustment for potential confounding factors in final multivariate analyses. Second, because of the retrospective nature of the study design and the recall bias, it is difficult to evaluate all prognostic factors exactly, especially, no enough information about patient’s response to platinum. Third, further investigations of genotype–phenotype associations and functional analysis for this SNP are warranted.
In summary, in the current cohort study with 1,030 ovarian cancer patients, we found that the EXO1 rs851797 polymorphism could predict clinical outcomes of EOC. In addition, age at menophania, FIGO stage, and complete cytoreduction might be independently prognostic factors for ovarian cancer. However, well-designed larger, prospective studies with functional analysis are warranted to validate our findings.
Abbreviations
EOC, epithelial ovarian cancer; SNP, single-nucleotide polymorphism; EXO1, Exonuclease 1; MMR, mismatch repair; FUSCC, Fudan University Shanghai Cancer Center; FIGO, International Federation of Gynecology and Obstetrics; UTR, untranslated region; MFE, minimum free energy; PFS, progression-free survival; OS, overall survival.
Acknowledgments
We would like to thank Qingyi Wei from Duke Cancer Institute and Lian Li from Tianjin Medical University Cancer Hospital for their supports on sample collection from Chinese EOC genome-wide association study. “Zhongshan Development Program” Recruitment (Grant No 016) and the National Science Fund for Young Scholars (Grant No 81402142).
Author contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. R Zang and T Shi were involved in study concept, design, and drafting of the manuscript. All the authors contributed to acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. T Shi, R Jiang, and P Wang contributed to statistical analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Hoskins WJ. Prospective on ovarian cancer: why prevent? J Cell Biochem Suppl. 1995;23:189–199. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Candido-dos-Reis FJ, Song H, Goode EL, et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21(3):652–627. | ||
Tran PT, Erdeniz N, Symington LS, Liskay RM. EXO1 – a multi-tasking eukaryotic nuclease. DNA Repair (Amst). 2004;3(12):1549–1559. | ||
Lee BI, Wilson DM. The RAD2 domain of human exonuclease 1 exhibits 5′ to 3′ exonuclease and flap structure-specific endonuclease activities. J Biol Chem. 1999;274(53):37763–37769. | ||
Goellner EM, Putnam CD, Kolodner RD. Exonuclease 1-dependent and independent mismatch repair. DNA Repair (Amst). 2015;32:24–32. | ||
Karras GI, Fumasoni M, Sienski G, Vanoli F, Branzei D, Jentsch S. Noncanonical role of the 9-1-1 clamp in the error-free DNA damage tolerance pathway. Mol Cell. 2013;49(3):536–546. | ||
Chen ZY, Zheng SR, Zhong JH, Zhuang XD, Zhou JY. Association between three exonuclease 1 polymorphisms and cancer risks: a meta-analysis. Onco Targets Ther. 2016;23(9):899–910. | ||
Zhang M, Zhao D, Yan C, Zhang L, Liang C. Associations between nine polymorphisms in EXO1 and cancer susceptibility: a systematic review and meta-analysis of 39 case-control studies. Sci Rep. 2016;8(6):29270. | ||
Duan F, Song C, Dai L, Cui S, Zhang X, Zhao X. The significance of Exo1 K589E polymorphism on cancer susceptibility: evidence based on a meta-analysis. PLoS One. 2014;9(5):e96764. | ||
Dong X, Li Y, Hess KR, Abbruzzese JL, Li D. DNA mismatch repair gene polymorphisms affect survival in pancreatic cancer. Oncologist. 2011;16(1):61–70. | ||
Dong X, Jiao L, Li Y, et al. Significant associations of mismatch repair gene polymorphisms with clinical outcome of pancreatic cancer. J Clin Oncol. 2009;27(10):1592–1599. | ||
Nogueira GA, Lourenco GJ, Oliveira CB, et al. Association between genetic polymorphisms in DNA mismatch repair-related genes with risk and prognosis of head and neck squamous cell carcinoma. Int J Cancer. 2015;137(4):810–818. | ||
Li R, Gu J, Heymach JV, et al. Hypoxia pathway genetic variants predict survival of non-small-cell lung cancer patients receiving platinum-based chemotherapy. Carcinogenesis. 2017;38(4):419–424. | ||
Lawrenson K, Iversen ES, Tyrer J, et al. Common variants at the CHEK2 gene locus and risk of epithelial ovarian cancer. Carcinogenesis. 2015;36(11):1341–1353. | ||
Chen K, Ma H, Li L, et al. Genome-wide association study identifies new susceptibility loci for epithelial ovarian cancer in Han Chinese women. Nat Commun. 2014;5:4682. | ||
Lee KR, Tavassoli FA, Prat J. Tumours of the ovary and peritoneum: surface epithelial-stromal tumours. In: Tavassoli FA, Devilee P, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Chapter 2. Lyon, France: IARC Press; 2003:117–145. | ||
Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer – shifting the paradigm. Hum Pathol. 2011;42(7):918–931. | ||
Hecht JL, Kotsopoulos J, Hankinson SE, Tworoger SS. Relationship between epidemiologic risk factors and hormone receptor expression in ovarian cancer: results from the Nurses’ Health Study. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1624–1630. | ||
He J, Qiu LX, Wang MY, et al. Polymorphisms in the XPG gene and risk of gastric cancer in Chinese populations. Hum Genet. 2012;131(7):1235–1244. | ||
Wei K, Clark AB, Wong E, et al. Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev. 2003;17(5):603–614. | ||
Jagmohan-Changur S, Poikonen T, Vilkki S, et al. EXO1 variants occur commonly in normal population: evidence against a role in hereditary nonpolyposis colorectal cancer. Cancer Res. 2003;63(1):154–158. | ||
Shi TY, Cheng X, Yu KD, et al. Functional variants in TNFAIP8 associated with cervical cancer susceptibility and clinical outcomes. Carcinogenesis. 2013;34(4):770–778. | ||
Tan H, Bao J, Zhou X. A novel missense-mutation-related feature extraction scheme for ‘driver’ mutation identification. Bioinformatics. 2012;28(22):2948–2955. | ||
Mazumder B, Seshadri V, Fox PL. Translational control by the 3′-UTR: the ends specify the means. Trends Biochem Sci. 2003;28(2):91–98. |
Supplementary material
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





