Back to Journals » Journal of Pain Research » Volume 16
Short-Term Collagen Nerve Wrapping Facilitates Motor and Sensory Recovery from Nerve Degeneration in a Sciatic Nerve Injury Rat Model
Authors Sonohata M, Doi A , Uchihashi K, Hashimoto A, Kii S, Inoue T, Mawatari M
Received 12 December 2022
Accepted for publication 8 May 2023
Published 20 May 2023 Volume 2023:16 Pages 1683—1695
DOI https://doi.org/10.2147/JPR.S401126
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qi Fang
Motoki Sonohata,1,2,* Atsushi Doi,3,* Kazuyoshi Uchihashi,4 Akira Hashimoto,2 Sakumo Kii,1 Takao Inoue,5 Masaaki Mawatari1
1Department of Orthopaedic Surgery, Faculty of Medicine, Saga University, Saga, Japan; 2Department of Orthopaedic Surgery, Saga Central Hospital, Saga, Japan; 3Department of Rehabilitation, Kumamoto Health Science University, Kumamoto, Japan; 4Department of Surgical Pathology, National Hospital Organization Saga Hospital, Saga, Japan; 5Organization of Research Initiatives, Yamaguchi University, Yamaguchi, Japan
*These authors contributed equally to this work
Correspondence: Atsushi Doi, Department of Rehabilitation, Kumamoto Health Science University, 324, Izumi, Kumamoto-kitaku, Kumamoto, 861-5598, Japan, Tel +81-96-275-2111, Email [email protected]
Purpose: This study used a sciatic nerve injury rat model to investigate the short-term effects of a polyglycolic acid (PGA)-collagen tube for nerve injury in continuity.
Materials and Methods: Sixteen female Wistar rats (6– 8 weeks) were used, and the left sciatic nerve was crushed with a Sugita aneurysm clip. Sciatic nerve model rats were randomly categorized into two groups (n = 8; control group, n = 8; nerve wrapping group). Then, we measured four sensory thresholds, magnetically stimulated the lumbar region to induce motor-evoked potentials (MEPs), and evaluated the sciatic nerve histopathologically.
Results: In the sensory thresholds, there were significant differences for the main effect in 250 and 2000 Hz stimulation (p = 0.048 and 0.006, respectively). Further, a significant difference was observed with 2000 Hz stimulation at 1 week (p = 0.003). In the heat stimulation, there were significant differences for the main effect in both weeks and groups (p = 0.0002 and 0.0185, respectively). The post-hoc test showed a significant difference between groups only in 2W (p = 0.0283). Three weeks after the surgery, both 2nd and 3rd MEPs waves-related latencies in the nerve wrapping group were significantly shorter than those in the control group (p = 0.0207 and 0.0271, respectively). Histological evaluation of the sciatic nerve revealed considerable differences in the number of axons between the two groups (p = 0.0352).
Conclusion: The short-term PGA-collagen tube nerve wrapping facilitated motor and sensory recovery from nerve degeneration in the sciatic nerve injury rat model.
Keywords: polyglycolic acid-collagen tube, nerve wrapping, sciatic nerve injury, sensory threshold, motor and sensory recovery
Introduction
Peripheral nerve bundles have two primary functions: motor and sensory neurotransmission.1 After nerve injuries occur, various symptoms, such as muscle atrophy and neuropathic pain, affect activities of daily living (ADL) and the mental condition.2,3
Injury of the nerve bundles is often associated with trauma, with an incidence of 2.8% in multi-trauma patients.2 The injured nerve axons can recover, which regenerate slowly at a rate of only 1–3 mm/day.4 If the injuries are far from the target organs, a long period of muscle atrophy, neuropathic pain, and functional disability will be induced because of the gradual decrease in the regenerative capacity after the denervation of target organs.5,6 In such cases, practical outcomes following nerve injury are poor.
Neuropathic pain is a severe public health problem, with a reported prevalence ranging from 3.3 to 8.2%,7 and 10% in the general population.8 Although neuropathic pain has been observed in various conditions, trauma-induced neuropathic pain is often accompanied by sensory loss, hyperalgesia, and allodynia.9 Such complex sensory disturbance-induced daily pain significantly reduces ADL. Therefore, surgical treatment targeting the injured peripheral nerve is critical.
Primary nerve repair is performed for nerve transection without nerve defects.10 Autologous nerve grafts, artificial nerve grafts, or nerve transfer surgeries are performed for nerve transections with defects. These surgical strategies are the gold standards for nerve restoration. Alternatively, in cases of severe nerve injury, such as crush injury and entrapment neuropathy, which is observed in 80% of peripheral nerve injuries, the only treatment options are nerve decompression, neurolysis, vein wrapping, or muscle wrapping.2 Vein wrapping, among them, has been shown to have good clinical results. It seems to promote M2 macrophage polarization as part of its mechanism.11 In recent years, the effectiveness of wrapping with absorbable artificial implants for nerve injury in continuity has been reported.12,13 The disadvantage of absorbable artificial implants is that patients must sacrifice their tissue and adapt even if insufficient tissue wraps around the damaged nerve. Although many artificial objects need to be evaluated, essential data are still lacking.
Nerbridge® (Toyobo Co., Osaka, Japan) is a polyglycolic acid (PGA) tube filled with collagen fibers. This tube is biocompatible, commercially available, and government-approved for practical use in Japan since March 2013. This tube comprises medical collagen (NMP Collagen PS; Nippon Meat Packers, Osaka, Japan). Collagen fibers are applied to the inside of the conduit, facilitating the guidance of blood vessels into the tube, thereby providing a favorable environment for the regeneration and guidance of peripheral nerves.14,15 The PGA tube dissolves and is absorbed within approximately three months. This PGA-collagen tube is mainly used for peripheral nerves in Japan.16 However, most previous research has investigated the long-term effects of collagen fibers as a bridge material after severe nerve injury, such as neurotmesis.14,17
Therefore, this study examined the short-term impact of a PGA-collagen tube wrapping on nerve injury in a rat sciatic nerve crush injury model by measuring sensory thresholds and motor nerve responses via histopathological evaluation.
Materials and Methods
Animals and Surgery
Sixteen female Wistar rats (6–8 weeks old, weighing approximately 200 g) were purchased from SLC Inc. (Hamamatsu, Japan). All animals were housed in a controlled laboratory environment at room temperature (22 ± 2 °C) in 50 ± 10% humidity under a 12 h light/dark cycle. Although 5 Rats were bred in a rat’s cage before surgery, each rat was separately bred in a mouse’s cage after the surgery. The Animal Research Committee of Kumamoto Health Science University (Permit number: Animal 19–12) approved all experimental protocols and animal maintenance procedures and conformed to the Animal Protection and Management Law of Japan and the Ethical Issue of the International Association for the Study of Pain.18
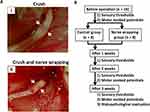
Animal surgery was performed under intraperitoneal injection of anesthetic agents (0.15 mg/kg medetomidine, 2 mg/kg midazolam, and 2.5 mg/kg butorphanol). The left lateral femoral region was opened after cleaning with an antiseptic solution. A unilateral muscular incision was made from the greater trochanter to the mid-femur, exposing the sciatic nerve. All left sciatic nerves were dissected from the surrounding tissue, and nerve crush injuries were inflicted using a Sugita aneurysm clip (1.3 mm width, Mizuho Ikakogyo, Tokyo, Japan).19 The clip was applied for 5 min with approximately 1.5 N of holding force (Figure 1Ai).20 We randomly categorized the rats into two groups after sciatic nerve injury. The first group received only a nerve crush using the clip (n = 8, control group). The second group was wrapped with Nerbridge® (Toyobo Co., Osaka, Japan; inner diameter; 1.5 mm, length: 5 mm) on the site of the nerve crush (n = 8, wrapping group; Figure 1Aii). Further, to wrap the Nerbridge on the injured nerve, we first put it in a Petri dish with saline for about 3 minutes. Then, after the Nerbridge became soft, we cut it longitudinally, and the Nerbridge was wrapped on the nerve. After that, the skin was closed with 4–0 nylon sutures. After the surgery was completed for each rat, atipamezole (1.5 mg/kg) was injected intraperitoneally. Rats recovered in the waking condition, and we returned each rat to its cage in the animal room. Two hand warmers were placed under the pet sheet in the cage to maintain body temperature.
Measurement of the Sensory Threshold with Electrical Stimulation
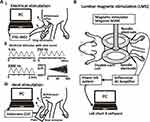
Sensory thresholds were measured using 5-, 250-, and 2000-Hz sine electrostimulation (STG-4002, Multichannel Systems Inc., Reutlingen, DE, Germany) before and 1–3 weeks after surgery (Figure 1A).21 The three different electrostimulation frequencies used in this study stimulated Aβ fibers (2000 Hz), Aδ fibers (250 Hz), and C fibers (5 Hz). An awake rat was briefly immobilized in the prone position in a plastic tube. Ring-type bipolar electrodes (Cadwell Shielded Tri-Lead Ring Electrode, Cadwell, Inc., WA, USA) were wrapped around the plantar surface of the left hind foot, and electrostimulation was applied to the left hind foot. A gradual increase in electrostimulation resulted in the withdrawal reflex of the rat’s hind limb. The STG-4002 was connected to a personalized computer (PC). We measured the time from the electrostimulation onset to the withdrawal reflex. The intensity at which the withdrawal reflex occurred was calculated (μA) (Figure 2Ai and Aii).21
Measurement of the Sensory Threshold with Heat Stimulus
A second sensory threshold was measured using heat stimulation before and 1–3 weeks after surgery (Figure 1B).21 Briefly, an awake rat was immobilized in a plastic tube. A probe with a 25 mm × 25 mm surface was placed on the plantar surface of the left hind foot to measure the sensory threshold, and heat stimuli (Intercross-210, Intercross Inc., Tokyo, Japan) were applied to the plantar surface, with the rat in a prone position. The Intercross-210 analyzer was connected to a personal computer (PC). We measured the time from the onset of heat stimulation to the observation of the withdrawal reflex (Figure 2Aiii).
Lumbar Magnetic Stimulation (LMS)-Induced Motor Evoked Potentials
Each rat was initially anesthetized with a mixture of agents (0.15mg/kg medetomidine, 2 mg/kg midazolam, and 2.5mg/kg butorphanol). The hair on the rats’ lower body was removed with hair clippers, and each rat was fixed with stereotaxic apparatus (Narishige, Tokyo, Japan), and their trunks were elevated with mini-jack (10cm✕10cm, MonotaRO, Amagasaki, Japan) until both the head and trunk became horizontal in position. Under these conditions, a double-corn coil (4610, Magstim Co Ltd, Spring Garden, UK), connected with a magnetic stimulator (M200, Magstim Co Ltd, Spring Garden, UK), was put on the lumbar region of the rat. A single magnetic stimulation was imposed on the rats, and motor evoked potentials (MEPs) were recorded into lab chart 8 software via differential AC Amplifier (Model 1700, AM systems, Sequim, WA, USA) and power lab system (AD Instruments, Dunedin, New Zealand). We used the magnetic stimulation on the lumbar region (lumbar magnetic stimulation; LMS)-induced MEPs to measure motor nerve responses. Two-needle electrodes for non-references (MLA1203 needle electrode, AD Instruments, Dunedin, New Zealand) were inserted into the middle portion of the lateral head at the bilateral gastrocnemius, respectively, to record bilateral responses from both gastrocnemius muscles simultaneously (Figure 2B). Both sides of reference electrodes (EM-275S, NORAXON, Arizona, USA) were attached to the plantar surface of their bilateral feet. Two ground electrodes (EM-275S, NORAXON, Arizona, USA) were put on the bilateral pelvic area. The low-cut filter for the recording was 300 Hz, and the high-cut filter was 500 Hz (Figure 2B).
We simultaneously measured the bilateral MEPs from the gastrocnemius muscle (Figure 3A). The recorded bilateral MEPs responses seemed stable and symmetrical (Figure 3A). Further, the MEPs responses had intensity dependency for the magnetic stimulation (Figure 3B). The responses were measured, and 1st negative wave, 2nd positive wave, and 3rd small negative wave were observed (Figure 3A–C). Both latencies and amplitudes of these three waves were analyzed with lab chart reader software (AD Instruments, Dunedin, New Zealand) (Figure 3Ci and Cii). The latencies were measured at the points of first wave basements (right latency 1 base, RL1 base; left latency base, LL1 base), first wave tops (right latency 1 top, RL1 top; left latency 1 top, LL1 top), second-wave base (RL2 base and LL2 base), second wave top (RL2 top and LL2 top), third wave base (RL3 base and LL3 base), and third wave top (RL3 top and LL3 top) (Figure 3Ci). The amplitudes were measured at RA1 (= RL1 top – RL1 base), RA2 (= RL1 top – RL2 base), RA3 (= RL2 top – RL2 base), RA4 (RL2 top – RL3 base), LA1 (= LL1 top – LL1 base), LA2 (= LL1 top – LL2 base), LA3 (= LL2 top – LL2 base), and LA4 (LL2 top – LL3 base) (Figure 3Cii); however, only LA1, LA2, LA3, and LA4 are demonstrated. Before sciatic nerve surgery, magnetic stimulation on the lumbar region induced symmetrical MEPs latencies and amplitudes (Supplemental Table 1). After finishing the MEPs measurements, atipamezole (1.5mg/kg) was injected intraperitoneally. We returned each rat to its cage in the animal room.
Histological Evaluation of the Sciatic Nerve
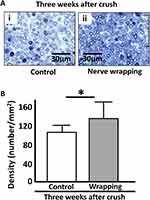
Three weeks after the operation, the sedated rats were euthanized. A specimen was taken from the sciatic nerve at 5 mm distal to the crushed lesion, fixed in 2.5% glutaraldehyde, and then fixed in 2% osmium tetroxide (OsO4; abcr GmbH, Karlsruhe, Germany). The specimen was routinely processed upon embedding. Sections of 1μm thickness were stained with toluidine blue and examined under light microscopy (BZ-X710; Keyence, Osaka, Japan). The number and diameter of the myelinated axons were measured using the BZ-X Analyzer software program (Keyence, Osaka, Japan). We calculated averaged myelin density (number/mm2) among the three randomly selected areas.
The data were analyzed using AnalySIS FIVE 2.8 (Olympus Soft Imaging Solutions GmbH, Munster, Germany). Morphometric analysis of the sciatic nerve sections, the number of myelinated nerve fibers, and the nerve diameter were measured in one area. The target area had the highest number of myelin among the three randomly selected areas.
All slides were photographed using a Keyence All-in-One Fluorescence Microscope BZ-X 710 (Keyence Corporation, Osaka, Japan) at a magnification of 120 × and analyzed per unit area of 10,800 μm2 (120μm×90μm) using a Keyence BZ-X Analyzer 1.4.0.1 (Keyence Corporation, Osaka, Japan). Nerve fiber diameters less than 1μm and larger than 10μm were excluded because the analyzer judged that noise was being counted. The diameter of the nerve fiber was the average of the major and minor axes.
Statistical Analysis
All numerical data are expressed as the mean ± standard deviation. All analyses were performed using either JMP Pro software (version 14.2.0, SAS Institute Japan Ltd, Tokyo, Japan) or EZR software (version 1.5.5).22 Student’s t-test was conducted to evaluate 5 Hz, 250 Hz, and 2000 Hz stimulation at pre-surgery, 1, 2, and 3 weeks postoperatively, and thermal stimulation at pre-surgery, 1, 2, and 3 weeks postoperatively between the two groups. Further analyses were made via two-way repeated measure ANOVA followed by Dunnett’s test for weeks and groups factor, respectively. We used the Wilcoxon rank sum test for the MEPs comparison between the two groups. To evaluate the normality distribution of continuous variables, after the Shapiro–Wilk test was conducted, the Student’s t-test was conducted to evaluate the number of axons or diameter of axons between the two groups.
Results
The Effects of Nerve Wrapping for Electro- and Heat-Stimulation-Induced Sensory Threshold
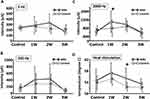
In the three types of electrical stimulation (Figure 4A–C, 5, 250, and 2000 Hz, respectively), there were significant differences for the main effect of weeks in 250 Hz (F(3,67) = 3.26, p = 0.048) and 2000 Hz stimulation (F(3,67) = 5.69, p = 0.006). However, multiple comparison results presented no statistical differences in the 250 Hz condition. Otherwise, a significant difference was observed in 1W compared with control in 2000 Hz stimulation (Figure 4C, p = 0.003). In the heat stimulation (Figure 4D), there were significant differences for the main effect of both weeks (F(3,67) = 10.1, p = 0.0002) and groups ((F(2,67) = 5.98, p = 0.0185). The post-hoc t-test showed a significant difference between groups only in 2W (Figure 4D, *p = 0.0283), although there were no significant differences among the time points.
The Effects of Nerve Wrapping for Both MEPs Amplitude and Latency
We compared the MEPs amplitudes between control and wrapping groups at 2 weeks after surgery. However, four kinds of amplitudes, including LA1, LA2, LA3, and LA4, had no significant differences between the two groups (Supplemental Figure 1). Further, no significant differences existed in MEPs latencies (Supplemental Figure 2). Three weeks after the surgery, we also compared the MEPs amplitudes between the control and wrapping groups (Figure 5A, n = 8). Four kinds of amplitudes, including LA1, LA2, LA3, and LA4, showed no significant differences between the two groups (Figure 5B, n = 8). Moreover, as per the latencies, LL1-related 4 parameters showed no remarkable difference between the two groups (Figure 6Bi–Biv). However, LL2 and LL3-related latencies in the wrapping group were significantly shorter than those in the control group: LL2 base (8.40 ± 1.64 ms vs 6.18 ± 0.64 ms, *p = 0.0207), LL2 top (10.09 ± 1.62 ms vs 7.74 ± 0.69 ms, *p = 0.0114), LL2 base – RL2 base (2.43 ± 1.45 ms vs 0.33 ± 0.43 ms, *p = 0.0207), LL2 top – RL2 top (2.39 ± 1.12 ms vs 0.45 ± 0.58 ms, *p = 0.00532), LL3 base (11.88 ± 1.93 ms vs 9.49 ± 0.84 ms, *p = 0.0271), LL3 top (13.85 ± 2.06 ms vs.11.31 ± 1.25 ms, *p = 0.0177), and LL3 base – RL3 base (1.19 ± 1.32 ms vs −0.15 ± 0.64 ms, *p = 0.0403) (Figure 6Bv–Bxii).
The Effects of Nerve Wrapping on Histopathological Evaluation of the Nerve Injury
A significant difference was observed in the number of axons between the two groups (*p = 0.0352 non-wrapping vs wrapping: 105 ± 13 axons vs 134 ± 34 axons; Figure 7A and B).
Discussion
The PGA-collagen tube (Nerbridge®) has already been used clinically, and its beneficial effects have been reported.16,23,24 However, in almost all animal studies and case reports, the PGA-collagen tube was used as a bridge material after the nerve defect injury, where the follow-up period is comparatively long.14,17,25,26 Here, we investigated the short-term effects of PGA-collagen tube wrapping after continuous peripheral nerve injury in rats. Three weeks after the surgery, the PGA-collagen tube was observed to facilitate recovery from nerve degeneration among the heat stimulation-induced sensory threshold, MEPs, and via pathological observation of the myelinated nerve fiber.
Previous animal studies using electrophysiological methods,14,17,27,28 electron microscopy,27,28 histological methods,27,28 morphometric analysis,15 gait analysis,15 and behavior analysis17 have reported the beneficial effects of PGA-collagen tubes after sciatic nerve surgery. The effectiveness of the PGA-collagen tube has been reported using electrophysiological methods,29 electromyography,23 2-point discrimination tests,29 electrostimulation-induced sensory thresholds,29 and gloss movement evaluation23,29 in clinical case reports. However, almost all of these previous reports investigated the long-term effects, showing that the impact on both sensory threshold and morphometric observation in this study might be first in discussing the short-term effects.
Nerve Wrapping and Sensory Effects
Interestingly, the sensory thresholds in four kinds of modalities had already recovered three weeks after surgery (Figure 4A–D). Generally, the axon diameter of sensory fibers is reported to be smaller than motor nerves.30 Further, these sensory fibers are classified with Aβ myelinated fibers (touch), Aδ myelinated fibers (fast pain), and C unmyelinated fibers (slow pain).30 If it is thought that the three different electrostimulation frequencies (2000 Hz, 250 Hz, and 5 Hz) reflect Aβ fibers, Aδ fibers, and C fibers, respectively,31 no statistical differences of the multiple comparisons between control and wrapping groups in both 5Hz (C fibers) and 250Hz (Aδ fibers) might occur from mild physical injuries by the clip (Figure 4A and B). Furthermore, mechanical stimulation, such as electro-stimulus, activates the mechanoreceptors.32 Hence, heat stimulus activates the mechanoreceptors and the polymodal receptors.33 Therefore, differences in the sensory effects for the wrapping between electro- and heat-stimulus might be because of differences of such activated receptors, and wrapping for heat-stimulus has a more prominent effect than that for electro-stimulus (Figure 4A–D).
Nerve Wrapping and Motor Nerve Effects
In general, MEPs result from injuries to the Aα fiber and are known to be variable.34 Previous reports show that lumbar magnetic stimulation-induced MEPs are recorded as a combination of negative and positive waves.35,36 In a human study, only one paper reported that magnetic stimulation on lumbar region-induced MEPs often had two sets of waves in healthy subjects; however, no evaluation for the second wave was carried out.37 In our study, however, lumbar magnetic stimulation-induced MEPs had three waves under the anesthetized condition (Figure 3). Although almost all MEP studies are performed as part of a clinical study, we used small animals in this study. Further, in human studies, non-reference electrodes are put on the skin as surface electrodes.36 On the contrary, in our research, we used needle non-reference electrodes and inserted them into the bilateral gastrocnemius muscles of the rats (see Materials and methods). Needle electrodes were used for two specific reasons. First, since rats’ skin is covered with hair, we could not obtain smooth skin perfectly even when the hair was removed with a shaver. Second is the difficulty of fixing the surface electrodes on the small area of the rat’s bilateral gastrocnemius. Generally, an electrophysiological recording with a needle electrode is thought to record a high signal-noise ratio. Therefore, the recordings with the needle electrodes might be why multiple waves appeared.
Lumbar magnetic stimulation is thought to stimulate the caudal equine in human studies.38 Researchers performed the experiments with humans and used twin coils,38 whereas a different paper used translumbosacral stimulation coils.39 In this research, we utilized a double-corn coil for rats. If the lumbar stimulation activates the action potentials on the caudal equine, the stimulation evokes 1st wave, showing that this stimulation is Aα fiber-related. If this occurs, we hypothesized two possibilities concerning the origin of the 2nd and 3rd waves, about RL2, LL2, RL3, and LL3. The first possibility is artificial noise. The second is an antidromic response containing central nervous system-related waves, such as F-waves.40 As per the first possibility, the artificial noise was suppressed after the sciatic nerve injury (Figure 4A and Supplemental Figure 1A). If both the 2nd and 3rd waves are antidromic responses, these waves should be unstable like F-waves.41 However, the LMS-induced three MEPs waves were very stable (Figure 3A), and the MEPs amplitudes were stimulation intensity-dependent (Figure 3B). Therefore, although the origin of the 2nd and 3rd sets of waves cannot perfectly deny the unknown possibility of the central nervous system-related waves, these waves do not seem to be artificial noises. We hypothesize that facilitating these waves’ recovery via nerve wrapping (Figure 6B) might denote neurophysiological inclusion.
Conversely, we could not demonstrate the exact mechanism of motor nerve recovery at the 1st wave. There are a couple of reasons why this might be. First, the follow-up period in this study might be too short because we followed only three weeks after surgery (Figures 1B, 6, and 7). Second, the physical compression affects the fibers with larger diameters more than other tiny fibers, showing that fibers with large diameters may be Aα-fibers. In contrast, fibers with small diameters include Aβ-, Aδ-, and C-fiber.34 It is thought that the functional recovery of the motor nerve (Aα-fiber) may be slow because the motor nerve might be more affected by the physical compression because of its larger diameter, showing that the recovery of the sensory nerve (Aδ- and C-fiber) (Figure 4) might be faster than that of the motor nerve which generated MEPs (Figure 6). Further, these wrapping-related effects of nerve recovery may correspond with the results of the histopathological data, ie, an increased number of myelinated axons (Figure 7).
Therefore, we might be unable to show the short-term effectiveness of nerve wrapping for the LL1 components included in the 1st MEPs wave (Figure 6Bi–iv).
Nerve Wrapping and Histopathological Effects
An increase in the number of myelinated axons shows the nerve-wrapping-induced facilitation of the recovery (Figure 7). However, we were not able to specify whether this increase is because of myelination of the motor or sensory nerves. As shown in the “Nerve wrapping and motor nerve effects”, the physical compression of fibers with large diameters, such as Aα-fiber, has a more prominent effect than that of other tiny fibers, such as Aβ-, Aδ-, and C-fiber,34 showing that the recovery of the motor nerve components has more delay (Figures 5 and 6). Therefore, the recovery of the axon might mainly come from the myelinated sensory fibers.
Possible Mechanisms of the PGA-Collagen Tube Wrapping-Induced Facilitation of Nerve Recovery
PGA-collagen tube wrapping-induced facilitation of nerve recovery may come from the activation of Schwann cells (SCs) and collagen-induced molecules.42 In this paragraph, we try to explain the roles of both SCs and collagen-induced molecules. Normal myelinated nerve fibers are covered with axons, which are composed of SCs.43 The activation of SCs is related to many functions, such as SCs migration,44 proliferation,45 differentiation, and myelination.46 Further, SCs activation seems to recruit macrophages47 and facilitate nerve growth factors.48 The recruitment of the macrophages especially induces M2 macrophages49 via serine/threonine-protein kinases (AKTs) and cAMP concentration-dependent protein kinase A (PKA).50 M2 macrophages release anti-inflammatory cytokines and facilitate nerve regeneration.51 Collagen-induced molecules, such as neural cell adhesion molecules 1 (NCAM1), appear to facilitate nerve bundle formation.52 Therefore, PGA-collagen tubes wrapping-induced SCs activation or collagen-induced molecules may facilitate nerve recovery in this study.42
Technical Considerations
In this study, three kinds of experiments, including measurement of motor nerve responses and sensory thresholds histopathological evaluation of the sciatic nerve, were performed to show the short-term effects of the PGA-collagen tube for nerve injury in continuity using a sciatic nerve injury rat model. However, we think behavior experiments like gait or motor behavior are more valuable in the recovery process than other evaluation methods.53 Nevertheless, scholars need to develop simpler achievable methods to get a quantitative evaluation.53
Clinical Application
Collagen nerve wrapping has been used in clinical practice, and the benefit of nerve wrapping has been widely addressed.23,24 However, since clinical-based research on nerve wrapping is potentially based on patients already undergoing peripheral nerve injury plus nerve wrapping, the research cannot be performed as a randomized control trial.23,24 Furthermore, since peripheral nerve injury is not always constant among patients, the beneficial effects of wrapping may not be easily and efficiently extrapolated. Therefore, to achieve translational research, scholars should try to make a simple compression injury model and a close-to-reality injury model and further investigate the effect of nerve wrapping.
Conclusion
As a short-term effect of the PGA-collagen tube for peripheral nerve injuries, the PGA-collagen tube facilitated the recovery from nerve degeneration at the heat stimulation-induced sensory threshold, motor evoked potentials, and upon pathological observation of the myelinated nerve fiber.
Acknowledgments
We thank Dr. Hiroharu Koga (Kumamoto Health Science University), Mr. Yasuyuki Teramoto (Kumamoto Kinoh Hospital), Mr. Tsukasa Kajihara (Miyuki Giken Kyusyu Co.) Dr. Kazuyuki Oda (Inrercross Co.), Mr. Soma Yamamoto (Bio Research Center Co. Ltd.) and Mr. Kazuyoshi Morita (Bio Research Center Co. Ltd.) for useful technical advice regarding the MEP experiment. This work is supported by both JPJS KAKENHI Grant Numbers 26350646 (AD) and 19K11383 (AD) and Kumamoto Health Science University special fellowship grant number 2020-C-02 (AD).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maugeri G, D’Agata V, Trovato B, et al. The role of exercise on peripheral nerve regeneration: from animal model to clinical application. Heliyon. 2021;7(11):e08281. doi:10.1016/j.heliyon.2021.e08281
2. Noble J, Munro CA, Prasad V, et al. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45(1):116–122. doi:10.1097/00005373-199807000-00025
3. Kim T-H, Yoon S-J, Lee W-C, et al. Protective effect of GCSB-5, an herbal preparation, against peripheral nerve injury in rats. J Ethnopharmacol. 2011;136:297–304. doi:10.1016/j.jep.2011.04.037
4. Sunderland S. Rate of regeneration of motor fibers in the ulnar and sciatic nerves. Arch Neurol Psychiatry. 1947;58:7–13. doi:10.1001/archneurpsyc.1947.02300300017002
5. Novak CB, Anastakis DJ, Beaton DE, et al. Relationships among pain disability, pain intensity, illness intrusiveness, and upper extremity disability in patients with traumatic peripheral nerve injury. J Hand Surg Am. 2010;35(10):1633–1639. doi:10.1016/j.jhsa.2010.07.018
6. Ahmed-Labib M, Golan JD, Jacques L. Functional outcome of brachial plexus reconstruction after trauma. Neurosurgery. 2007;61:1016–1023. doi:10.1227/01.neu.0000303197.87672.31
7. Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. doi:10.1016/j.pain.2010.07.031
8. van Hecke O, Austin SK, Khan RA, et al. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662. doi:10.1016/j.pain.2013.11.013
9. Chu XL, Song XZ, Li Q, et al. Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation. Neural Regen Res. 2022;17:2185–2193. doi:10.4103/1673-5374.335823
10. Pabari A, Lloyd-Hughes H, Seifalian AM, et al. Nerve conduits for peripheral nerve surgery. Plast Reconstr Surg. 2014;133(6):1420–1430. doi:10.1097/PRS.0000000000000226
11. Hirosawa N, Uchida K, Kuniyoshi K, et al. Vein wrapping promotes M2 macrophage polarization in a rat chronic constriction injury model. J Orthopaed Res. 2018;36(8):2210–2217. doi:10.1002/jor.23875
12. Shintani K, Uemura T, Takamatsu K, et al. Protective effect of biodegradable nerve conduit against peripheral nerve adhesion after neurolysis. J Neurosurg. 2018;129(3):815–824. doi:10.3171/2017.4.JNS162522
13. Kokkalis ST, Mavrogenis AF, Vottis C, et al. Median nerve biodegradable wrapping: clinical outcome of 10 patients. Acta Orthop Belg. 2016;82:351–357.
14. Nakamura T, Inada Y, Fukuda S, et al. Experimental study on the regeneration of peripheral nerve gaps through a polyglycolic acid–collagen (PGA–collagen) tube. Brain Res. 2004;1027(1–2):18–29. doi:10.1016/j.brainres.2004.08.040
15. Toba T, Nakamura T, Shimizu Y, et al. Regeneration of canine peroneal nerve with the use of a polyglycolic acid–collagen tube filled with laminin‐soaked collagen sponge: a comparative study of collagen sponge and collagen fibers as filling materials for nerve conduits. J Biomed Mater Res. 2001;58(6):622–630. doi:10.1002/jbm.1061
16. Zukawa M, Osada R, Kimura T. Clinical outcome and ultrasonographic evaluation of treatment using polyglycolic acid-collagen tube for chronic neuropathic pain after peripheral nerve injury. J Orthop Sci. 2019;24(6):1064–1067. doi:10.1016/j.jos.2019.07.017
17. Yoshitani M, Fukuda S, Itoi S, et al. Experimental repair of phrenic nerve using a polyglycolic acid and collagen tube. J Thorac Cardiovasc Surg. 2007;133(3):726–732. doi:10.1016/j.jtcvs.2006.08.089
18. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi:10.1016/0304-3959(83)90201-4
19. Kato N, Nemoto K, Arino H, et al. Treatment of the chronic inflammation in peripheral target tissue improves the crushed nerve recovery in the rat: histopathological assessment of the nerve recovery. J Neurol Sci. 2002;202(1–2):69–74. doi:10.1016/S0022-510X(02)00209-5
20. Iwatsuki K, Arai T, Ota H, et al. Targeting anti-inflammatory treatment can ameliorate injury-induced neuropathic pain. PLoS One. 2013;8(2):e57721. doi:10.1371/journal.pone.0057721
21. Doi A, Sakasaki J, Tokunaga C, et al. Both ipsilateral and contralateral localized vibratory stimulations modulated pain-related sensory thresholds on the foot in mice and humans. J Pain Res. 2018;11:1645–1657. doi:10.2147/JPR.S162379
22. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi:10.1038/bmt.2012.244
23. Nakamura Y, Takanari K, Ebisawa K, et al. Repair of temporal branch of the facial nerve with novel polyglycolic acid-collagen tube: a case report of two cases. Nagoya J Med Sci. 2020;82(1):123–128. doi:10.18999/nagjms.82.1.123
24. Seo K, Terumitsu M, Inada Y, et al. Prognosis after surgical treatment of trigeminal neuropathy with a PGA-c tube: report of 10 cases. Pain Med. 2016;17(12):2360–2368. doi:10.1093/pm/pnw088
25. Fujimaki H, Matsumine H, Osaki H, et al. Dedifferentiated fat cells in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Regen Ther. 2019;11:240–248. doi:10.1016/j.reth.2019.08.004
26. Oatari M, Uehara M, Shimizu F. Evaluation of the effects of a polyglycolic acid-collagen tube in the regeneration of facial nerve defects in rats. Int J Artif Organs. 2018;41:664–669. doi:10.1177/0391398818783860
27. Kiyotani T, Nakamura T, Shimizu Y, et al. Experimental study of nerve regeneration in a biodegradable tube made from collagen and polyglycolic acid. Asaio j. 1995;41(3):M657–61. doi:10.1097/00002480-199507000-00092
28. Kiyotani T, Teramachi M, Takimoto Y, et al. Nerve regeneration across a 25-mm gap bridged by a polyglycolic acid-collagen tube: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 1996;740(1–2):66–74. doi:10.1016/S0006-8993(96)00848-7
29. Inada Y, Morimoto S, Moroi K, et al. Surgical relief of causalgia with an artificial nerve guide tube: successful surgical treatment of causalgia (Complex Regional Pain Syndrome Type II) by in situ tissue engineering with a polyglycolic acid-collagen tube. Pain. 2005;117(3):251–258. doi:10.1016/j.pain.2005.05.033
30. Perl ER. Ideas about pain, a historical view. Nat Rev Neurosci. 2007;8(1):71–80. doi:10.1038/nrn2042
31. Koga K, Furue H, Rashid MH, et al. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain. 2005;1:13. doi:10.1186/1744-8069-1-13
32. Hao J, Bonnet C, Amsalem M, et al. Transduction and encoding sensory information by skin mechanoreceptors. Pflugers Arch. 2015;467(1):109–119. doi:10.1007/s00424-014-1651-7
33. Perl ER. Cutaneous polymodal receptors: characteristics and plasticity. Prog Brain Res. 1996;113:21–37.
34. Vleggeert-Lankamp CL, van den Berg RJ, Feirabend HK, et al. Electrophysiology and morphometry of the Aalpha- and Abeta-fiber populations in the normal and regenerating rat sciatic nerve. Exp Neurol. 2004;187(2):337–349. doi:10.1016/j.expneurol.2004.01.019
35. Herdmann J, Bielefeldt K, Enck P. Quantification of motor pathways to the pelvic floor in humans. Am J Physiol. 1991;260(5 Pt 1):G720–3. doi:10.1152/ajpgi.1991.260.5.G720
36. Matsumoto H, Octaviana F, Terao Y, et al. Magnetic stimulation of the cauda equina in the spinal canal with a flat, large round coil. J Neurol Sci. 2009;284(1–2):46–51. doi:10.1016/j.jns.2009.04.003
37. Rao SS, Coss-Adame E, Tantiphlachiva K, et al. Translumbar and transsacral magnetic neurostimulation for the assessment of neuropathy in fecal incontinence. Dis Colon Rectum. 2014;57(5):645–652. doi:10.1097/DCR.0000000000000069
38. Maccabee PJ, Lipitz ME, Desudchit T, et al. A new method using neuromagnetic stimulation to measure conduction time within the cauda equina. Electroencephalogr Clin Neurophysiol. 1996;101:153–166. doi:10.1016/0924-980X(95)00264-L
39. Matsumoto H, Hanajima R, Terao Y, et al. Magnetic-motor-root stimulation: review. Clin Neurophysiol. 2013;124(6):1055–1067. doi:10.1016/j.clinph.2012.12.049
40. Fisher MA. F-waves--physiology and clinical uses. Sci World J. 2007;7:144–160. doi:10.1100/tsw.2007.49
41. Fisher MA. H reflexes and F waves. Fundamentals, normal and abnormal patterns. Neurol Clin. 2002;20:339–60, vi. doi:10.1016/S0733-8619(01)00004-4
42. Li X, Zhang X, Hao M, et al. The application of collagen in the repair of peripheral nerve defect. Front Bioengine Biotechnol. 2022;10:973301. doi:10.3389/fbioe.2022.973301
43. Salzer JL. Schwann cell myelination. Cold Spring Harb Perspect Biol. 2015;7:a020529. doi:10.1101/cshperspect.a020529
44. Min Q, Parkinson DB, Dun X-P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia. 2021;69:235–254. doi:10.1002/glia.23892
45. Pan B, Guo D, Jing L, et al. Long noncoding RNA Pvt1 promotes the proliferation and migration of Schwann cells by sponging microRNA-214 and targeting c-Jun following peripheral nerve injury. Neural Regeneration Res. 2023;18:1147–1153. doi:10.4103/1673-5374.353497
46. Qian T, Qiao P, Lu Y, et al. Transcription factor SS18L1 regulates the proliferation, migration and differentiation of Schwann cells in peripheral nerve injury. Front Vet Sci. 2022;9:936620. doi:10.3389/fvets.2022.936620
47. Huang T, Qin J-Q, Huo X-K, et al. Changes in content of macrophage migration inhibitory factor secreted by Schwann cells after peripheral nerve injury. Di Yi Jun Yi Da Xue Xue Bao. 2002;22:493–495.
48. Anwar H, Rasul A, Iqbal J, et al. Dietary biomolecules as promising regenerative agents for peripheral nerve injury: an emerging nutraceutical-based therapeutic approach. J Food Biochem. 2021;45:e13989. doi:10.1111/jfbc.13989
49. Govindappa PK, Elfar JC. Erythropoietin promotes M2 macrophage phagocytosis of Schwann cells in peripheral nerve injury. Cell Death Dis. 2022;13:245. doi:10.1038/s41419-022-04671-6
50. Chen P, Cescon M, Zuccolotto G, et al. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015;129:97–113.
51. Liu P, Peng J, Han G-H, et al. Role of macrophages in peripheral nerve injury and repair. Neural Regeneration Res. 2019;14:1335–1342. doi:10.4103/1673-5374.253510
52. He Q-R, Cong M, Chen Q-Z, et al. Expression changes of nerve cell adhesion molecules L1 and semaphorin 3A after peripheral nerve injury. Neural Regeneration Res. 2016;11:2025–2030.
53. Alvites RD, Branquinho MV, Sousa AC, et al. Combined use of chitosan and olfactory mucosa mesenchymal stem/stromal cells to promote peripheral nerve regeneration in vivo. Stem Cells Int. 2021;2021:6613029. doi:10.1155/2021/6613029
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.