Back to Journals » International Journal of General Medicine » Volume 16
Shikonin Inhibits Candida albicans Biofilms via the Ras1-cAMP-Efg1 Signalling Pathway
Authors Pang C, Chen J, Yang L, Yang Y, Qi H, Li R, Cao Y, Miao H
Received 15 April 2023
Accepted for publication 16 June 2023
Published 23 June 2023 Volume 2023:16 Pages 2653—2662
DOI https://doi.org/10.2147/IJGM.S417327
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Chong Pang,1,2 Jianshuang Chen,1,2 Lan Yang,3 Yang Yang,1 Haihua Qi,4 Ran Li,1 Yingying Cao,5 Hao Miao1
1School of Basic Medicine, Chengde Medical University, Chengde, Hebei, People’s Republic of China; 2Hebei Key Laboratory of Nerve Injury and Repair, Chengde, Hebei, People’s Republic of China; 3Hebei Key Laboratory of Research and Development for Chinese Medicine, Chengde Medical University, Chengde, Hebei, People’s Republic of China; 4Department of Dermatology, Affiliated Hospital of Chengde Medical University, Chengde, Hebei, People’s Republic of China; 5Shanghai Skin Disease Hospital, School of Medicine, Tongji University, Shanghai, People’s Republic of China
Correspondence: Hao Miao, School of Basic Medicine, Chengde Medical University, Chengde, Hebei, People’s Republic of China, Email [email protected] Yingying Cao, Shanghai Skin Disease Hospital, School of Medicine, Tongji University, Shanghai, People’s Republic of China, Email [email protected]
Objective: To investigate the influence of shikonin (SK) on the formation of Candida albicans biofilms and discuss the possible mechanism.
Methods: The inhibition of the formation of C. albicans biofilms by SK was observed by scanning electron microscopy. A silicone film method and a water–hydrocarbon two-phase assay were performed to investigate the effects of SK on cell adhesion. Real-time reverse-transcription polymerase chain reaction was used to analyse the expression of genes related to cell adhesion and Ras1–cyclic adenosine monophosphate (cAMP) – enhanced filamentous growth protein 1 (Efg1) signalling pathway. Finally, the level of cAMP in C. albicans was detected and exogenous cAMP rescue experiment was conducted.
Results: The results showed that SK could destroy the typical three-dimensional structure of the biofilms, inhibit cell surface hydrophobicity and cell adhesion, downregulate the expression of Ras1-cAMP-Efg1 signalling pathway-related genes (ECE1, HWP1, ALS3, RAS1, CYR1, EFG1 and TEC1) and effectively reduce the production of key messenger cAMP in the Ras1-cAMP-Efg1 pathway. Meanwhile, exogenous cAMP reversed the inhibitory effect of SK on biofilms formation.
Conclusion: Our results suggest that SK exhibits potential anti-C. albicans biofilms effects related to the inhibition of Ras1-cAMP-Efg1 pathway.
Keywords: shikonin, Ras1-cAMP-Efg1 signalling pathway, Candida albicans, biofilms
Introduction
Candida albicans is a type of fungus that is part of the normal human microbiota, which is a collection of microorganisms that live on and in the human body. It is commonly found in the mouth, intestines, and genital tract of healthy individuals. However, under certain conditions, such as a weakened immune system, antibiotic use, or hormonal imbalances. C. albicans can overgrow and cause infections, particularly deep fungal infections.1 In addition, recent research has shown that biofilms, which are structured communities of microorganisms that attach to human organs or medical devices, are becoming a more common source of C. albicans infections. The formation of C. albicans biofilms is a multifaceted process that entails several developmental stages. It begins with the adhesion of yeast cells to the substrate, followed by cell proliferation, elevation of hyphal growth, and production of extracellular matrix materials.2,3 Once formed, C. albicans biofilms are highly adapted to the environment and less sensitive to stimuli such as changes in hydrogen peroxide and pH. More importantly, they develop a high tolerance to the human immune system and various antifungal drugs. Studies have shown that C. albicans biofilms show a 1000-fold greater resistance to antifungals than planktonic cells.4,5 Therefore, once a biofilm is formed in the body, it is difficult to completely eliminate and can pose great challenges to treatment. The formation of biofilms is highly regulated and involves multiple interconnected signaling pathways, Ras1-cAMP-Efg1 signaling pathway is one of the most important.6,7
Shikonin (SK), an extract from the roots of Lithospermum erythrorhizon, is a naphthoquinone. Previous studies have demonstrated that SK have various pharmacological activities including antifungal.8,9 Especially against some species of fluconazole-resistant C. albicans, it has been shown to have 8 to 32 times more antifungal activity than fluconazole.10 Additionally, SK was also found to be the first natural compound reported to execute antifungal activity directly via boosting H3K56ac mediated by HST3.11 Moreover, SK has an inhibitory effect on biofilms causing highly resistant C. albicans,12 but the underlying mechanism involved has not been fully clear.
The formation of biofilms is a major cause of antifungal treatment failure, and the development of novel antifungal drugs that can effectively inhibit biofilms is urgently needed.13 Consistent with our previous published results, we further verified the potent inhibitory effect of SK on C. albicans biofilms using scanning electron microscopy experimental observations in this study. In previous study,12 the possible mechanism of SK against C. albicans biofilms has been described, it was through the enhancement production of farnesol, which can reduce CSH in a dose-dependent manner, by which SK may hinder the initial adhesion of C. albicans cells and thus prevent the formation of biofilms. However, this study did not provide an in-depth analysis of the above aspect, mainly to investigate the effect of SK on the biofilms formation relevant signalling pathways, and it is complementary to such studies as it enables a clearer understanding of the mechanism of SK against C. albicans biofilms at the genetic level. We believe our findings will provide new ideas for the clinical treatment of chronic refractory fungal infections caused by C. albicans biofilms.
Materials and Methods
Fungal Strains, Culture Media and Chemical Compounds
The American Type Culture Collection (ATCC) strain of C. albicans SC5314 was used in many fungal studies. C. albicans cells were cultured on Sabouraud dextrose agar medium (1% peptone, 4% glucose, and 1.8% agar) and incubated in Yeast Peptone Dextrose (YPD) liquid medium (1% yeast extract, 2% peptone, and 2% glucose) at 30 °C with oscillation at 200 r/min. For all in vitro experiments, 6.4 mg/mL SK (purity > 99%, Beijing Solarbio Science and Technology Co., Ltd., dissolved in DMSO) was used as a stock solution and added to the culture medium to obtain the desired concentration with the concentration of solvent DMSO was uniform Less than 1%. cAMP standards were purchased from Beijing Solarbio Science and Technology Co., Ltd., and RPMI-1640 medium (Gibco, USA) was used for biofilms formation experiments.
Reagents
The following reagents were used: a fungal RNA extraction kit (TIANZ, Beijing, China), a cDNA synthesis kit (TaKaRa Biotechnology, Dalian, China), SYBR Green I stain (TaKaRa Biotechnology, Dalian, China), a cAMP enzyme immunoassay kit (Kingsley Biotechnology, Nanjing, China), XTT (Sigma) and menadione (Sigma).
Observation of the Effect of SK on Biofilms Formation Using Scanning Electron Microscopy
A 1-mL volume of the culture solution (1.0 × 106 CFU/mL) was inoculated into 6-well plates; each well contained a pre-placed 1-cm-long silicone catheter to be used to develop biofilms, which had been soaked in 75% ethanol for 24 h. The catheter was inoculated with C. albicans and incubated statically at 37°C for 120 min to allow adhesion. After removing non-adhesion cells, the catheters were incubated with fresh RPMI 1640 medium at 37°C for 24 h. For SK treatment group, 8 μg/mL SK was added with the fresh RPMI 1640 medium after 120 min of adhesion. Next, the catheter was washed three times with sterile PBS and placed in a fixative consisting of 2.5% (vol/vol) glutaraldehyde in 0.15 M sodium cacodylate buffer (pH 7.2) for 2 h. Then, the catheter was rinsed three times with sterile PBS and dehydrated for about 10 min each with 30% ethanol, 70% ethanol, and anhydrous ethanol, respectively, and dried at room temperature overnight. And then, it was attached with double-sided tape to a sample table, sprayed with gold by an HVS-GS vacuum vaporizer and observed and photographed by a scanning electron microscope,14 with observation multiples of 3500 ×, 2000 × and 1000 ×.
Effects of SK on the Cell Adhesion of C. albicans
Investigation of the Effects of SK on the Adhesion of C. albicans to Cells Using the Silicone Film Method
C. albicans can form biofilms on the surfaces of inert materials or other organisms, such as artificial organ implants or devices/catheters implanted in vivo. Such biofilms can be formed on the surfaces of inert materials mainly because of the strong adhesive effect of C. albicans on inert materials. SK was added at different concentrations during the early stages (120 min) of adhesion to compare the biofilms formation ability of C. albicans on silicone film.15 The experiment was performed as follows: Cells of C. albicans (1.0 × 106 CFU/mL in RPMI 1640 medium) were added to a 6-well culture plate, and a silicone film that was prepared by soaking in fetal bovine serum and incubated for 24 h was pre-placed in each well. SK at 4, 8, 16 μg/mL concentrations was added simultaneously and incubated during 120 min of adhesion at 37 °C and 100 r/min oscillations; then, the medium was aspirated, non-adherent cells were removed, and fresh medium was added to the adherent cells. The plates were further incubated at 37 °C for 24 h under gentle stirring until a mature biofilm was formed. Then the silicone films with attached biofilms were removed from the wells, dried overnight and weighed. The total biomass of biofilms was calculated by subtracting the weight of the silicone prior to biofilm growth from the weight of the silicone after the drying period, with adjustment for the weight of control silicone film exposed to no cells.
Cellular Surface Hydrophobicity (CSH) Assay
The CSH of C. albicans biofilms was determined using a water–hydrocarbon biphasic assay.12 C. albicans biofilms were scraped from the surface of the silicone films using a sterile spatula. The suspension (optical density [OD] 600 = 1.0) was prepared with YPD broth; 1.2 mL of the suspension was placed in a clean glass tube for each group and covered with 0.3mL of n-octane. The suspensions were vortexed for 3 min, and the OD600 values of the aqueous phase were measured immediately after the separation of the two phases at room temperature. The OD600 value of the YPD broth without n-octane was used as a negative control, and the CSH was calculated as relative hydrophobicity = (OD600control group − OD600experimental group) / OD600control group. Assays were performed in triplicate.
Real-Time Reverse-Transcription Polymerase Chain Reaction Experiments
Real-time reverse transcription-PCR (RT-PCR) was performed as described previously.16 Fungal RNA was extracted according to the method described in the fungal RNA extraction kit (TIANZ, Beijing, China). The extracted RNA was resuspended in diethyl pyrocarbonate-treated water. The OD260 and OD280 were measured. First-strandcDNA was obtained using a cDNA synthesis kit for RT-PCR (TaKaRa Biotechnology, Dalian, China), and a real-time PCR was performed using a real-time PCR system (7500, Applied Biosystems). Finally, SYBR green I (TaKaRa Biotechnology, Dalian, China) is used for real-time monitoring of amplification products. The primers are shown in Table 1. The PCR protocol consisted of a denaturation programme (90 °C, 10s), a 40-cycle amplification and quantification programme (95 °C, 10s [denaturation]; 60 °C, 20s [annealing]; 72 °C, 30s [extension]), a melting curve programme (60 °C – 95 °C, with a heating rate of 0.1 °C/s) and a final cooling to 40 °C. The results were analysed using an ABI 7500 SDS software system (Applied Biosystems), with 18S rRNA as the internal reference standard. The levels of gene expression were reported as ploidy changes (the 2−(ΔΔCt) method). Assays were performed in triplicate.
 |
Table 1 Primers Used in This Study |
Measurement of cAMP Levels
C. albicans cells (1 mL, 2.0×106 CFU/mL) were added to 6-well culture plates, each containing a pre-placed silicone film (prepared as described above 1.4.1). They were adhered for 120 min, and then incubated at 37 °C for 4 h (for adherence) or 24 h (for biofilms formation) with 4, 8, 16 μg/mL concentrations of SK, which were added under oscillation at 100 r/min. C. albicans cells were scraped from the silicone film using a cell scraper, rinsed three times in PBS buffer and transferred to a 1.5 mL microcentrifuge tube containing 0.5 g glass beads and 500 µL 10% trichloroacetic acid. They were vortexed briefly and frozen immediately in liquid nitrogen. After centrifugation at 12,700 g for 3 min, trichloroacetic acid was extracted four times with water-saturated ether. The cAMP content in the supernatant was measured using a cAMP enzyme immunoassay kit (Kingsray Biotechnology, Nanjing, China) according to the kit manufacturer’s instructions for analysing cAMP in the supernatant.17 The experiments were performed in triplicate.
Determination of the Effects of cAMP Re-Supplementation on the Anti-Biofilms Effect of SK by the XTT Method
A cell suspension of 1.0×106 CFU/mL was prepared using RPMI 1640 medium, and 100 μL of the solution was inoculated into 96-well polystyrene plates and incubated at 37 °C for 120 min. After aspirating the medium and removing the non-adherent cells, the cells were divided into the following eight groups: control group, 5 mM cAMP group, 4 μg/mL SK group, 8 μg/mL SK group, 16 μg/mL SK group, 4 μg/mL SK + 5 mM cAMP group, 8 μg/mL SK + 5 mM cAMP group and 16 μg/mL SK + 5 mM cAMP group. Incubation was continued at 37 °C for 24 h. The nonadherent cells were washed off with PBS, and XTT–methanone–quinone solution was added. Then, the samples were placed in the dark for 2 h at 37°C. The OD values (directly reflecting the change in the metabolic activity of the periplasmic cells) were measured at 490 nm with a microtiter plate reader.18,19 Data were expressed as percentages of biofilms formation in treated sample vs untreated control. The experiments were performed in triplicate.
Statistical Analysis
Statistical processing software SPSS 19.0 was used for the statistical analysis. All data were tested for normality using the Shapiro–Wilks test before the statistical analysis. Measurement data conforming to a normal distribution are expressed as mean ± standard deviation (SD), and enumeration data are expressed as rates. A one-way analysis of variance was used to compare groups, and P < 0.05 was used as the significance test criterion.
Results
SK Inhibits the Formation of C. albicans Biofilms
Consistent with our previous results,12 scanning electron microscopy analysis further detected the antibiofilm activity of SK. In the control group, the normal mature biofilms presented a dense irregular three-dimensional network, mainly composed of true hyphae. With 8 µg/mL SK, the filamentous structure of the biofilms to each other was disrupted; it mainly comprised pseudohyphae and yeast-state cells, and very few true hyphae could be observed (Figure 1). The result further confirmed that SK had a strong inhibitory effect on the biofilms formation of C. albicans.
 |
Figure 1 Morphology alteration of C. albicans SC5314 biofilms after treated by 8 μg/mL SK. Notes: The inset in the 3500×, 2000×, 1000× panels show the area that was magnificated. |
SK Concentration-Dependent Inhibition of C. albicans Cell Adhesion
As adherence is a fundamental prerequisite for promoting biofilms formation, we investigated the impact of SK on the adhesion of C. albicans cells. In the control group, a thick opaque capsule was formed on the silicone film. However, with the increasing concentration of SK added during the adhesion period, the biofilms on the silicone film gradually became thinner and transparent. When the SK concentration reached 16 µg/mL, only a few C. albicans cells adhered to the silicone film, and essentially, no biofilm formation was observed (Figure 2). The result was also confirmed by biofilms biomass determination, indicating that SK significantly inhibited the adhesion of C. albicans to the silicone film.
We investigated the impact of SK treatment on the cellular surface hydrophobicity (CSH), given that CSH is a critical factor for cell adhesion. Our results, shown in Figure 3, indicate a negative correlation between SK concentration and the CSH of C. albicans biofilms. The relative CSH of the untreated C. albicans biofilms was higher (approximately 0.77), and the CSH of the biofilms formed by SK treatment was significantly reduced to 0.69 (P < 0.05) at 2 µg/mL SK; after this, it decreased with increasing drug concentration to 0.47 (P < 0.05) at 4 µg/mL SK, 0.35 (P < 0.01) at 8 µg/mL, 0.15 (P < 0.01) at 16 µg/mL, below 0.05 (P < 0.01) at 32 µg/mL and almost 0 (P < 0.01) at 64 µg/mL. The results showed that SK decreased the CSH of the biofilms in a concentration-dependent manner.
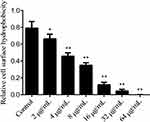 |
Figure 3 Effects of different concentrations of SK on cell surface hydrophobicity (CSH) of C. albicans SC5314 biofilms. Notes: Compared with control, *P < 0.05, **P < 0.01. |
SK Affects the Expression of Biofilms-Associated Genes in the Ras1-cAMP-Efg1 Signalling Pathway
To understand the molecular basis for SK with inhibit action on C. albicans biofilms, we investigated the expression of genes related to adhesion, hyphae growth and biofilms formation, especially those related to the Ras1-cAMP-Efg1 pathway through the real time PCR methods. Compared with the control group, the expression levels of ECE1, HWP1, ALS3, RAS1, EFG1, TEC1 and CYR1, which are involved in positive regulation of biofilms formation biological processes and also crucial members of the Ras1-cAMP-Efg1 pathway, were downregulated by 2.17-, 2.38-, 2.94-, 1.35-, 2.32-, 2.13- and 2.08-fold in the 8 µg/mL SK treatment group, respectively (Figure 4). This result suggested that the potential mechanism of inhibition of biofilms formation by SK might be associated with the Ras1-cAMP-Efg1 pathway.
SK Inhibits Biofilms Formation by Reducing cAMP Levels in C. albicans
To delve deeper into the impact of SK on the Ras1-cAMP-Efg1 pathway, we assessed the production of cAMP - The critical messenger molecule of this pathway - in C. albicans exposed to SK at 4- and 24-hours post-treatment. The result showed that SK could significantly decrease cAMP production in a concentration-dependent manner at 4- and 24- h (P< 0.01) (Figure 5). The result suggested that SK significantly decreases cAMP production at both the adhesion (4 h) and maturation (24 h) stages of biofilms formation in C. albicans.
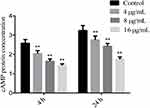 |
Figure 5 Effects of SK on the production of cAMP protein of C. albicans SC5314 biofilms. Notes: Compared with control, **P < 0.01. |
After analyzing the aforementioned results, we hypothesized that SK’s ability to hinder C. albicans biofilms formation could be linked to the suppression of the Ras1-cAMP-Efg1 pathway. To confirm our assumption, we performed experiments involving the rescue of exogenous cAMP. As shown in Figure 6, the formation rates of the biofilms in the 4, 8 and 16 µg/mL SK-alone groups were 65%, 42% and 26%, respectively. But, when exogenous cAMP was added at the same time, the formation rates of the biofilms were promoted to 77% (P < 0.05), 60% (P < 0.01) and 51% (P < 0.01), respectively; Given that 5 mM cAMP alone had no effect on the biofilms, the results in above indicated that cAMP could significantly reverse the inhibitory effect of SK on biofilms formation. Together, these data suggested that SK might inhibit C. albicans biofilms through the Ras1-cAMP-Efg1 pathway.
Discussion
In this study, the antibiofilm effect of SK was further confirmed by SEM. Compared with the normal C. albicans biofilms, with true hyphae criss-crossing, a dose of 8µg/mL of SK resulted in a significant decrease in cell density and severe defects in filamentation. Our utilization of SEM provided a clearer and more detailed visualization of the three-dimensional network structure of C. albicans biofilms, which allowed us to demonstrate the disruptive effects of SK on this biofilms structure. By showcasing this enhanced visualization, we believe our study adds valuable insights into the mechanistic understanding of the action of SK on C. albicans biofilms.
Adhesion of single C. albicans cells in the basal layer is the initial stage of C. albicans biofilms formation and plays an important role in the formation of C. albicans biofilms.20 Therefore, in this study, we specifically focused on investigating the influence of SK on cell adhesion through experiments such as the silicone film method and cell surface hydrophobicity assay. Unlike previous study that added drugs after the adhesion stage of biofilms formation, we introduced SK during the 120 min cell adhesion stage. This approach allowed us to examine the direct impact of SK on the initial adhesion process. Our results indicated that SK exhibited a significant inhibitory effect on the early cell adhesion process of C. albicans biofilms formation, and this effect was closely related to its effect on the cell surface hydrophobicity of C. albicans, as previous studies have found that the ability of the fungus to adhere to the surface of inert materials such as plastic is related to its cell surface hydrophobicity (CSH).21,22 It has been reported that CSH plays an important role in the biofilm adhesion phase of C. albicans, and the larger the CSH value, the stronger the cell adhesion and the stronger the biofilms formation capacity.23 Our experimental results showed SK could reduce CSH in a dose-dependent manner, which is consistent with literature reports. Therefore, we speculated that influencing cell CSH and thus inhibiting cell adhesion could be a possible mechanism for SK to disrupt biofilm formation.
To further explore the molecular basis of SK inhibition in C. albicans biofilms, we examined the expression levels of genes related to cell adhesion and yeast filamentous state transformation, namely ECE1, HYR1, HWP1, and ALS3. Our findings indicated significant downregulation of these genes, all of which are regulated by the Ras1-cAMP-Efg1 signaling pathway,24 as reported in the literature. The Ras1-cAMP-Efg1 pathway is a crucial regulator of C. albicans biofilms, and is activated by extracellular environmental factors that stimulate the Ras1 protein, which in turn activates Cyr1 to produce the second messenger, cAMP. Thus, the protein kinase A (PKA) complex is activated, PKA complex phosphorylates and activates transcription factors Efg1 and Tec1, thereby controlling adhesion, mycelial development, biofilm formation, and other biological processes.24 Several natural products, including magnolol and sodium new houttuyfonate, have been shown to inhibit C. albicans adhesion, yeast-hyphae state transformation, and biofilm formation by downregulating the Ras1-cAMP-Efg1 pathway.6,25
Our research furtherly uncovered that SK effectively decreased the expression of genes linked to the Ras1-cAMP-Efg1 signalling pathway, namely RAS1, CYR1, EFG1, and TEC1. RAS1 is a gene that encodes a small GTPase involved in the regulation of multiple cellular processes, including cell growth and differentiation. It is a critical component of the Ras1-cAMP-Efg1 pathway, where it functions as a key regulator of Efg1 and other downstream genes expression.26 EFG1 (embedded filamentation protein 1) is a gene that encodes a transcription factor that regulates hyphal growth and biofilms formation in C. albicans. It is a downstream target of the Ras1-cAMP pathway and is required for proper biofilms formation. TEC1 (transcriptional effector of Candida) is a gene that encodes a transcription factor that is necessary for the expression of downstream genes involved in hyphal growth and biofilms formation. It is a downstream target of Efg1 and is also activated by Ras1-cAMP pathway. Both the EFG1 and TEC1 positively regulate biofilms-specific genes expression, such as ECE1, ALS3 and HWP1.27,28 ECE1 (enhanced Candida virulence gene) is a key regulator of biofilms formation in C. albicans. It encodes a protein that is involved in the production of hyphae, which are necessary for the formation of mature biofilms. The ALS3 (agglutinin-like sequence 3) is a gene that encodes a protein which contributes to cell invasion and subsequent host cell damage.29 HWP1 (hyphal wall protein 1) encodes a mammalian transglutaminase-targeted cell surface glycoprotein that is necessary for the adhesion of hyphae to surfaces and the formation of biofilm matrix.30 CYR1 (adenylate cyclase) is a gene that encodes an enzyme responsible for the production of cAMP, a second messenger involved in the regulation of cellular processes. When Cyr1p is disrupted, C. albicans’ growth rate decreases, and cells are confined to the yeast state.31 Overall, these genes are critical for the regulation of various cellular processes during C. albicans biofilms formation, including cell adhesion, hypha growth as well as virulence. Therefore, the downregulation of these genes by SK suggests that it may inhibit the formation of biofilms in C. albicans by disrupting these cellular processes. Additionally, our study demonstrated that SK substantially decreased the biofilms cAMP levels of C. albicans, and the anti-biofilm effect of SK was significantly reduced after cAMP back-supplementation. Together, we speculate that the mechanism of SK inhibition of C. albicans biofilms may be related to the blocking of the Ras1-cAMP-Efg1 signalling pathway.
Conclusion
SK may affect the expression levels of cell adhesion and yeast filamentous state transformation-related factors (ECE1, HWP1, ALS3, RAS1, CYR1, EFG1 and TEC1) downstream of the Ras1-cAMP-Efg1 signalling pathway by decreasing cAMP levels, thereby affecting the surface hydrophobicity and adhesion ability of C. albicans cells, playing an anti-C. albicans biofilms role and destroying the typical three-dimensional structure of C. albicans biofilms. These findings suggest that SK has potential as an alternative antifungal agent for the treatment of biofilm-associated infections. Further studies are needed to explore the in vivo efficacy and safety of SK as an antifungal agent.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was funded by Science and Technology Project of Hebei Education Department (QN2021004), 2021 Research Start-up Fund for High-level Talents of Chengde Medical University (202109) and Neurobiology Department of Chengde Medical University (071006).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang T, Shao J, Da W, et al. Strong synergism of palmatine and fluconazole/itraconazole against planktonic and biofilm cells of candida species and efflux-associated antifungal mechanism. Front Microbiol. 2018;9:2892. doi:10.3389/fmicb.2018.02892
2. Gulati M, Nobile CJ. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect. 2016;18:310–321. doi:10.1016/j.micinf.2016.01.002
3. Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol. 2011;9:109–118. doi:10.1038/nrmicro2475
4. Tobudic S, Kratzer C, Lassnigg A, Presterl E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses. 2012;55:199–204. doi:10.1111/j.1439-0507.2011.02076.x
5. Zarei Mahmoudabadi A, Zarrin M, Kiasat N. Biofilm Formation and Susceptibility to Amphotericin B and Fluconazole in Candida albicans. Jundishapur J Microbiol. 2014;7:e17105. doi:10.5812/jjm.17105
6. Sun L, Liao K, Wang D. Effects of magnolol and honokiol on adhesion, yeast-hyphal transition, and formation of biofilm by Candida albicans. PLoS One. 2015;10:e0117695. doi:10.1371/journal.pone.0117695
7. Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71:348–376. doi:10.1128/MMBR.00009-06
8. Lan W, Wan S, Gu W, Wang H, Zhou S. Mechanisms behind the inhibition of lung adenocarcinoma cell by shikonin. Cell Biochem Biophys. 2014;70:1459–1467. doi:10.1007/s12013-014-0083-5
9. Liao Z, Yan Y, Dong H, Zhu Z, Jiang Y, Cao Y. Endogenous nitric oxide accumulation is involved in the antifungal activity of Shikonin against Candida albicans. Emerg Microbes Infect. 2016;5:e88. doi:10.1038/emi.2016.87
10. Miao H, Zhao L, Li C, et al. Inhibitory effect of Shikonin on Candida albicans growth. Biol Pharm Bull. 2012;35:1956–1963. doi:10.1248/bpb.b12-00338
11. Liao Z, Zhu Z, Li L, et al. Metabonomics on Candida albicans indicate the excessive H3K56ac is involved in the antifungal activity of Shikonin. Emerg Microbes Infect. 2019;8(1):1243–1253. doi:10.1080/22221751.2019.1657362
12. Yan Y, Tan F, Miao H, Wang H, Cao Y. Effect of Shikonin Against Candida albicans Biofilms. Front Microbiol. 2019;10:1085. doi:10.3389/fmicb.2019.01085
13. De Vita D, Friggeri L, D’Auria FD, et al. Activity of caffeic acid derivatives against Candida albicans biofilm. Bioorg Med Chem Lett. 2014;24:1502–1505. doi:10.1016/j.bmcl.2014.02.005
14. Zhao LX, Li DD, Hu DD, et al. Effect of tetrandrine against Candida albicans biofilms. PLoS One. 2013;8:e79671. doi:10.1371/journal.pone.0079671
15. Nobile CJ, Andes DR, Nett JE, et al. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006;2:e63. doi:10.1371/journal.ppat.0020063
16. Sun LM, Cheng AX, Wu XZ, Zhang HJ, Lou HX. Synergistic mechanisms of retigeric acid B and azoles against Candida albicans. J Appl Microbiol. 2010;108:341–348. doi:10.1111/j.1365-2672.2009.04429.x
17. Miwa T, Takagi Y, Shinozaki M, et al. Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot Cell. 2004;3:919–931. doi:10.1128/EC.3.4.919-931.2004
18. Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475–2479. doi:10.1128/AAC.45.9.2475-2479.2001
19. Jung WH, Stateva LI. The cAMP phosphodiesterase encoded by CaPDE2 is required for hyphal development in Candida albicans. Microbiology. 2003;149:2961–2976. doi:10.1099/mic.0.26517-0
20. Montelongo-Jauregui D, Saville SP, Lopez-Ribot JL. Contributions of Candida albicans Dimorphism, Adhesive Interactions, and Extracellular Matrix to the Formation of Dual-Species Biofilms with Streptococcus gordonii. mBio. 2019;10. doi:10.1128/mBio.01179-19
21. Reynolds TB, Fink GR. Bakers’ yeast, a model for fungal biofilm formation. Science. 2001;291:878–881. doi:10.1126/science.291.5505.878
22. Suchodolski J, Muraszko J, Korba A, Bernat P, Krasowska A. Lipid composition and cell surface hydrophobicity of Candida albicans influence the efficacy of fluconazole-gentamicin treatment. Yeast. 2020;37:117–129. doi:10.1002/yea.3455
23. Diaz-Garcia J, Arendrup MC, Canton R, et al. Candidemia Candida albicans clusters have higher tendency to form biofilms than singleton genotypesdagger. Med Mycol. 2020;58:887–895. doi:10.1093/mmy/myaa002
24. Lim CS, Wong WF, Rosli R, Ng KP, Seow HF, Chong PP. 2-dodecanol (decyl methyl carbinol) inhibits hyphal formation and SIR2 expression in C. albicans. J Basic Microbiol. 2009;49:579–583. doi:10.1002/jobm.200900035
25. Wu J, Wu D, Zhao Y, et al. Sodium New Houttuyfonate Inhibits Candida albicans Biofilm Formation by Inhibiting the Ras1-cAMP-Efg1 Pathway Revealed by RNA-seq. Front Microbiol. 2020;11:2075. doi:10.3389/fmicb.2020.02075
26. Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol. 2008;67:47–62. doi:10.1111/j.1365-2958.2007.06013.x
27. Panariello BHD, Klein MI, Pavarina AC, Duarte S. Inactivation of genes TEC1 and EFG1 in Candida albicans influences extracellular matrix composition and biofilm morphology. J Oral Microbiol. 2017;9:1385372. doi:10.1080/20002297.2017.1385372
28. Deveau A, Piispanen AE, Jackson AA, Hogan DA. Farnesol induces hydrogen peroxide resistance in Candida albicans yeast by inhibiting the Ras-cyclic AMP signaling pathway. Eukaryot Cell. 2010;9:569–577. doi:10.1128/EC.00321-09
29. Liu Y, Filler SG. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell. 2011;10:168–173. doi:10.1128/EC.00279-10
30. Ene IV, Bennett RJ. Hwp1 and related adhesins contribute to both mating and biofilm formation in Candida albicans. Eukaryot Cell. 2009;8:1909–1913. doi:10.1128/EC.00245-09
31. Burgain A, Pic E, Markey L, Tebbji F, Kumamoto CA, Sellam A. A novel genetic circuitry governing hypoxic metabolic flexibility, commensalism and virulence in the fungal pathogen Candida albicans. PLoS Pathog. 2019;15:e1007823. doi:10.1371/journal.ppat.1007823
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



