Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Shen-Qi-Jie-Yu-Fang has antidepressant effects in a rodent model of postpartum depression by regulating the immune organs and subsets of T lymphocytes
Authors Qu M, Tang Q, Li X, Zhao R, Li J, Xu H, Gao Y, Mao Y
Received 5 March 2015
Accepted for publication 10 April 2015
Published 23 June 2015 Volume 2015:11 Pages 1523—1540
DOI https://doi.org/10.2147/NDT.S83964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Miao Qu,1 Qisheng Tang,1 Xiaoli Li,1 Ruizhen Zhao,1 Jingya Li,1 Hong Xu,2 Yushan Gao,2 Yingqiu Mao3
1Third Affiliated Hospital, 2School of Basic Medical Sciences, 3Center of Scientific Research, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
Background: Shen-Qi-Jie-Yu-Fang (SJ Fang) is a herbal preparation used in traditional Chinese medicine, and is a potentially important new therapeutic agent in postpartum depression (PPD). Previously, we have elucidated the effects of SJ Fang on hormone receptors and monoamine neurotransmitters involved in the hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes in PPD rats. However, the immune-modulating effects of SJ Fang in PPD are still unknown. In this study, we explored the effects of SJ Fang on the immune organs and subsets of T lymphocytes in PPD rats.
Methods: PPD was created in Sprague-Dawley rats by inducing hormone-simulated pregnancy followed by hormone withdrawal. After hormone withdrawal, the PPD rats were then treated with fluoxetine at 1, 2, and 4 weeks, and the SJ Fang rats were also treated at 1, 2, and 4 weeks. Depressive behavior in the rats was evaluated by the forced swim test, sucrose consumption test, and open field test. The thymus index and spleen index were calculated. Hematoxylin-eosin staining was used to identify pathological features in the thymus and spleen. CD3, CD4, and CD8 lymphocyte subsets were analyzed by flow cytometry.
Results: Both fluoxetine and SJ Fang decreased immobility time, increased sucrose consumption, an horizontal and vertical movement. After 4 weeks of treatment with fluoxetine or SJ Fang, the thymus index and spleen index were significantly higher than at baseline, and the morphology of the thymus and spleen were returning to normal. Two weeks after hormone withdrawal, subsets of T lymphocytes indicated a shift from immune activation to immune suppression, which was reversed by 4 weeks of treatment with fluoxetine or SJ Fang.
Conclusion: It is suggested that T-cell mediate immune responses which may play a role in the etiopathology of postpartum depression. SJ Fang had an antidepressant effect on the immune system in rats with PPD.
Keywords: spleen index, thymus index, Hematoxylin and eosin staining, CD4+/CD8+ ratio, immune activation, immune suppression
Introduction
Postpartum depression (PPD) is a serious postpartum psychological mood disorder that affects both mothers and their children. A diagnosis of PPD has a significant adverse impact on women with PPD and their families.1 The majority of mothers with PPD are reluctant to seek treatment because of the risk of medication adversely affecting the infant via breastfeeding. Therefore, alternative treatment needs to be considered to alleviate the symptoms of depression with the least side effects for both mother and baby. Traditional Chinese medicine, eg, Shen-Qi-Jie-Yu-Fang (SJ Fang), may be an effective therapy for PPD.
SJ Fang was established in traditional Chinese medicine theory and subjected to clinical studies 10 years ago by Tang, who considered that the pathogenesis of PPD included both qi and blood deficiency, blockade of static blood and turbidity, dysfunction of brain essence and spirit.2 The solution to the key point was tonifying and replenishing heart and spleen, harmonizing qi and dispelling stasis, nourishing brain and enlivening spirit. Thus, SJ Fang was proposed to have a role in the treatment of PPD. When used clinically, SJ Fang has been shown to be able to improve symptoms of depression in PPD.
Our team has researched the ability of SJ Fang to regulate the neuroendocrine system in PPD rats. Basing on the “ovarian steroid withdrawal” hypothesis,3–6 onset of PPD is thought to be due to the sudden decrease in estrogen and progesterone levels after delivery. Clinical reports3,7–10 and animal studies11–13 support this hypothesis. More specifically, it is thought that PPD is caused by changes in levels of estrogen, progesterone, monoamine neurotransmitters, their receptors, metabolic products, and other biological factors within the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes.14 In previous research, also using PPD rats, Yang et al found that SJ Fang can significantly increase expression of estradiol, progesterone, gonadotropin-releasing hormone, follicle-stimulating hormone, and luteinizing hormone in the HPG axis and decrease levels of corticotropin-releasing hormone, adrenocorticotropic hormone, and corticosterone in the HPA axis.15 Further, our research has demonstrated that SJ Fang upregulates expression of estradiol receptor-α and estradiol receptor-β in the prefrontal cortex, hippocampus, and hypothalamus.16 SJ Fang can increase levels of monoamine neurotransmitters, ie, 5-hydroxytryptamine, norepinephrine, and dopamine, in the prefrontal cortex and hippocampus. We have also shown that SJ Fang can increase the metabolic products of 5-hydroxyindoleacetic acid and dihydroxyphenylacetic acid in the prefrontal cortex and hippocampus.17
The cytokine network and the inflammatory response system are now thought to be involved in the pathophysiology of major depressive disorder.18–21 The etiology of PPD, as a subtype of major depressive disorder, has also been proposed to be associated with the immune system and cytokines.22 Further, hyperfunction in HPA axis altered in immune system and cytokines have been proposed to play a role in PPD.23–26 Further, in the gestational period, the maternal occurred immunological tolerance with the allogenic fetus and T lymphocyte response to fetal antigens.27 T lymphocyte subsets include helper/inducer (CD4+) T-cells and suppressor/cytotoxic (CD8+) T-cells, which coordinate the immune response. T lymphocytes induce the immune balance is the essential condition in maintain the function with maternal. In such application, flow cytometry was used to provide absolute counts, percentages and ratios of these lymphocyte subsets.
Although there is both clinical and experimental evidence that the immune system is dysfunctional in PPD,28–32 the status of T lymphocyte subpopulations remains unknown. Further, the ability of SJ Fang to modulate the immune system in PPD is still not well understood. In this study, we investigated the effects of SJ Fang on the immune organs and T lymphocyte subpopulations in PPD rats. We also compared the immunological effects of SJ Fang with those of fluoxetine.
Materials and methods
Ethics statement
The experimental protocols used in this research were approved by the Beijing Institute of Animal Ethics Committee (Beijing, People’s Republic of China). The procedures used were carried out in accordance with the Guidelines for Animal Experimentation of Beijing University of Chinese Medicine (Beijing, People’s Republic of China). All effort was made to keep the number of animals used in this study to a minimum and to minimize their suffering.
Preparation of SJ Fang decoction
The SJ Fang decoction used in this study contained 20 g of Radix astragali, 12 g of Codonopsis pilosula, 15 g of fried Semen Ziziphi spinosae, 15 g of Fructus corni, 15 g of Radix curcumae, 10 g of Pericarpium citri reticulatae, 10 g of Citrus chirocarpus, and 10 g of Angelica sinensis. The granules containing SJ Fang were sourced from Tcmages Pharmaceuticals Co Ltd (Beijing, People’s Republic of China), and authenticated by Dr Lu at the Third Affiliated Hospital of Beijing University of Chinese Medicine, where voucher specimens were deposited. Two bags of SJ Fang granules (containing 107 g of crude drug, equivalent to an adult dosage for 1 day) were mixed and dissolved in 85.6 mL of double-distilled water to a concentration of 1.25 g/mL. The solution was stored at 4°C, and heated before gavage.
Animals and drug treatment
Ninety female Sprague-Dawley rats (SCXK 2012-0001, Charles River Laboratories International Inc, Beijing, People’s Republic of China) weighing approximately 190–210 g were group-housed in a temperature-controlled colony room (21°C±2°C), maintained on a 12-hour light/dark cycle (lights on from 7:30 am to 7:30 pm), and given free access to laboratory food and water, except during surgery and behavioral assessments. After acclimation to their new environment for 7 days, their behavior was assessed to record the behavior scores by the open field test (OFT). Basing on the similar scores, the rats were randomly divided into five groups: normal rats (naive + double-distilled water, n=6), sham-operated rats (ovariectomized control + double-distilled water, n=6), PPD rats (hormone-simulated pregnancy [HSP] + double-distilled water, n=6), SJ Fang rats (HSP + SJ Fang, n=6), and fluoxetine rats (HSP + fluoxetine hydrochloride, n=6). Except for those in the normal and sham-operated groups, all rats were ovariectomized bilaterally using aseptic techniques while under 4% chloral hydrate anesthesia. As in our previous study,33 the experimental rats were treated with SJ Fang 1.25 g/mL and fluoxetine hydrochloride (2090A, Eli Lilly Suzhou Pharmaceutical Co Ltd, USA) 0.25 mg/mL. After establishing HSP, the normal rats, sham rats, and PPD rats were given 2 mL of double-distilled water via the intragastric route. Rats in the SJ Fang group received 2 mL of SJ Fang via the same route. Rats in the fluoxetine group received 2 mL of fluoxetine. The double-distilled water, SJ Fang, and fluoxetine were administered by gavage daily at 8:30 am for 1, 2, and 4 weeks, respectively.
Procedure used to establish HSP
The procedure used to establish HSP was performed as described previously.11,12 Seven days after surgery, the ovariectomized rats were started on subcutaneous injections of vehicle or hormone at approximately 8:30 am for 23 days (Table 1). The sham-operated rats received 0.3 mL of sesame oil daily for 16 days and then 0.1 mL of sesame oil for 7 days. The PPD rats, SJ Fang rats, and fluoxetine rats were administered a low dose of estradiol benzoate (Hangzhou Animal Medicine Factory, Hangzhou, People’s Republic of China) as 0.1 mL (2.5 μg) and 0.2 mL (4 mg) of progesterone (Shanghai General Pharmaceutical Co Ltd, Shanghai, People’s Republic of China) daily for 16 days, followed by a high dose of estradiol benzoate in 0.1 mL (50 μg) for a further 7 days. On day 24, the injections were terminated to initiate hormone withdrawal.
  | Table 1 Experimental timeline |
Forced swim test
The apparatus consisted of a plexiglas cylindrical container (60 cm height ×25 cm diameter) filled to a depth of 35 cm with tap water at 24°C–25°C. This depth was sufficient to ensure that animals could not touch the bottom of the container with their hind paws or their tails.34 On day 1, the rats were placed in the container with water for 15 minutes, then removed from the water, dried, kept warm for 30 minutes, and put back in their cages. On day 2, the rats were placed into the water under the same conditions for 5 minutes, and the amount of time spent immobile, swimming, and struggling was recorded. Immobility included floating and making only those movements necessary to keep the head above water. Swimming included pedaling, circling, and other active movements in the water. Struggling included climbing and upward-directed movements of the forepaws against the walls of the cylinder. A video camera was mounted above the cylinder and an opaque curtain surrounded the platform to block all visual cues.
Sucrose consumption test
The sucrose consumption test, whereby the rats were allowed to drink 1% sucrose water after fluid deprivation for 24 hours, was used to evaluate anhedonia in response to reward. The sucrose intake was measured with the consumption over an hour, by recording the bottle weight (g) prior to and immediately after 1 hour.
Open field test
The OFT was used to explore motivation and anxiety behavior in a novel environment. The open field apparatus consisted of an 80×80 cm arena with 40 cm high walls. The apparatus was painted black except for 2 mm white lines. The arena was divided into 25 squares of equal size. The room was kept dark during the test. Each rat was placed in the central area within the arena and recorded for 5 minutes by a video camera positioned above the apparatus. The numbers of horizontal and vertical movements were counted by two observers blinded to the experimental protocol. Horizontal movement was recorded as the number of grid lines crossed with all four paws, and vertical movement was recorded as the number of times each rat reared to stand on its hind limbs. The apparatus was cleaned with 70% alcohol between testing of each rat.
Body weight
Body weight was calculated once a week during the HSP and gavage periods. A behavioral evaluation was done before the rats were euthanized. Body weight was measured before the behavioral assessment and on the day of euthanasia.
Thymus index and spleen index
Six rats in each group were anesthetized with 4% chloral hydrate (0.4 mL/100 g). The spleen and thymus were isolated and wet-weighed immediately. The immune organ index was calculated as: thymus/spleen index (mg/g) = weight of thymus or spleen (mg)/body weight of rat (g).
Hematoxylin-eosin staining of thymus and spleen
Tissue samples from the thymus and spleen were immersed in 4% paraformaldehyde for 48 hours. After fixation, each sample was washed using tap water five times for 1 hour. Three days later, each sample was sliced into 5 mm sections, and then routinely dehydrated in a graded series of ethanol (70%, 80%, 90%, 95%, and 100%) for 1 hour each, and 100% ethanol for 2 hours. The samples were dipped into xylene twice for 40 minutes, immersed once in paraffin for 1 hour, 2 hours, and left overnight. The tissues were embedded in liquid paraffin to make the paraffin blocks. The paraffin blocks were cut to sections of approximately 5 μm to adhere to microscope slides. After being dried at 60°C in an oven for 1 hour, the sections were transferred the slides through the following solutions: twice in xylene for 15 minutes, twice in 100% ethanol, and once in 95%, 90%, 80%, and 70% ethanol for 5 minutes each, and then in distilled water for 5 minutes. Finally, the sections were stained with hematoxylin-eosin.
Percentages of subsets of T lymphocytes
The CD3, CD4, and CD8 lymphocyte subsets in peripheral arterial blood were analyzed by flow cytometry (BD FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA). The rats were anesthetized and euthanized. Abdominal aortic blood was collected into a sodium-heparin tube for immunofluorescence staining. 100 μL of anticoagulated blood was drawn from the heparinized blood. This anticoagulated blood was added with membrane surface antibodies of anti-CD3 phycoerythrin, anti-CD4 fluorescein isothiocyanate, and anti-CD8 phycoerythrin (all from BD Pharmingen, San Diego, CA, USA), and incubated at room temperature for 15 minutes in the dark. Next, 2 mL of red blood cell lysis solution (Solarbio Bioscience and Technology Co Ltd, Shanghai, People’s Republic of China) was added to the antibody mixture, followed by incubation at room temperature for 20 minutes. The mixture was then centrifuged at 350× g for 5 minutes, after which the supernatant was discarded. Next, 2 mL of cell staining buffer (Biolegend, San Diego, CA, USA) was added twice to wash by centrifugation at 350× g for 5 minutes, after which the supernatant was discarded. The immunofluorescence-stained lymphocyte subsets were resuspended in 0.2 mL of 1% paraformaldehyde-phosphate-buffered saline fixation buffer and analyzed by fluorescence-activated cell sorting with CellQuest software. The gating strategy enabled us to count the numbers of CD3+, CD4+, and CD8+ T-cells. The ratio of CD4+ to CD8+ T lymphocytes (CD4+/CD8+) was used to calculate the immunoreactivity index.
Statistical analysis
The data are presented as the mean ± standard deviation. The statistical analysis was performed using Statistical Package for the Social Sciences version 16.0 software (SPSS Inc, Chicago, IL, USA). Post hoc multiple comparison was performed. The mean values were compared using one-way analysis of variance after normal distribution and homogenous variance tests. The least squares difference method was used to compare differences between two groups. P-values of <0.05 and <0.01 were considered to be statistically significant.
Results
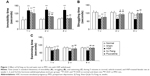
The antidepressant-like activity of SJ Fang was assessed in rats by measuring changes in immobility time in the forced swim test (Figure 1A). Immobility time in the PPD rats was significantly increased after the HSP withdrawal phase in weeks 1 [F(4,25)=15.589, P<0.01], 2 [F(4,25)=45.534, P<0.01], and 4 [F(4,25)=31.476, P<0.01]. After treatment with SJ Fang, there was a significant decrease in immobility time during the forced swim test at 2 weeks (P<0.01) and 4 weeks (P<0.01). Comparison with the positive control rats indicated no difference between SJ Fang and fluoxetine with regard to antidepressant-like activity (P>0.05). Both struggling time (Figure 1B) and swimming time (Figure 1C) were significant decreased at weeks 1, 2, and 4 after HSP withdrawal (P<0.01). Struggling time in the group of PPD rats treated with SJ Fang increased after 1 week of treatment [F(4,25)=19.509], as was swimming time [F(4,25)=6.248]. Both struggling time and swimming time were significantly increased in the PPD rats after treatment with SJ Fang for 2 weeks [F(4,25)=22.125, P<0.01; F(4,25)=17.001, P<0.01] and 4 weeks [F(4,25)=8.833, P<0.01; F(4,25)=11.972, P<0.01].
As shown in Figure 2, repeated measures analysis of variance showed significant differences between the five treatment groups. Sucrose consumption in PPD rats was lower than in rats in the normal and sham groups (both P<0.01). Rats treated with SJ Fang for 2 weeks [F(4,25)=24.323, P<0.01] and 4 weeks [F(4,25)=17.838, P<0.01] were significantly higher than PPD rats. Rats treated with fluoxetine showed significantly increased sucrose consumption when compared with PPD rats. There was no statistically significant difference between rats treated with SJ Fang and those treated with fluoxetine at 2 weeks or 4 weeks (P>0.05).
As shown in Figure 3, measurements of horizontal and vertical movements in the five groups showed significant differences at all three observation points. Horizontal and vertical movements in PPD rats were significantly fewer than in normal and sham rats at weeks 1, 2, and 4 of the hormone withdrawal phase. After treatment with SJ Fang (Figure 3A), horizontal movements in PPD rats were significantly increased in week 1 [F(4,25)=44.604, P<0.01], week 2 [F(4,25)=81.380, P<0.01], and week 4 [F(4,25)=65.534, P<0.01], as were vertical movements (Figure 3B) at week 2 [F(4,25)=29.646, P<0.01] and week 4 [F(4,25)=7.031, P<0.01]. When compared with the positive control rats, there was no difference between SJ Fang and fluoxetine with regard to improvement of horizontal and vertical movements (P>0.05).
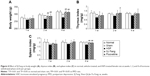
As shown in Figure 4A, body weights in the PPD rats were lower than in normal and sham rats. Growth rates were significantly slower in PPD rats after the HSP withdrawal phase (P<0.01). Body weight in the SJ Fang and fluoxetine groups was higher than in PPD rats after the HSP withdrawal phase, and was significantly higher at 2 weeks [F(4,25)=5.506, P<0.01] and 4 weeks [F(4,25)=18.231, P<0.01].
The thymus index (Figure 4B) and spleen index (Figure 4C) were both lower in PPD rats than in normal rats. Compared with normal and sham rats, the thymus index in PPD rats was significant decreased at 1 week after hormone withdrawal [F(4,25)=5.784, P<0.01]. The spleen index was also significant decreased in PPD rats at 4 weeks after hormone withdrawal [F(4,25)=12.310, P<0.01]. Both the thymus index and spleen index were increased after treatment with SJ Fang or fluoxetine for 1, 2, and 4 weeks. The spleen index in the SJ Fang and fluoxetine groups was significant higher than in PPD rats after treatment for 4 weeks (P<0.01).
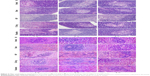
Histopathology showed pathological changes in the thymus and spleen in PPD rats (Figure 5), which could be attenuated by SJ Fang. As shown in Figure 5A, the boundary between the cortex and medulla was not clearly observed in the PPD rat thymus. A thinner cortex and a thicker medulla were seen in the PPD rats. Thymocytes were decreased in number, arranged in a loose and disordered manner, and their nuclei were lighter on staining with hematoxylin-eosin. Corpuscles and vascular proliferation could be found in the thymic tissue of PPD rats at 1, 2, and 4 weeks after hormone withdrawal. After treatment with SJ Fang for 2 weeks, the cortex of the thymus was thicker and the medulla was thinner. The boundary between the cortex and medulla was observed to become gradually clearer. Thymocytes also increased in number and became closely aligned. The thymic structures in PPD rats approached those of a normal thymus after treatment with SJ Fang for 4 weeks.
As shown in Figure 5B, the boundary between the red pulp and white pulp was not clear in the spleen of the PPD rat. The splenic corpuscles were atrophied and structurally disordered. The splenic sinusoids were extended in mass and the splenic cords were narrow. Lymphocytes were decreased in number and vascular hyperplasia could be seen in the PPD rat spleen at 1, 2, and 4 weeks after hormone withdrawal. After treatment with SJ Fang for 2 weeks, the boundary between the red pulp and white pulp in the spleen gradually became clear. The splenic sinusoids were in order, the splenic cords were wide and thick, and lymphocytes were increased and arranged intensively. Splenic structures in the PPD rats were approaching normal after 4 weeks of treatment with SJ Fang.
CD3+ T-cell counts were higher in PPD rats (Figure 6) than in normal or sham rats at 1 week after HSP withdrawal [F(4,25)=0.567, P>0.05]. However, compared with normal and sham rats, CD3+ T-cell counts were significantly decreased in PPD rats by 2 weeks [F(4,25)=8.194, P<0.01] and increased by 4 weeks [F(4,25)=21.447, P<0.01] after HSP withdrawal. After treatment with SJ Fang, CD3+ T-cell counts were decreased in PPD rats by 1 week (P>0.05) and significantly so in 4 weeks (P<0.01). Compared with HSP rats, CD3+ T-cell counts were increased in the rats treated with SJ Fang by 2 weeks (P<0.05).
As shown in Figure 7, CD4+ T-cell counts were significantly increased in PPD rats by week 1 [F(4,25)=1.851, P<0.05] and week 4 after HSP withdrawal [F(4,25)=2.298, P<0.05] when compared with normal and sham rats. However, CD4+ T-cell counts were lower in PPD rats than in normal and sham rats at 2 weeks after HSP withdrawal [F(4,25)=0.649, P>0.05]. Further, CD4+ T-cell counts in rats treated with SJ Fang or fluoxetine were both higher than in PPD rats at 2 weeks after HSP withdrawal (P>0.05), and lower than values at 1 week (P>0.05). After 4 weeks of treatment with SJ Fang, CD4+ T-cell counts were significantly decreased in PPD rats (P<0.05). There was no difference between SJ Fang and fluoxetine in terms of their effect on CD4+ T-cell counts (P>0.05).
As shown in Figure 8, CD8+ T-cell counts were lower in PPD rats than in normal or sham rats at 1 week [F(4,25)=0.263, P>0.05] and significantly increased at 4 weeks [F(4,25)=18.660, P<0.01] after HSP withdrawal. However, compared with normal and sham rats, CD8+ T-cell counts in PPD rats were significantly decreased at 2 weeks [F(4,25)=5.877, P<0.01] after HSP withdrawal. After treatment with SJ Fang, CD8+ T cell counts were higher at both 1 and 2 weeks than in PPD rats (P>0.05). However, CD8+ T-cell counts in rats treated with SJ Fang were significantly decreased at 4 weeks (P<0.01). Compared with normal and sham rats, SJ Fang rats was decreased in 2 weeks (P<0.05).
As shown in Figure 9, the CD4+/CD8+ ratio was significantly higher in PPD rats than in normal or sham rats at week 1 [F(4,25)=2.290, P>0.05] and week 2 [F(4,25)=1.991, P<0.05] after HSP withdrawal. However, compared with normal and sham rats, the CD4+/CD8+ ratio in PPD rats was significantly decreased at week 4 [F(4,25)=5.877, P<0.01] after HSP withdrawal. After SJ Fang treatment for 1 and 2 weeks, CD4+/CD8+ ratios were both lower than HSP rats (P<0.05). However, the CD4+/CD8+ ratio in rats treated with SJ Fang was significantly increased at week 4 (P<0.01).
Discussion
The present report provides new experimental evidence of antidepressant effects and dual-directional immunomodulation activity of SJ Fang in rats with PPD induced by HSP withdrawal. This study indicates that SJ Fang can alleviate depressive-like behaviors, ie, immobility, anhedonia, and motivation, in rats with PPD. Treatment with SJ Fang increased body weight, along with the thymus index and spleen index. According to regulating the CD4+/CD8+, SJ Fang and fluoxetine could exert antidepressant effects in PPD rats.
SJ Fang improved depressive-like behaviors
Hormone withdrawal from high levels of HSP is a widely used in animal models of PPD. There are a number of reports indicating that PPD rats show loss of body weight35 and depressive-like symptomatology manifesting as decreased mobility in the forced swim test,11,12,36 anhedonia in the sucrose consumption test,37,38 and decreased exploratory motivation and increased anxiety status in the OFT.11 Additionally, some researchers have shown that this depressive-like symptomatology can be alleviated by antidepressant therapy. Green et al used the HSP model and found that treating PPD rats with estrogen and a selective estrogen receptor β agonist could increase body weight, mobility in the forced swim test, and anhedonia in the sucrose consumption test.37 Craft et al observed the immobility time of rats in the forced swim test on postpartum days 1–7 in the antidepressant drug treatment with nomifensine, desipramine, and sertraline.39 The results showed that nomifensine, but not desipramine or sertraline, significantly decreased immobility on postpartum day 2. In our study, HSP-exposed rats showed loss of body weight, increased immobility in the forced swim test, decreased preference for sucrose consumption, and performed fewer active movements. Treatment with SJ Fang for 1, 2, and 4 weeks had significant antidepressant-like effects in PPD rats. Interestingly, the antidepressant effect of SJ Fang in the present study mimics the effects of fluoxetine.
SJ Fang elevated immune function
To determine the effects of SJ Fang on immune function and tissue changes, we investigated immune organ indices and histopathology in PPD rats after treatment for 1, 2 and 4 weeks. To a certain degree, the thymus index and spleen index reflect immune function in the body. In our study, SJ Fang improved both hypofunctional immune status and pathological structures in the thymus and spleen.
SJ Fang regulated the immune response
Our finding of a dynamic redistribution of T lymphocytes in PPD rats indicates a change in the T lymphocyte subsets involved in the immune response and in immunocompetent cells. SJ Fang could regulate variations in levels of these cells in PPD rats, returning them to normal levels. Lymphocytes coordinate the immune response and play a central role in cell-mediated immunity.40 A change in numbers of T lymphocytes can lead to disorders in cellular immune function.41 T lymphocytes (CD3+ T-cells) are one of the major types of immune cells, which develop and matured in the thymus. The main T lymphocyte subsets are helper/inducer (CD4+) T-cells and suppressor/cytotoxic (CD8+) T-cells. CD4+ T-cells induce and proliferate rapidly, secreting cytokines that send signals and maintain an active immune response, while CD8+ T-cells kill foreign cells and keep the immune reaction within reasonable limits. A reduction in the CD4+/CD8+ ratio is an important indicator of immune deficiency.42 Recently, observation of T-cell subtypes in patients with first-onset postpartum psychosis showed that total T-cells and some T-cell subtypes were significantly decreased by 4 weeks postpartum, but that CD8+ T-cell levels were not different from those in healthy postpartum control subjects.43 The present study shows that T lymphocyte subsets in PPD rats were significantly different at 1, 2, and 4 weeks after HSP withdrawal. One week after HSP withdrawal, PPD rats had increased numbers of CD4+ T-cells and CD4+/CD8+ ratios, which were due to high levels of CD4+ T-cells with helper activity. This phase was characterized as immune activation. Notably, by 2 weeks after HSP withdrawal, PPD rats showed decreased numbers of CD3+ and CD8+ T-cells, and an increase in the CD4+/CD8+ ratio which was due to low CD8+ T-cell counts with a predominant suppressor/cytotoxic function. This phase was characterized as immune activation that would transform to immune suppression. However 4 weeks after HSP withdrawal, PPD rats showed an increased in CD3+ T-cells, CD4+ T-cells, and CD8+ T-cells, along with a decrease CD4+/CD8+ ratio. This phase was shifted in immune suppression with high CD8+ T-cells counts. These results demonstrate that both cell-mediated immune activation and suppression were related pathological processes occurring in different phases of PPD. Therefore, our data support the view that the immune system reaches a distinct activated point, particularly with regard to T-cells, in the postpartum period. Across the female lifespan, the risk of exacerbation of immune disease is elevated during the postpartum period,44–46 and there is no definite evidence of alteration of T-cell subsets in PPD. However, there is some research on T-cell activation in postpartum women and in mood disorders. Weetman considers that many postpartum immune syndromes, eg, postpartum thyroiditis, can be attributed to activation of the T-cell system in particular.47 Moreover, activation of the T-cell system has been shown to be involved in susceptibility to mood disorder.48–51 Further, activation of the immune response results in regulation of proinflammatory cytokines. We consider that an imbalance in proinflammatory cytokines plays a role in the etiopathology of mood shifts in PPD, which were seen in a study by Cheng and Pickler.52 Maes et al reported that activation of the inflammatory response, as implied by increased levels of serum interleukin-6 and the interleukin-1-receptor antagonist, may cause PPD.53,54 Boufidou et al discovered a positive correlation between levels of interleukin-6 in cerebrospinal fluid, levels of tumor necrosis factor-alpha in cerebrospinal fluid and serum, and PPD.26 In contrast, other researchers have reported a change in T lymphocyte subpopulations in major depression,55,56 imply a possible mechanism characterized by immune suppression.57 Other reports have mentioned reduced numbers of lymphocytes,58–60 CD4+ T-cells,61 and CD8+ T-cells62,63 being seen in depressed patients. There are also reports of proportionately equal reductions in numbers of CD4+ T-cells and CD8+ T-cells in depressed patients,62 with no difference in the CD4+/CD8+ ratio.62,64,65 A murine model of depression found a reduction in T-cell populations and shift in the CD4+/CD8+ ratio toward cytotoxic T-cells.66 Depressive-like behavior in mice and rats was associated with a decrease in CD4+, accumulation of CD8+, and decrease in the CD4+/CD8+ ratio.67,68 Finally, our findings demonstrate that SJ Fang can regulate both immune activation and immune suppression in different phases of PPD in rats that have undergone hormone withdrawal, and is not significantly different from fluoxetine in this respect.
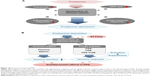
In summary, accumulating evidence suggests that dysfunction of the immune system is involved in the pathogenesis of PPD. As shown in Figure 10A, this dysfunction lies in activation of HPA axis, cytokines, the inflammatory response, and the innate immune response. In this study, we established that SJ Fang exerts an antidepressant effects by regulating the organs of the immune system and T lymphocyte subsets in rats with PPD (Figure 10B). However, there are some limitations to our study, including the different sustained time in PPD all-stages between human and rats. Further, we need a novel animal model of PPD that can mimic human pregnancy, and the postpartum and lactation periods. Our hypothesis that SJ Fang has a regulating effect on the organs of the immune system and T lymphocyte subsets needs to explored further in human patients with PPD. The transformation between immune activation and inhibition involved in the pathology stage of PPD should be verified in humans.
Conclusion
SJ Fang may be an effective therapeutic option for improving the depressive-like behavior associated with PPD. SJ Fang has a dual-directional immunoregulating effect on the immune system, which is associated with enhanced function of the thymus and spleen and T-cell subsets. These observations should be investigated further by measuring more immune variables relevant to pro/anti-inflammatory cytokines, cytokine receptors, and helper T-cells. Moreover, the role of SJ Fang in regulating immune activation and inhibition shifts in PPD need to be explored further. In this study, we demonstrated that PPD induces immune hypofunction and an alteration of immune activation to inhibition. Our findings provide new evidence of the pharmacological mechanism of action of SJ Fang in PPD. SJ Fang may be a suitable therapeutic option for women with PPD.
Acknowledgments
All authors read the manuscript, contributed to its correction, and approved the final version. This study was supported by the Doctoral Fund of the Ministry of Education of China (20130013130002) and the Program for Innovative Research Team in Beijing University of Chinese Medicine (2011-CXTD-26).
Disclosure
The authors report no conflicts of interest in this work.
References
Dennis CL. Treatment of postpartum depression. Part 2: a critical review of nonbiological interventions. J Clin Psychiatry. 2004;65(9):1252–1265. | ||
Yang X, Tang Q. Investigation of treatment on postpartum depression with methods of reinforcing deficiency and removing phlegm-dampness and blood stasis, nourishing brain and inducing resuscitation. | ||
Douma SL, Husband C, O’Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv Nurs Sci. 2005;28(4):364–375. | ||
Studd J, Panay N. Hormones and depression in women. Climacteric. 2004;7(4):338–346. | ||
Hendrick V, Altshuler LL, Suri R. Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics. 1998;39(2):93–101. | ||
Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–930. | ||
Sichel DA, Cohen LS, Robertson LM, Ruttenberg A, Rosenbaum JF. Prophylactic estrogen in recurrent postpartum affective disorder. Biol Psychiatry. 1995;38(12):814–818. | ||
Gregoire AJ, Kumar R, Everitt B, Henderson AF, Studd JW. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006):930–933. | ||
Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62(5):332–336. | ||
Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44(3):234–246. | ||
Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122(1):1–9. | ||
Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol Behav. 2004;83(3):505–513. | ||
Green AD, Galea LA. Adult hippocampal cell proliferation is suppressed with estrogen withdrawal after a hormone-simulated pregnancy. Horm Behav. 2008;54(2008):203–211. | ||
Zhu Y, Tang Q, Yang X, et al. A review: biological mechanisms of postpartum depression. | ||
Yang X, Tang Q, Zhu M, et al. Research of influence of Shenqijieyu prescription on changes of neuroendocrine system-related hormones in rats with postpartum depression. | ||
Zhao R, Yang X, Tang Q, et al. Research of influence of Shenqijieyu prescription on expression and distribution of Er in brain tissue in rats with postpartum depression. | ||
Hou X, Tang Q, Li X, et al. Research of influence of Shenqijieyu prescription on changes of monoamine neurotransmitter and metabolite in rats with postpartum depression. | ||
Pavón L, Sandoval-López G, Eugenia Hernández M, et al. Th2 cytokine response in major depressive disorder patients before treatment. J Neuroimmunol. 2006;172(2006):156–165. | ||
Maes M, Meltzer HY, Bosmans E, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34(4):301–309. | ||
Sutcigil L, Oktenli C, Musabak U, et al. Pro- and anti-inflammatory cytokine balance in major depression effect of sertraline therapy. Clin Dev Immunol. 2007;2007:76396. | ||
Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009;42(5):182–188. | ||
Corwin EJ, Johnston N, Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol Res Nurs. 2008;10(2):128–133. | ||
Corwin EJ, Pajer K. The psychoneuroimmunology of postpartum depression. J Womens Health (Larchmt). 2008;17(9):1529–1534. | ||
Maes M, Lin AH, Ombelet W, et al. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology. 2000;25(2000):121–137. | ||
Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology. 2007;32(2):133–139. | ||
Boufidou F, Lambrinoudaki I, Argeitis J, et al. CSF and plasma cytokines at delivery and postpartum mood disturbances. J Affect Disord. 2009;115(2009):287–292. | ||
Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PA. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. Immunology. 2012;189(2):1072–1080. | ||
Skalkidou A, Sylvén SM, Papadopoulos FC, et al. Risk of postpartum depression in association with serum leptin and interleukin-6 levels at delivery a nested case-control study within the UPPSAT cohort. Psychoneuroendocrinology. 2009;34(2009):1329–1337. | ||
Dunn A, Paul S, Corwin E. Perineal injury during childbirth increases inflammatory response and risk of postpartum depression. Brain Behav Immun. 2014;40:2. | ||
Weigelt K, Bergink V, Burgerhout KM, Pescatori M, Wijkhuijs A, Drexhage HA. Downregulation of inflammation-protective microRNAs 146a and 212 in monocytes of patients with postpartum psychosis. Brain Behav Immun. 2013;29(2013):147–155. | ||
Licinio J. Potential diagnostic markers for postpartum depression point out to altered immune signaling. Mol Psychiatry. 2010;15(1):1. | ||
Corwin EJ, Paul S, Pajer K, McCarthy D, Weber M, Lowe N. Dysregulation of psychoneuroimmune function in women symptomatic of postpartum depression. Brain Behav Immun. 2014;40:1. | ||
Li X, Yang X, Tang Q, et al. Research of influence of Shenqijieyu prescription on changes of monoamine neurotransmitter and metabolite in rats with postpartum depression. | ||
Suda S, Segi-Nishida E, Newton SS, Duman RS. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol Psychiatry. 2008;64(4):311–319. | ||
Brummelte S, Pawluski JL, Galea LA. High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: a model of post-partum stress and possible depression. Horm Behav. 2006;50(3):370–382. | ||
Beckley EH, Finn DA. Inhibition of progesterone metabolism mimics the effect of progesterone withdrawal on forced swim test immobility. Pharmacol Biochem Behav. 2007;87(2007):412–419. | ||
Green AD, Barr AM, Galea LA. Role of estradiol withdrawal in “anhedonic” sucrose consumption: a model of postpartum depression. Physiol Behav. 2009;97(2):259–265. | ||
Navarre BM, Laggart JD, Craft RM. Anhedonia in postpartum rats. Physiol Behav. 2010;99(2010):59–66. | ||
Craft RM, Kostick ML, Rogers JA, White CL, Tsutsui KT. Forced swim test behavior in postpartum rats. Pharmacol Biochem Behav. 2010;96(4):402–412. | ||
Ahmadi KR, Hall MA, Norman P, et al. Genetic determinism in the relationship between human Cd4+ and Cd8+ T lymphocyte populations? Genes Immun. 2001;2(2001):381–387. | ||
Scherrenburg J, Piriou ER, Nanlohy NM, van Baarle D. Detailed analysis of Epstein-Barr virus-specific Cd4+ and Cd8+ T cell responses during infectious mononucleosis. Clin Exp Immunol. 2008;153(2):231–239. | ||
Stefanski V. Social stress in laboratory rats, behavior, immune function and tumor metastasis. Physiol Behav. 2001;73(3):385–391. | ||
Bergink V, Burgerhout KM, Weigelt K, et al. Immune system dysregulation in first-on set postpartum psychosis. Biol Psychiatry. 2013;73(10):1000–1007. | ||
Shi X, Li C, Li Y, et al. Circulating lymphocyte subsets and regulatory T cells in patients with postpartum thyroiditis during the first postpartum year. Clin Exp Med. 2009;9(4):263–267. | ||
Langer-Gould A, Gupta R, Huang S, et al. Interferon-gamma-producing T cells, pregnancy, and postpartum relapses of multiple sclerosis. Arch Neurol. 2010;67(1):51–57. | ||
Häupl T, Østensen M, Grützkau A, Radbruch A, Burmester GR, Villiger PM. Reactivation of rheumatoid arthritis after pregnancy: increased phagocyte and recurring lymphocyte gene activity. Arthritis Rheum. 2008;58(10):2981–2992. | ||
Weetman AP. Immunity, thyroid function and pregnancy: molecular mechanisms. Nat Rev Endocrinol. 2010;6(6):311–318. | ||
Santiago FM, Moynihan JA. Brain behavior and immunity: twenty years of T cells. Brain Behav Immun. 2007;21(7):872–880. | ||
Wong ML, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry. 2008;13(8):800–812. | ||
Drexhage RC, Hoogenboezem TH, Versnel MA, Berghout A, Drexhage HA, Nolen WA. The activation of monocyte and T cell networks in patients with bipolar disorder. Brain Behav Immun. 2011;25(6):1206–1213. | ||
Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25(2):221–229. | ||
Cheng CY, Pickler RH. Perinatal stress, fatigue, depressive symptoms, and immune modulation in late pregnancy and one month postpartum. Scientific World Journal. 2014;2014:652630. | ||
Maes M, Libbrecht I, Lin A, et al. Immune activation in the early puerperium is related to postpartum affective symptoms. Psychoneuroendocrinology. 2000;25(2):121–137. | ||
Maes M, Ombelet W, Verkerk R, Bosmans E, Scharpé S. Effects of pregnancy and delivery on the availability of plasma tryptophan to the brain: relationships to delivery-induced immune activation and early post-partum anxiety and depression. Psychol Med. 2001;31(5):847–858. | ||
Darko DF, Rose J, Gillin JC, Golshan S, Baird SM. Neutrophilia and lymphopenia in major mood disorders. Psychiatry Res. 1988;25(3):243–251. | ||
Irwin M. Psychoneuroimmunology of depressive disorder: mechanisms and clinical implications. Brain Behav Immun. 2002;16(1):1–16. | ||
Zorrilla EP, Luborsky L, McKay JR, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15(3):199–226. | ||
Schleifer SJ, Keller SE, Meyerson AT, Raskin MJ, Davis KL, Stein M. Lymphocyte function in major depressive disorder. Arch Gen Psychiatry. 1984;41(5):484–486. | ||
Murphy D, Gardner R, Greden JF, Carroll BJ. Lymphocyte numbers in endogenous depression. Psychol Med. 1987;17(2):381–385. | ||
Robertson MJ, Schacterle RS, Mackin GA, et al. Lymphocyte subset differences in patients with chronic fatigue syndrome, multiple sclerosis and major depression. Clin Exp Immunol. 2005;141(2):326–332. | ||
Krueger R, Levy E, Cathcart E, Fox B, Black P. Lymphocyte subsets in patients with major depression: preliminary findings. Advances. 1984;1(1):5–9. | ||
Denney DR, Stephenson LA, Penick EC, Weller RA. Lymphocyte subclasses and depression. J Abnorm Psychol. 1988;97(4):499–502. | ||
Fortes C, Farchi S, Forastiere F, et al. Depressive symptoms lead to impaired cellular immune response. Psychother Psychosom. 2003;72(5):253–260. | ||
Calabrese JR, Skwerer RG, Barna B, et al. Depression, immunocompetence, and prostaglandins of the E series. Psychiatry Res. 1986;17(1):41–47. | ||
Kronfol Z, House JD. Depression, cortisol, and immune function. Lancet. 1984;1(8384):1026–1027. | ||
Tallerova AV, Kovalenko LP, Kuznetsova OS, Durnev AD, Seredenin SB. Correcting effect of Ladasten on variations in the subpopulation composition of T lymphocytes in C57bl/6 mice on the experimental model of an anxious-depressive state. Bull Exp Biol Med. 2014;156(3):335–337. | ||
Idova GV, Al’perina EL, Gevorgyan MM, Zhukova EN, Kulikov AV, Yur’ev DV. T-lymphocyte subpopulation composition and the immune response in depression-like behavior in ASC mice. Neurosci Behav Physiol. 2013;43(8):946–950. | ||
Guan SZ, Liu JW, Fang EF, Ng TB, Lian YL, Ge H. Chronic unpredictable mild stress impairs erythrocyte immune function and changes T-lymphocyte subsets in a rat model of stress-induced depression. Environ Toxicol Pharmacol. 2014;37(1):414–422. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







 [China Journal of Traditional Chinese Medicine and Pharmacy]. 2012;27(4):1131–1135. Chinese.
[China Journal of Traditional Chinese Medicine and Pharmacy]. 2012;27(4):1131–1135. Chinese. [Beijing Journal of Traditional Chinese Medicine]. 2013;32(3):168–176. Chinese.
[Beijing Journal of Traditional Chinese Medicine]. 2013;32(3):168–176. Chinese.