Back to Journals » Patient Preference and Adherence » Volume 17
Shared Decision-Making in Allergen Immunotherapy (AIT) Options Using a Questionnaire for Respiratory Allergic Patients: A Delphi Consensus Study
Authors Antón M, Cabañes N, Fernández-Meléndez S , Fernández-Nieto M, Jiménez-Ferrera G, Letrán A, Méndez-Brea P, Montoro J, Moreno F, Mur-Gimeno P, Rodríguez-Vázquez V, Rosado A, Sánchez-Guerrero I, Vega-Chicote JM, Vidal C
Received 16 March 2023
Accepted for publication 10 May 2023
Published 24 July 2023 Volume 2023:17 Pages 1771—1782
DOI https://doi.org/10.2147/PPA.S409466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Mónica Antón,1 Nieves Cabañes,2 Salvador Fernández-Meléndez,3 Mar Fernández-Nieto,4 Gloria Jiménez-Ferrera,5 Antonio Letrán,6 Paula Méndez-Brea,7 Javier Montoro,8,9 Francisco Moreno,6 Pilar Mur-Gimeno,10 Virginia Rodríguez-Vázquez,7 Ana Rosado,11 Inmaculada Sánchez-Guerrero,12 Jose Mª Vega-Chicote,3 Carmen Vidal7,13
1Allergy Department, Hospital Universitario San Juan de Alicante, Alicante, Spain; 2Allergy Department, Hospital Universitario de Toledo, Toledo, Spain; 3Allergy Department, Hospital Civil de Málaga, Malaga, Spain; 4Allergy Department, Hospital Universitario Fundación Jiménez Díaz, Madrid, Spain; 5Allergy Department, Hospital Universitario de Badajoz, Badajoz, Spain; 6Allergy Unit, Centro médico Asisa Doctor Lobatón, Cádiz, Spain; 7Allergy Department, Complejo Hospitalario Clínico Universitario de Santiago, Santiago de Compostela, Spain; 8Allergy Department, Hospital de Llíria, Valencia, Spain; 9Department of Medicine, Universidad Católica de Valencia San Vicente Mártir, Valencia, Spain; 10Allergy Department, Hospital de Santa Bárbara, Puertollano, Ciudad Real, Spain; 11Allergy Department, Hospital Universitario Fundación Alcorcón, Alcorcón, Madrid, Spain; 12Allergy Department, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain; 13Department of Medicine, Universidad de Santiago de Compostela, Santiago de Compostela, Spain
Correspondence: Carmen Vidal, Allergy Department, Complejo Hospitalario Universitario de Santiago, Santiago de Compostela, 15706, Spain, Tel +34 981 951752, Email [email protected]
Purpose: The objective of this study was to develop and validate a questionnaire, through a Delphi consensus, to be used by allergists in their routine clinical practice to assess the preferences of patients starting allergen immunotherapy (AIT) treatment using an objective approach.
Patients and Methods: A Delphi consensus-driven process was used. The scientific committee, composed of 15 allergists, led the study and participated in the preparation of the questionnaire. Two-hundred panelists from different Spanish regions were invited to complete a 16-item questionnaire on a nine-point Likert scale covering six topic blocks. Consensus was achieved if ≥ 66.6% of panelists reached agreement or disagreement.
Results: Of the 200 experts invited to participate in the Delphi process, a total of 195 (97.5%) answered the questionnaire. The panel experts reached a consensus on “agreement” on a total of 12 of the 16 (75.0%) items, covering a total of six categories: (a) patient knowledge (2 questions), (b) barriers to patient adherence (3 questions), (c) patient behavior (4 questions), (d) future actions (3 questions), (e) treatment costs (2 questions), and (f) final patient preferences (2 questions).
Conclusion: This Delphi consensus study validated a set of twelve recommended questions for patients objectively assessing their preferences and suitability for the most common AIT options available. The questionnaire intends to assist allergists in making an objective, unconditioned decision regarding the best AIT option for each patient, after informing them about the different routes.
Keywords: allergen immunotherapy, Delphi consensus, shared decision-making, questionnaire
Introduction
Respiratory allergic illness is a global health concern affecting both industrialized and developing countries,1 with an estimated prevalence of more than 20% of the worldwide population.2 Allergic rhinitis and asthma are the most common respiratory allergic diseases, affecting hundreds of millions and 235 million individuals, respectively,2–5 with asthma causing 455,000 deaths in 2019.3,4
Antihistamines and corticosteroids are the first-line therapy for allergic respiratory disorders. However, they are often insufficient to control symptoms. Allergen immunotherapy (AIT) treats respiratory allergic disease by modulating basic immunological pathways to induce immune tolerance, relieve symptoms, and improve disease control. This treatment has shown long-term efficacy, resulting in improved prognosis and quality of life.6 Furthermore, AIT is the only treatment that modifies the natural history of allergic sensitization and may prevent asthma development.7,8 AIT therapies are commonly administered subcutaneously or sublingually for a 3–5 year period.9,10 Subcutaneous injections (SCIT) should always be administered at the doctor’s and/or nurse´s office, allowing 30 minutes to monitor adverse reactions. Therefore, patients receiving SCIT should attend the clinic during the 3–5 years of treatment.9,10 In contrast, the sublingual drops or tablets (SLIT-drops or SLIT-tablets) can be self-administered at home, only requiring daily patient involvement. Direct head-to-head studies comparing SLIT and SCIT are uncommon and small;11 however, SLIT and SCIT differ in local reactivity. Current data indicate that the risk of systemic reactions with SCIT is quite low, but near-fatal and fatal anaphylaxis can occur.9,10 In contrast, SLIT has a higher incidence of local reactions, but severe anaphylactic events are very rare.9,10 Regarding the utilization of the most common routes, SLIT and SCIT prescription patterns vary by country.12 In Spain, 85.5% of adults and 77.8% of children were administered SCITs,13,14 although a recent study indicates that patients prefer SLIT.15 Furthermore, few studies have been performed on patients’ preferences, with mixed results.15,16 Hence, prescription patterns in Spain are not supported by clinical evidence or patient preferences. Altogether, these observations suggest a need for objective tools for the decision-making process on the route of AIT administration.
Consideration of patients’ viewpoints and preferences and joint decision-making increase treatment adherence.17–20 The cultural, social, and democratic growth of the last several decades has led to a transformation in the physician-patient interaction, where various decisions can be taken collaboratively, as reflected in the latest clinical guidelines.21 Previous research has shown diverse patients preferences and benefits of the different AIT treatments.15,22–26 Mode of administration, efficacy, risk of adverse events, and direct and indirect expenditures may determine which AIT patients prefer.15 In this regard, SCIT treatment is burdensome due to time spent on each appointment and travel expenses, affecting patients’ adherence.22
Despite the potential benefits of shared AIT decision-making, a physicians’ guide on patient-related criteria and preferences is missing. Furthermore, AIT prescription patterns and patient preferences seem disconnected. The objective of this study was to develop and validate a questionnaire, through a Delphi consensus, that can be used by allergists in their routine clinical practice to objectively assess the preferences of patients starting AIT treatment. In order to express the need for reaching an agreement, the project was called ALLIANCE (ALIANZA in its Spanish version).
Materials and Methods
Study Design
A Delphi consensus is a structured technique used in different fields to collect relevant information on a specific issue, which consists of a series of questions targeted at experts.27 The key features of this method are anonymized responses and controlled feedback.
The ALIANZA project was carried out in four phases. In Phase 1, a literature review was performed.26,28–32 During Phase 2, the Delphi questionnaire was developed based on the review of the literature and the scientific committee’s experience. In phases 3 and 4, the Delphi questionnaire was answered by a panel of experts, results were analyzed, and the manuscript was prepared.
Study Phases
A diagram of the study phases is presented in Figure 1. In the initial phase, conducted between March and June 2021, the literature regarding adherence and patients’ preferences and shared decisions on allergy and AIT treatment was reviewed, with a special focus on factors related to the socioeconomic and health system factors and on aspects related to the characteristics of the patient and the treatment. In the second phase, the scientific committee developed the Delphi questionnaire based on the literature review (June 2021). The questions were generated based on the relevant factors identified in the literature review, and considering patients’ preferences and situation. The final version of the Delphi questionnaire included 16 items written as questions to be asked by allergists to patients when informing them about the different AIT routes. The questions included in the Delphi were answered on a nine-point Likert scale, where 1 was “Strongly disagree” and 9 “Strongly agree”. The third phase was the Delphi phase, conducted between September and October 2021, in which the panelists selected by the expert panel answered the questionnaire. During Phase 4 (October to November 2021), the scientific committee analyzed the results of the Delphi phase, resulting in the final ALIANZA questionnaire, and the manuscript was prepared.
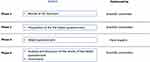 |
Figure 1 Diagram of study phases. Abbreviations: AIT, allergen immunotherapy; Cns, consensus measure; GPPR, General Data Protection Regulation; SCIT, subcutaneous injections; SLIT, sublingual. |
Scientific Committee
The project was led by a scientific committee that was comprised of a team of fifteen allergists from sites located across the Spanish territory, experts in the physician-patient relationship. All the members of the scientific committee have proven experience using SLIT and SCIT with a median experience in the allergy field of 25 years (IQR, 19–30) and a median prescription of allergen immunotherapy of 180 (IQR, 120–200) per year.
Expert Panel
A group of 200 allergy experts were invited among the 1200 allergists of Spain, ensuring representation of all regions of Spain. The criteria for their selection included professional knowledge and experience in the field of allergy and AIT treatment. The geographic distribution of the panelists is displayed in Supplementary Table 1.
Consensus Definition
A statement was considered consensual if the panelists’ votes outside one of the three-point regions ([1–3], [4–6], [7–9]) containing the median were less than one-third of the responses (<33.3%). The median value defined group consensus: majority “disagreement” if the median was within 1–3 and majority “agreement” if it was within 7–9. Cases with a 4–6 median were considered “doubtful”. When one-third or more of panelists scored [1–3] and another third scored [7–9], “discordance” was considered. The remaining assertions without concordance or discordance were considered as “undetermined” consensus.
Items were recommended when the voting result reached consensus in the “agreement” region (votes outside region [7–9] were <33.3%). Strong consensus was defined as 80% of panelists rating the statement 7 or above. When 67–79% of panelists evaluated a statement 7 or above, a moderate consensus was reached. Statements that did not reach this level of agreement were interpreted as “undetermined”.
Alternatively, we used the consensus measure (Cns) developed by Tasle et al for the analysis of ordinal scales.33 For its calculation, we adapted the original formula, developed for 5-point scales, to apply it to a 9-point scale (Supplementary Figure 1). Using the Cns, results of ≥0.80 indicated consensus agreement.
Data Analysis
SPSS Statistics version 20 (IBM; Armonk, NY, USA) was used to create and analyze the database. The median and the percentage of responses in the 7–9 range were calculated, and their values were used to define consensus. For the Cns, the formula was calculated using SAS Version 9.4 software (SAS Institute, Inc, Cary, NC, USA).
Ethical Aspects
The study was carried out according to the Helsinki Declaration. Data from the Delphi questionnaire were anonymized during processing and analysis; therefore, personal data were dissociated from the results in compliance with the EU General Data Protection Regulation (GDPR).
Results
A total of 16 questions were developed by the scientific committee as part of a questionnaire to be administered by the physician when informing the allergy patient about the AIT (Table 1). The questions covered a total of six categories: (a) patient knowledge (2 questions), (b) barriers to patient adherence (3 questions), (c) patient behavior (4 questions), (d) future actions (3 questions), (e) treatment costs (2 questions), and (f) final patient preferences (2 questions). Of the 200 experts invited to participate in the Delphi process, a total of 195 (97.5%) answered the questionnaire. The panel experts reached a consensus on “agreement” on a total of 12 of the 16 questions (75.0%), with a strong consensus on 10 questions (85.71%) and a moderate consensus on 2 questions (14.29%). Key results for each block are detailed below.
 |
Table 1 Level of Consensus Reached with Proposed Questions |
Patients’ Knowledge
A 100% consensus was obtained across block a, with agreement on the 2 questions, and both were categorized into the strong consensus range (Table 1, Block a). The panelists agreed that the physician should ask the patients whether they are familiar with the different routes of AIT administration, as well as whether the knowledge of the pros/cons of each of the different routes of AIT administration. For both questions, experts agreed that the physician must provide information to the patient on both routes of administration, including efficacy, safety, adverse effects, dosage, and costs.
Barriers to Patients’ Adherence
The panelists reached a consensus agreement on all 3 questions (100%) of block b, of which 1 (33.33%) had a moderate consensus and 2, a strong consensus (66.67%) (Table 1, Block b). The question regarding the barrier of treatment adherence related to the costs of travel to the health center and/or hospital, in terms of time and/or money, was the one that reached a moderate consensus (question 4). The panelists agreed and showed a strong consensus on the questions of barriers to adherence related to the work/school/family constraints or commitments (questions 3 and 5).
Patients’ Behavior
Agreement was reached on 1 (25.0%) of the 4 block c questions, with strong consensus (Table 1, Block c). The panelists strongly recommended that the physicians should ask the patients if they followed the prescribed medication guideline for the treatment of their allergy (question 9). Instead, the experts did not agree on the relevance of asking the patients about aspects of their behavior, related to their organizational skills and ability/method to remember medical appointments and/or treatment schedules, and if they feel that they are adequately compliant with medication prescribed for other pathologies (if applicable).
Patients’ Future Attitudes
A total of 2 of the 3 questions (66.67%) reached consensus agreement by the panelists, as shown in Table 1 (Block d), both with strong consensus. The question related to the concern of the patient about having to keep the treatment in the refrigerator if required and having to maintain the cold chain while travelling reached an indeterminate agreement by the experts consulted at the Delphi (question 12). On the contrary, it was strongly recommended that patients be asked about future compliance with aspects related to subcutaneous (question 10) and sublingual (question 11) treatment.
Treatment Costs
Consensus was reached on 2 questions in block e (100%), with 1 considered a strong consensus and the other a moderate consensus (Table 1, Block e). The panelists strongly recommended that the physicians ask the patients if they consider that the cost of acquiring the treatment at the pharmacy is a potential limitation to continue with the treatment for a period of 3 years. The experts moderately agreed on asking the patients if they consider that the indirect cost derived from the administration of the AIT (such as travel and loss of work or school hours) may have an impact on their choice of administration route.
Patient Preferences
The results of the block regarding final patient preferences are shown in Table 1 (Block f). The total of the 2 questions reached an agreement, both with strong consensus. The experts consulted in the Delphi strongly recommended that physicians ask the patients about AIT treatment delivery route preferences, taking into account side effects and efficacy, security and characteristics of the two AIT routes of administration.
Final ALIANZA Questionnaire
Finally, based on the results obtained, a patient questionnaire to assess patients’ preferences regarding AIT route was validated (Table 2). The questionnaire, which includes a total of 12 questions, considered socioeconomic and health system factors, as well as aspects related to patient and treatment characteristics.
 |
Table 2 Validated ALIANZA Questionnaire |
Discussion
To our knowledge, this is the first study to validate a questionnaire aiming to guide physicians in making shared decisions regarding the optimal AIT for patients with respiratory allergy. A total of 195 physicians, allergy specialists based in Spain, validated the questions of the questionnaire through a Delphi methodology. The experts reached a consensus on questions to be asked to capture patients’ preferences regarding AIT routes to guide decisions on AIT therapy alternatives in the allergist’s office (Table 2).
Within the process of incorporating patients’ preferences, it is essential to be aware of patients’ knowledge.34,35 In this case, patients’ knowledge about the different AIT options is very important for deciding the best treatment alternative. It is probably for this reason that a 100% agreement and a strong consensus were obtained for questions assessing patients’ knowledge (Block a).
The reasons for the lack of treatment adherence by patients are varied and depend on individual factors, requiring a case-by-case assessment.36,37 Aside from improving individual patient outcomes, overall treatment adherence helps to determine the best treatment modalities and reduces the burden of disease on society.38 Consequently, factors associated with patient adherence should be carefully considered to guide decisions regarding the best therapeutic option for each patient. Accordingly, panelists agreed on the three questions related to exploring potential barriers to AIT adherence. The aim of assessing patients’ behavior regarding treatment adherence should be for physicians and patients to take these factors into consideration when deciding the AIT treatment. Like with any other treatment, adherence to AIT is crucial to ensure effectiveness. SCIT requires monthly visits to the health center, while SLIT does not require regular visits to the doctor. Several studies have concluded that non-adherence to an AIT schedule and early discontinuation are common problems.24,39–47 These could be reduced by engaging patients in the decision-making process for the selection of AIT route of administration, as some studies have concluded.26,40
Patient behavior is directly related to the definition of adherence, according to the World Health Organization.48 However, the experts consulted agreed on recommending only one of the four suggested questions on patients’ behavior. The three questions that did not reach agreement address issues of a personal nature or about other therapies, which some of the experts consulted may not have considered necessary for the evaluation of the AIT decision. Conversely, the panelists strongly recommended that the physicians should ask the patient if they followed the prescribed medication guideline for their allergy treatment. Although a doubt as to whether the patient’s answers regarding adherence to treatment are always truthful cannot be ruled out,49,50 if patients acknowledge a lack of treatment compliance, physicians and patients can develop together a therapeutic strategy that will help them comply with AIT in the future.
Patients’ attitudes and beliefs impact medication adherence.51 For this reason, three questions on patients’ future attitudes have been included, two of which have been recommended by the experts, related to SCIT and SLIT treatment. In particular, the questions that were considered relevant were related to actions required specifically for adherence to the SCIT or SLIT. SCIT has the inconvenience of requiring visits to the physician’s office, whereas compliance with SLIT is contingent on the patient decision to take the recommended doses.51 Patients responding affirmatively to only one of these two impediments will therefore have expressed their preferences, and the physician will be able to guide them appropriately.17,18
The cost of medications is one of the factors that may impact treatment adherence. Cost-related medication non-adherence is a problem that has been described by several studies in the literature, and which affects a wide range of patients.52–55 In the field of AIT, there is also evidence regarding the possible impact of treatment costs on adherence to treatment regimens.45,56–59 The experts consulted in this Delphi are probably aware of the importance of this factor and have therefore agreed to recommend that patients be asked if they consider that the cost of acquiring the treatment at the pharmacy is a potential limitation to continue with the treatment for a period of 3 years (the typically recommended AIT treatment period). In addition to the direct costs of treatment, if the treatment requires visits to the health center, such as for SCIT administration, various indirect costs can be added, including the cost of travel itself and the time lost for other activities (work, social, school, etc.). In fact, the inconvenience related to patient time and travel is the most frequently cited reason for SCIT discontinuation in several studies.45,59–61 In this regard, experts have recommended asking the patient if they consider that the indirect cost derived from the administration of the AIT may have an impact on their choice of administration route.
The two final questions directly asked patients about their preferred AIT option and were both recommended by the experts. The shared decision-making process should end with a final assessment and decision after discussing all the information with the patient,62,63 and this is reflected in the position of the panelists.
Finally, it is worth mentioning that there was a total of four items on which no consensus was reached. Therefore, additional research and studies must be conducted on these topics in order to provide further evidence. Future expert discussions should be held with the purpose of reaching an agreement on these concerns.
The Delphi consensus technique is widely used in health studies as a method to obtain an agreement from experts on topics where the published body of evidence is incomplete.27,64 One of the limitations of the study is related to the definition of consensus, which is not standard for the Delphi technique.65 To overcome this issue, we used two different approaches to determine consensus. In one of them, we defined two categories of consensus, moderate and strong, and in the other one, we considered the use of Cns, a method proposed by Tastle et al.33 The Cns has been used in studies using the Delphi technique on questions/statements graded on a 5-point scale66,67 and, therefore, we adapted the formula for a 9-point scale. Both methods yielded similar results in terms of agreement, strengthening the agreed questions. To our knowledge, this is the first time that the results of both methodologies are shown on a 9-point scale Delphi study. Future studies may validate the equivalence of the results of both methods. Additionally, due to the methodology and the characteristics of the experts who answered the Delphi questionnaire, it is necessary to point out certain limitations of the recommendations obtained through this technique. In this study, the panelists were exclusively from Spain and, thus, their experience with the patient was focused on the Spanish healthcare system. Therefore, although we believe that this questionnaire could be used in other healthcare settings, the validation of the questionnaire presented here should be considered only for the Spanish healthcare system. Clinicians with experience in other healthcare systems should assess whether each question in this paper can be translated into their clinical allergy practice and adapt or discard those that they do not consider appropriate. They should also verify that the validated questionnaire presented here complies with the ethical and regulatory framework of the country where the physician-patient relationship is assessed.68 However, regarding the representativeness of the panelists in the Spanish context, the high number of allergy specialists responding to the Delphi questionnaire should be noted. In Spain, it is estimated that there are around 1200 allergy specialists, which means that the 195 included in this study account for a percentage of more than 15%. In addition, the unique characteristics of the Spanish healthcare system, fragmented into autonomous communities, has also been taken into account, with representatives from all the territories. Therefore, although the extrapolation of the results to other healthcare systems should be done with caution, the high representation in the Delphi process means that it can be applied anywhere within the Spanish territory.
Conclusion
Patients’ perspectives and preferences should be considered for a shared decision-making process between the patient and the allergologist to promote treatment compliance and AIT adherence. This Delphi consensus study validated a set of twelve recommended questions for patients with the intention of assessing their preferences and suitability for the most common AIT options available. The questionnaire enables an objective approach to the patient, thus resulting in a decision not conditioned by other external factors, but by the patient’s preference after being informed of the characteristics of the different routes. This questionnaire, validated by 195 Spanish allergy experts, can be used in the daily clinical practice at the allergist’s consultation and may help improve treatment adherence and, therefore, health-related outcomes. The intention of the questionnaire is not to decide the treatment based on a specific number of questions answered but to be aware of the patient`s needs and expectations.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics
Review and approval for this research were not needed because the study does not include information on specific patients. It is a review of suggested questions which could be theoretically asked to patients in clinical settings.
Acknowledgments
The authors wish to thank the panelists for their participation in the Delphi questionnaire (Annex I). The authors also would like to thank Grupo Draft for their collaboration with meetings logistics and the technical assistance, Bioclever 2005 for providing statistical analysis and study management support, and the i2e3 Procomms team, specially Jesús Loureiro PhD and Sara Cervantes PhD, for providing medical writing support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by ALK-Abelló S.A.
Disclosure
MA has received funding for educational and research activities from Alk-Abelló, Allergy therapeutics, Astra, Chiesi, GSK, Inmunotek, Kenko, Novartis, Roxall and Sanofi. NC has received funding for educational and research activities from ALK-Abelló, Allergy Therapeutics and Diater. MFN has received funding for educational and research activities from Alk-Abelló, Allergy Therapeutics and Roxall. AL has received funding for educational and research activities from Allergy Therapeutics, AstraZeneca, Diater, GSK, Leti, Novartis and Stallergenes Greer. PMB has received funding for educational and research activities from ALK-Abelló, Allergy Therapeutics, Chiesi, GSK, and Stallergens. JM has received funding for educational and research activities from Chiesi, Faes, GSK and Sanofi. FM has received funding for educational and research activities from Inmunotek. VRV has received funding for educational and research activities from ALK-Abelló, Allergy Therapeutics, GSK and Sanofi. AR has received funding for educational and research activities from ALK-Abelló, Stallergens and Roxall. ISG has received funding for educational and research activities from ALK, Allergy Therapeutics, ASTRA, Chiesi, Diater, GSK, Inmunotek, LETI, Probeltepharma, Roxall and Stallergenes. JMVC has received funding for educational and research activities from ALK-Abelló, Allergopharma, Allergy Therapeutics, Astra-Zeneca, Chiesi, Diater, GSK, Hal, Inmunotek, Leti, Novartis, Sanofi, Stallergenes and Teva. CV has received funding for educational and research activities from ALK-Abelló, Allergy Therapeutics, Astra-Zeneca, GSK, HAL, Industry Roxal, Leti and Stallergenes-Greer. The authors report no other conflicts of interest in this work.
References
1. Ring J, Akdis C, Behrendt H, et al. Davos declaration: allergy as a global problem. Allergy. 2012;67(2):141–143. doi:10.1111/j.1398-9995.2011.02770.x
2. Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. 2014;7(1):12. doi:10.1186/1939-4551-7-12
3. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
4. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783–800. doi:10.1016/S0140-6736(17)33311-1
5. Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Prim. 2020;6(1):95. doi:10.1038/s41572-020-00227-0
6. Mortuaire G, Michel J, Papon JF, et al. Specific immunotherapy in allergic rhinitis. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(4):253–258. doi:10.1016/j.anorl.2017.06.005
7. Canonica GW, Bachert C, Hellings P, et al. Allergen immunotherapy (AIT): a prototype of precision medicine. World Allergy Organ J. 2015;8(1):31. doi:10.1186/s40413-015-0079-7
8. Zhang W, Lin C, Sampath V, Nadeau K. Impact of allergen immunotherapy in allergic asthma. Immunotherapy. 2018;10(7):579–593. doi:10.2217/imt-2017-0138
9. James C, Bernstein DI. Allergen immunotherapy: an updated review of safety. Curr Opin Allergy Clin Immunol. 2017;17(1):55–59. doi:10.1097/ACI.0000000000000335
10. Gunawardana NC, Durham SR. New approaches to allergen immunotherapy. Ann Allergy Asthma Immunol. 2018;121(3):293–305.
11. Chaaban MR, Mansi A, Tripple JW, Wise SK. SCIT versus SLIT: which one do you recommend, Doc? Am J Med Sci. 2019;357(5):442–447. doi:10.1016/j.amjms.2019.02.004
12. Mahler V, Esch RE, Kleine-Tebbe J, et al. Understanding differences in allergen immunotherapy products and practices in North America and Europe. J Allergy Clin Immunol. 2019;143(3):813–828. doi:10.1016/j.jaci.2019.01.024
13. Ojeda P, Ibáñez MD, Olaguibel JM, Sastre J, Chivato T. Alergológica 2015: a national survey on allergic diseases in the Spanish pediatric population. J Investig Allergol Clin Immunol. 2018;28(5):321–329. doi:10.18176/jiaci.0308
14. Ojeda PM, Sastre J, Olaguibel JM, Chivato T. Alergólogica 2015: a national survey on allergic diseases in the adult Spanish population. J Investig Allergol Clin Immunol. 2018;28(3):151–164. doi:10.18176/jiaci.0264
15. Bøgelund M, Ingelmo AR, Ruiz JMA, et al. Preference for sublingual immunotherapy with tablets in a Spanish population with allergic rhinitis. Clin Transl Allergy. 2022;12(2):e12118. doi:10.1002/clt2.12118
16. Chester JG, Bremberg MG, Reisacher WR. Patient preferences for route of allergy immunotherapy: a comparison of four delivery methods. Int Forum Allergy Rhinol. 2016;6(5):454–459. doi:10.1002/alr.21707
17. Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9–18. doi:10.1016/j.pec.2011.02.004
18. Sandman L, Granger BB, Ekman I, Munthe C. Adherence, shared decision-making and patient autonomy. Med Health Care Philos. 2012;15(2):115–127. doi:10.1007/s11019-011-9336-x
19. Blaiss MS, Steven GC, Bender B, Bukstein DA, Meltzer EO, Winders T. Shared decision making for the allergist. Ann Allergy Asthma Immunol. 2019;122(5):463–470.
20. Brazier JE, Dixon S, Ratcliffe J. The role of patient preferences in cost-effectiveness analysis: a conflict of values? Pharmacoeconomics. 2009;27(9):705–712. doi:10.2165/11314840-000000000-00000
21. GEMA 5.1. Guía Española Para El Manejo Del Asma [Spanish Guideline for Asthma Treatment]; 2021. Available from: https://www.semg.es/images/2021/Documentos/GEMA_5.1.pdf.
22. Tankersley M, Winders T, Aagren M, et al. Subcutaneous immunotherapy takes more than the time in the clinic. Curr Med Res Opin. 2021;37(11):1925–1931. doi:10.1080/03007995.2021.1976126
23. Tankersley M, Winders T, Aagren M, et al. Preference for immunotherapy with tablets by people with allergic rhinitis. Patient Prefer Adherence. 2021;15:2539–2549. doi:10.2147/PPA.S338337
24. Cox LS, Hankin C, Lockey R. Allergy immunotherapy adherence and delivery route: location does not matter. J Allergy Clin Immunol Pract. 2014;2(2):156–160. doi:10.1016/j.jaip.2014.01.010
25. Damm K, Volk J, Horn A, et al. Patient preferences in allergy immunotherapy (AIT) in Germany - a discrete-choice-experiment. Health Econ Rev. 2016;6(1):32. doi:10.1186/s13561-016-0110-x
26. Sánchez J. Adherence to allergen immunotherapy improves when patients choose the route of administration: subcutaneous or sublingual. Allergol Immunopathol. 2015;43(5):436–441. doi:10.1016/j.aller.2014.04.011
27. Niederberger M, Spranger J. Delphi technique in health sciences: a map. Front Public Health. 2020;8:457. doi:10.3389/fpubh.2020.00457
28. De la Hoz B, Colás C, Rodríguez Rodríguez M; Grupo Freedom. Calidad de vida en pacientes con rinitis alérgica: estudio comparativo con la hipertensión arterial en el ámbito de atención primaria [Quality of life in patients with allergic rhinitis: comparative study with hypertension in primary care]. An Sist Sanit Navar. 2009;32(2):169–181. doi:10.23938/ASSN.0157
29. Latre Gorbe C. Atención al paciente ambulatorio, comunicación y entrevista clínica [Outpatient care, communication and clinical interview]. SEFH. 2018;Tema 10:1–71.
30. Valle-Rodríguez F, López-García AI, Rivero-Yeverino D, et al. Eficacia y seguridad de la inmunoterapia subcutánea para alérgenos inhalables en pacientes con alergia respiratoria [The effectiveness and safety of subcutaneous immunotherapy for inhalable allergens in patients with respiratory allergies]. Rev Alerg Mex. 2019;66(3):301–307. doi:10.29262/ram.v66i3.617
31. Sackett DL, Haynes RB, Gibson ES, Hackett BC, Taylor PW, Roberts RS. Randomised clinical trial of strategies for improving medication compliance in primary hypertension. Lancet. 1975;305(7918):1205–1207. doi:10.1016/S0140-6736(75)92192-3
32. Galve E, Guijarro-Herraiz C, Masana-Marin L, Cordero-Fort A. Consenso sobre los objetivos y pautas de actuación en el control del colesterol ligado a lipoproteínas de baja densidad en pacientes de muy alto riesgo cardiovascular [Consensus on the objectives and guidelines for action in cholesterol control linked to low-density lipoproteins in patients with very high cardiovascular risk]. Clin Invest Arterioscler. 2016;28(1):31–42.
33. Tastle WJ, Wierman MJ, Dumdum UR. Ranking ordinal scales using the consensus measure. Issues Inf Syst. 2005;6(2):96–102.
34. Baca-Dietz D, Wojnar DM, Espina CR. The shared decision-making model: providers’ and patients’ knowledge and understanding in clinical practice. J Am Assoc Nurse Pract. 2020;33(7):529–536. doi:10.1097/JXX.0000000000000401
35. Elwyn G, Dehlendorf C, Epstein RM, Marrin K, White J, Frosch DL. Shared decision making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Ann Fam Med. 2014;12(3):270–275. doi:10.1370/afm.1615
36. Kvarnström K, Airaksinen M, Liira H. Barriers and facilitators to medication adherence: a qualitative study with general practitioners. BMJ Open. 2018;8(1):e015332. doi:10.1136/bmjopen-2016-015332
37. Chan AHY, Cooper V, Lycett H, Horne R. Practical barriers to medication adherence: what do current self- or observer-reported instruments assess? Front Pharmacol. 2020;11:572. doi:10.3389/fphar.2020.00572
38. Reisacher WR, Visaya JM. Patient adherence to allergy immunotherapy. Curr Opin Otolaryngol Head Neck Surg. 2013;21(3):256–262. doi:10.1097/MOO.0b013e32835f8048
39. Antico A. Long-term adherence to sublingual therapy: literature review and suggestions for management strategies based on patients’ needs and preferences. Clin Exp Allergy. 2014;44(11):1314–1326. doi:10.1111/cea.12362
40. Bender BG, Lockey RF. Solving the problem of non-adherence to immunotherapy. Immunol Allergy Clin North Am. 2016;36(1):205–213. doi:10.1016/j.iac.2015.08.014
41. Feinstein AR. On white-coat effects and the electronic monitoring of compliance. Arch Intern Med. 1990;150(7):1377–1378. doi:10.1001/archinte.1990.00390190043003
42. Passalacqua G, Musarra A, Pecora S, et al. Quantitative assessment of the compliance with once-daily sublingual immunotherapy in children (EASY project: evaluation of a novel SLIT formulation during a year). Pediatr Allergy Immunol. 2007;18(1):58–62. doi:10.1111/j.1399-3038.2006.00471.x
43. Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Randomized controlled open study of sublingual immunotherapy for respiratory allergy in real-life: clinical efficacy and more. Allergy. 2004;59(11):1205–1210. doi:10.1111/j.1398-9995.2004.00508.x
44. Röder E, Berger MY, de Groot H, Gerth van Wijk R. Sublingual immunotherapy in youngsters: adherence in a randomized clinical trial. Clin Exp Allergy. 2008;38(10):1659–1667. doi:10.1111/j.1365-2222.2008.03060.x
45. Hsu NM, Reisacher WR. A comparison of attrition rates in patients undergoing sublingual immunotherapy vs subcutaneous immunotherapy. Int Forum Allergy Rhinol. 2012;2(4):280–284. doi:10.1002/alr.21037
46. Kiel MA, Röder E, Gerth van Wijk R, et al. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132(2):353–60.e2. doi:10.1016/j.jaci.2013.03.013
47. Sieber J, De Geest S, Shah-Hosseini K, Mösges R. Medication persistence with long-term, specific grass pollen immunotherapy measured by prescription renewal rates. Curr Med Res Opin. 2011;27(4):855–861. doi:10.1185/03007995.2011.559538
48. WHO. Adherence to Long Term Therapies: Evidence for Action. WHO; 2003.
49. Møldrup C, Stein J, Søndergaard B. “Patients don’t lie”; a view on adherence in asthma. Pharm World Sci. 2010;32(6):795–798. doi:10.1007/s11096-010-9439-0
50. Vogel L. Why do patients often lie to their doctors? Can Med Assoc J. 2019;191(4):E115. doi:10.1503/cmaj.109-5705
51. Kelly M, McCarthy S, Sahm LJ. Knowledge, attitudes and beliefs of patients and carers regarding medication adherence: a review of qualitative literature. Eur J Clin Pharmacol. 2014;70(12):1423–1431. doi:10.1007/s00228-014-1761-3
52. Kaul S, Avila JC, Mehta HB, Rodriguez AM, Kuo Y-F, Kirchhoff AC. Cost-related medication nonadherence among adolescent and young adult cancer survivors. Cancer. 2017;123(14):2726–2734. doi:10.1002/cncr.30648
53. Gupta D, Ehrlich JR, Newman-Casey PA, Stagg B. Cost-related medication non-adherence in a nationally representative US population with self-reported glaucoma. Ophthalmol Glaucoma. 2021;4(2):126–130. doi:10.1016/j.ogla.2020.08.010
54. Nili M, Adelman M, Madhavan SS, LeMasters T, Dwibedi N, Sambamoorthi U. Asthma-chronic obstructive pulmonary disease overlap and cost-related medication non-adherence among older adults in the United States. J Asthma. 2022;59(3):484–493. doi:10.1080/02770903.2020.1868497
55. Lu ZK, Xiong X, Brown J, Horras A, Yuan J, Li M. Impact of Cost-related medication non-adherence on economic burdens, productivity loss, and functional abilities: management of cancer survivors in Medicare. Front Pharmacol. 2021;12:706289. doi:10.3389/fphar.2021.706289
56. Silva D, Pereira A, Santos N, Plácido JL. Costs of treatment affect compliance to specific subcutaneous immunotherapy. Eur Ann Allergy Clin Immunol. 2014;46(2):87–94.
57. Pajno GB, Vita D, Caminiti L, et al. Children’s compliance with allergen immunotherapy according to administration routes. J Allergy Clin Immunol. 2005;116(6):1380–1381. doi:10.1016/j.jaci.2005.07.034
58. Ruiz FJ, Jiménez A, Cocoletzi J, Durán E. Cumplimiento y abandono de la inmunoterapia [Compliance with and abandonment of immunotherapy]. Rev Alerg Mex. 1997;44(2):42–44. Spanish.
59. Vaswani R, Liu Y-C-C, Parikh L, Vaswani S. Inadequate health insurance coverage: a major factor in premature discontinuation of subcutaneous immunotherapy for allergic rhinitis. Ear Nose Throat J. 2011;90(4):170–173. doi:10.1177/014556131109000408
60. Rhodes BJ. Patient dropouts before completion of optimal dose, multiple allergen immunotherapy. Ann Allergy Asthma Immunol. 1999;82(3):281–286. doi:10.1016/S1081-1206(10)62609-9
61. More DR, Hagan LL. Factors affecting compliance with allergen immunotherapy at a military medical center. Ann Allergy Asthma Immunol. 2002;88(4):391–394. doi:10.1016/S1081-1206(10)62370-8
62. Elwyn G. Shared decision making: what is the work? Patient Educ Couns. 2021;104(7):1591–1595. doi:10.1016/j.pec.2020.11.032
63. Bae J-M. Shared decision making: relevant concepts and facilitating strategies. Epidemiol Health. 2017;39:e2017048. doi:10.4178/epih.e2017048
64. Niederberger M, Köberich S. Coming to consensus: the Delphi technique. Eur J Cardiovasc Nurs. 2021;20(7):692–695. doi:10.1093/eurjcn/zvab059
65. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. doi:10.1016/j.jclinepi.2013.12.002
66. Cos F-X, Gómez-Huelgas R, Gomez-Peralta F. Are there different viewpoints about the management of type 2 diabetes mellitus and comorbidities? A multidisciplinary Spanish qualitative research. Diabetes Ther Res Treat Educ Diabetes Relat Disord. 2022;13(1):189–203.
67. Dongre AR, Norcini J. Strengths, weaknesses, and suggestions for improvement in postgraduate assessment in community medicine in India: a Delphi study. Indian J Community Med. 2021;46(3):464–468. doi:10.4103/ijcm.IJCM_776_20
68. Raina RS, Singh P, Chaturvedi A, Thakur H, Parihar D. Emerging ethical perspective in physician-patient relationship. J Clin Diagn Res. 2014;8(11):XI01–XI04. doi:10.7860/JCDR/2014/10730.5152
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
