Back to Journals » Cancer Management and Research » Volume 11
Sex-related differences in urothelial cell carcinoma of the bladder in Germany
Authors Scheller T , Hofmann R, Hegele A
Received 26 July 2018
Accepted for publication 16 November 2018
Published 28 December 2018 Volume 2019:11 Pages 309—316
DOI https://doi.org/10.2147/CMAR.S181532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Thomas Scheller, Rainer Hofmann, Axel Hegele
Philipps University Hospital, Department of Urology, Marburg, Germany
Background: Urothelial cell carcinoma (UCC), also called transitional cell cancer, occurs significantly more often in males than in females. Essential for the prognosis of recovery is depth of infiltration (muscle-invasive or non-muscle invasive) and tumor-differentiation at initial diagnosis. The current study aimed to explore sex-related differences after initial diagnosis of UCC in Germany.
Methods: We identified patients who underwent transurethral resection of the bladder tumor (TUR-BT). Data were retrospectively analyzed, including TNM classification, histopathological grading, risk group according to the European Association of Urology (EAU), use of photodynamic diagnosis (PDD), and early intravesical chemotherapy (IVC).
Results: A total of 539 male and 190 female patients with UCC underwent TUR-BT. Approximately 75% were non-muscle invasive bladder cancer (NMIBC). Females evidenced significantly higher rates of muscle-invasive bladder cancer (MIBC; P=0.04). Carcinoma in situ (CIS) was significantly more common among males (P=0.01). Recurrence and progression rates showed no significant sex differences – only in the small subgroup of EAU low-risk NMIBC females, we found a significantly higher progression rate (P=0.03). In a Cox proportional hazards model, we found for MIBC, an HR for progression of 6.5 (95% CI, 1.29–33.2; P=0.02) after a median follow-up of 56 months. Use of PDD or IVC showed no significant differences in recurrence and progression between females and males.
Conclusion: Females were significantly more likely to suffer from MIBC at the time of first diagnosis. In NMIBC, males showed a significantly higher prevalence of CIS and EAU low-risk NMIBC females showed significantly higher rates of progression. Sex was not associated with recurrence rates in NMIBC. PDD and IVC were equally effective in both sexes. Based on the collected data we suggest to further investigate possible sex differences in UCC with therapeutical impact. Additional prospective multicenter studies are needed to evaluate both sex-related long-term disease courses and effectiveness of therapies.
Keywords: sex, urothelial cancer, NMIBC, bladder cancer, gender, TCC, UCC, UCB
Introduction
Bladder cancer is not a rare disease as it is the sixth most common carcinoma in the European Union and the eleventh most common worldwide.1 German epidemiological data by the Robert Koch Institute (RKI) indicate a difference in frequency of occurrence between males and females.2 Among males, it is the fourth most common carcinoma; however, in females, it is only the tenth most common carcinoma. Histologically, over 90% of cases are urothelial cell carcinomas (UCCs). A critical factor for prognosis of the disease is the depth of infiltration. About 75% of first diagnosed UCCs are in a non-muscle invasive state.3 Studies with a focus on sex differences in non-muscle invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) are limited. We examined possible sex-specific differences in histopathological aspects, affiliation with risk groups, and recurrence and progression behaviors. We also investigated whether intravesical chemotherapy (IVC) instillation or initial resection using photodynamic diagnosis (PDD) influenced these factors, in particular, sex-specific factors.
Materials and methods
Study design and participants
Patients who underwent transurethral resection of the bladder tumor (TUR-BT) at the Department of Urology and Pediatric Urology at Marburg University Hospital (Germany) between December 2004 and July 2012 were identified. We retrospectively analyzed patient records and created a database for a total of 1,296 interventions. Variables included in the analysis were histopathological findings, TNM, and tumor differentiation, as well as the use of PDD or IVC with Mitomycin C (MMC) 40 mg in between 6 hours after TUR-BT.
Tumor stage, grade, risk group
Tumors were staged using the most recent TNM classification of the Union International Center le Cancer.4 We graded tumors according to the WHO’s 1973 classification.5 Based on the risk group criteria established by the European Association of Urology (EAU), we assigned patients with NMIBC to low, intermediate, and high-risk groups.6
Statistical analyses
We analyzed the data in their entirety and by sex using SPSS for Mac Version 22® (IBM Corporation, Armonk, NY, USA). Statistical analyses included Pearson’s correlation coefficient, Mann–Whitney U-test, ANOVA, and chi-squared test of independence. We chose to perform a Kaplan–Meier analysis with log-rank tests and a Cox proportional hazards model. Study variables included sample demographics (ie, age, sex and age at first diagnosis), tumor characteristics (ie, histopathology, TNM, WHO grading, and size at first diagnosis, multilocal vs unilocal disease at first diagnosis, and EAU risk group), frequency of recurrence, frequency of tumor progression, frequency of recurrence after IVC, and frequency of recurrence after primary resection with usage of PDD. The significance level was set at P<0.05 for both one and two-tailed tests.
Ethics approval
This study was conducted in accordance with the principles of the Declaration of Helsinksi. Prior to the start of our retrospective evaluation and data collection, the Ethics Committee of the Philipps University Hospital Marburg informed us that no written ethical assessment was necessary due to the character of the examination. The data which we collected from archived patient’s files and used in the study, had been anonymized during the process of collection. The requirement for patients’ consent was waived by the Ethics Committee of the Philipps University Hospital Marburg.
Results
A total of 729 patients (♀: ♂ 1: 2.8) underwent TUR-BT at the Department of Urology and Pediatric Urology at Marburg University Hospital (Germany) between December 2004 and July 2012. We diagnosed 479 patients with UCC (♀: ♂ 1: 3.6) and excluded 75 patients who presented with a relapse. The mean follow-up period was 70 months.
We identified 404 patients with a first diagnosed UCC, 312 were male, 92 were female (♀:♂ 1: 3.4). MIBC at the time of initial diagnosis was statistically more common among females (25%, n=23) than males (16%, n=50), which corresponded to a female to male ratio (♀:♂) of 1:0.64 (P=0.04). In the MIBC group, T3 stage was significantly increased in the female cohort (P=0.02).
Since we focused on NMIBC in this work, we excluded patients with MIBC at initial diagnosis (n=73) from further analysis. The first diagnosed NMIBC group (n=331) resulted in 262 male and 69 female patients (♀: ♂ 1:3.8). We retrospectively followed their clinical course over a mean of 70 months. Patients’ distribution and distribution of infiltration depths are shown in Figures 1 and 2.
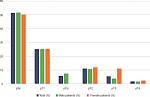  | Figure 2 Distribution of UCC depth of infiltration on first diagnosis. Abbreviation: UCC, urothelial cell carcinoma. |
Tumor stage, tumor grading, and EAU risk group
The subgroup analysis of NMIBC revealed a significantly higher incidence of carcinoma in situ (CIS) in males (P=0.01). In tumor differentiation, a trend toward an increased occurrence of G3 lesions in males was observed (P=0.06). There was no association between sex and EAU risk group classification (low P=0.85; intermediate P=0.72; and high P=0.81, Table 1).
  | Table 1 Risk group subgroup analysis Abbreviation: EAU, European Association of Urology. |
Recurrence and progression
There was no statistically significant relationship between sex and recurrence rates (P>0.05). The small subgroup of female patients in the EAU low-risk group (n=6) showed a trend toward more frequent relapses (P=0.076). Furthermore, 14.5% of females (n=10) and 8% of males (n=21) evidenced tumor progression without reaching statistical significance (P=0.1). Among female patients, only those classified as EAU low risk showed progression more frequently compared with male patients (P=0.03, Table 1). Again, the small subgroup of only 6 female patients must be emphasized.
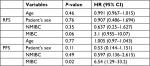
We chose to perform a Kaplan–Meier analysis with log-rank tests and a Cox proportional hazards model on recurrence-free survival (RFS) and progression-free survival (PFS). We examined the covariables “age at first diagnosis”, “MIBC or NMIBC”, and “Patient’s sex” (Table 2). We found for MIBC an HR for progression of 6.5 (95% CI, 1.29–33.2; P=0.02) after a median follow-up of 56 months. This was the only result which reached statistical significance. The Kaplan–Meier plots are shown in Figures 3 and 4. The log-rank tests showed no statistically significant difference between female and male patients (RFS P=0.93/PFS P=0.116).
No further statistically significant relationship between sexes, age, and MIBC/NMIBC could be found for RFS and PFS.
IVC and PDD
In our cohort, 169 patients (134 male and 35 female) received IVC with MMC. PDD was performed during 69 (54 male and 15 female) initial TUR-BTs, whereas 262 (208 male and 54 female) used only white light resection. Patients receiving IVC showed a lower recurrence rate (14.8% vs 21.9%) without reaching statistical significance (P=0.06). IVC reduced recurrence from 20.6% to 17.1% in females and from 22.2% to 14.2% in males, though these findings did not reach statistical significance (P>0.05). Resection using PDD reduced the incidence of recurrence from 15.6% to 11.7%. No significant sex differences were found (P>0.05).
Discussion
Data concerning sex differences in UCC are limited. Previous studies have highlighted various sex differences in UCC. Most studies showed that MIBC at first diagnosis is more often found in female sex. In multiple studies, female patients also showed poorer cancer-specific and overall survival.7–10 Our work aimed to add to this growing body of research by further clarifying sex-specific differences in NMIBC. In particular, we were interested in recurrence and progression rates of the disease, as well as differences in response to IVC, the efficacy of PDD, and risk stratification according to EAU criteria.
In our cohort, MIBC was significantly more common at the time of the first diagnosis in females (P=0.04). Our data are in accordance with other studies like Soave et al examining 517 patients with UCC treated with radical cystectomy.11 Similar to our results, they found a higher proportion of female patients with pT2 and pT3 UCC. Shariat et al analyzed the available literature from 1966 to 2009.7 In their review, they tried to find theories to explain the higher proportion of male patients who suffered from bladder cancer in general as well as the higher percentage of MIBC in the female sex. One theory is the influence of sex hormones. The review concluded that, so far, no sufficient explanation has been found. Henning et al retrospectively investigated possible sex differences concerning referral patterns in UCC.12 They found that female patients tend to have prolonged treatment for urinary tract infection before being referred to a urologist, maybe leading to delayed diagnosis of UCC. Henning et al stated this as a possible explanation for the gender gap in outcomes both in NMIBC and MIBC. Thorstenson et al examined 31,283 patients in Sweden who were followed up in a central registry after initial diagnosis.8 There was also a significantly higher incidence of MIBC in the female sex. However, the analysis of Horstmann et al, which found a statistically insignificant increase in MIBC in male patients, should be mentioned. Unfortunately, no explanation for these strongly differing numbers was given.13
We found no significant sex differences in NMIBC concerning EAU risk groups. In a large multicenter retrospective study (n=5,122) by Rieken et al, 12.3% of the patients were stratified to low risk, 45% to intermediate, and 42.7% to high risk.14
By comparison, in our cohort at initial diagnosis according to EAU criteria, 8.2% low, 38% intermediate, and 53.8% high-risk tumors were found.
An explanation for these slightly differing numbers could be regional differences as well as a possible selection bias of the Urology University Hospital Marburg as a supraregional “high-volume” center.
Unfortunately, a separate analysis by sex did not take place in the work of Rieken et al; hence, a further comparison is impossible. Even after extensive literature research, no work could be identified, which explored EAU, European Organization for Research and Treatment of Cancer (EORTC), or Spanish Urological Club for Oncological Treatment (CUETO) risk group classification by sex. In our study, there was no significant correlation between sex and risk group classification. Based on our data, we can state first that sex is not associated with the risk profile of newly diagnosed NMIBC.
A fundamental issue in the therapy of NMIBC is the prevention of recurrence. Previous data have not provided consistent recurrence rates. In our sample, 18% of patients developed UCC recurrence over the follow-up period of 70 months. In contrast, Xylinas et al compared the effectiveness of risk stratification according to EORTC and CUETO in a sample of 4,689 patients.15 At a median follow-up of 57 months, 45% of patients had relapsed. Walczak et al found a similar relapse rate in their sample of 243 patients over a follow-up period of 46 months (ie, 41% relapses).16 In their meta-analysis of seven EORTC studies (n=2,596), Sylvester et al established a relapse rate of 47.8%.17 The investigations of the CUETO showed a recurrence rate of 33.5%.15 The discrepancy of data may be associated with the inclusion criteria of the current study. We only included patients who presented with an initial diagnosis of UCC as they showed an increased risk of disease recurrence and excluded those who had already relapsed. Another possible explanation for the relatively low proportion of relapses is an improvement in diagnosis, advances in therapy, an increase in resection quality, and the influence of the now regularly performed Re-TURBT.
We further investigated whether belonging to an EAU risk group increased the likelihood of relapse among the entire sample and by sex and could not demonstrate statistically significant relationships (18.8% females vs 18.1% males). Females in our low-risk group showed a recurrence in 50% of cases, but only a trend can be discussed, and the subsample size (n=6) was rather small. In our cohort, we found no significant sex differences in recurrence rate and RFS in NMIBC.
Another challenge in the treatment of NMIBC is the identification of patients at risk for disease progression. Previous studies showed considerable differences in disease progression rates. In our cohort, 9.4% (n=31) progressed. In contrast, other studies reported progression rates of 10.7%–18%, some of which had been defined differently. For instance, Sylvester et al reported a progression rate of 10.7% into a muscle-invasive stage in a large patient population (n= 2,596). Other groups define progression as histopathological progression to a more advanced stage. Data about sex differences concerning NMIBC progression and time to progression are rare. In our cohort, 14.5% of females and 8% of males developed disease progression (P=0.1). However, featuring the NMIBC EAU risk groups, we found that females in the low-risk group showed a significantly higher incidence of disease progression, although the subsample size (n=6) was, again, rather small (P=0.03). These results could be accidental. It would be of interest to further explore this finding using a larger patient sample. Furthermore, we found no sex differences concerning PFS in our cohort.
In our study, IVC was not significantly associated with recurrence rates among the entire sample and by sex. Specifically, of the 169 patients who received IVC, 14.8% relapsed. In contrast, 21.9% of the 160 patients who did not receive IVC, relapsed. Compared to other studies, our recurrence rates are relatively low. Malmström et al reported a 43.4% disease recurrence in their sample of 2,820 patients after IVC; however, the study did not include a control group.18 Solsona et al examined the effect of IVC in a sample of 131 low-risk NMIBC observing a non-significant decrease in relapse rates (40.3% vs 51.9%).19 Again, a possible explanation for the relatively low proportion of relapses in our cohort may be an improvement in the diagnosis, advances of therapy, and an increase in resection quality.
In female and male patients, IVC reduced recurrence rate not significantly (female 17.1% vs 20.6%, male 14.2% vs 22.2%). Given that the subgroup sizes were rather small, it is unclear if our findings are secondary to a genuine lack of association or a lack of power. To date, there are no data available that examined sex-specific relapse rates after IVC. Further prospective and randomized studies are needed to clarify possible differences.
Furthermore, we could not determine a significant difference in the progression rate if using IVC (7.1% vs 11.9%, P=0.1) and no sex differences. Perlis et al performed a meta-analysis on the controversial issue of progression risk reduction through IVC.20 They conclude, due to the weak data and biased study situations, the evidence regarding statements on IVC and progression risk reduction is “very low”.
Our examination of relapse rates when PDD was used during the initial TUR-BT did not yield any significant results among the entire sample and by sex. Specifically, when TUR-BT was performed in conjunction with PDD, 11.7% of patients relapsed compared with 15.6% when PDD was not used. Neither female nor male patients’ usage of PDD reduced the recurrence rate significantly (female 13.3% vs 20.3%, male 13% vs 19.2%) also without sex differences. No publications could be found in the literature, which examined sex-differences in relapse rates with use of PDD to compare our findings. However, in concordance with our findings, O’Brien et al similarly did not find an association between PDD use and a decrease in recurrence rates after 12 months.21
In our cohort, we found no significant difference concerning NMIBC progression when using PDD. Furthermore, no sex differences were evident when using PDD. Comparing our results with a meta-analysis on PDD-assisted TUR-BT by Rink et al, a similar picture emerges.22 This research showed no evidence for a lower rate of progression in PDD-treated patients. It also addressed the aforementioned difficulties of small subsamples, the problems of various biases, as well as different definitions of “progression”, and different primary endpoints.
Conclusion
We can show that female patients are significantly more likely to have MIBC at the time of initial diagnosis, especially tumors with the tumor infiltration depth pT3. In NMIBC, males showed a significantly higher prevalence of CIS. Neither sex exhibited more frequent recurrences. Only the female NMIBC low-risk group showed a significantly increased progression rate. PDD and IVC were equally effective in both sexes, without showing significant sex-specific differences. Our data are encouraging to elucidate possible sex differences in UCC with therapeutical impact. Due to limited data, further prospective and multicenter studies should also be performed focusing on possible causalities.
Author contributions
Thomas Scheller, Rainer Hofmann, and Axel Hegele contributed to the design and implementation of the research, the analysis of the results, and the writing of the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11 (Internet); 2013. Available from: http://globocan.iarc.fr. Accessed December 4, 2018. | ||
Robert-Koch-Institut (Hrsg), Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg). Krebs in Deutschland für 2013/2014 [Cancer in Germany 2013/2014]. Berlin: 2017. | ||
Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–241. | ||
Union internationale contre le cancer (UICC). TNM Classification of Malignant Tumours, 8th Edition. 8th ed. 2017. | ||
Mostofi F, Sobin L, Torloni H. Histological Typing of Urinary Bladder Tumours. In. Geneva: World Health Organization; 1973. | ||
Babjuk M M, Burger M, Compérat E, et al. EAU Guidelines on Non-muscle-invasive Bladder Cancer: Limited Update 2017. EAU Guidelines in Non-muscle-invasive Bladder cancer 2017; 2018. Available from: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer. Accessed December 4, 2018. | ||
Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz PI, Meryn S, Bochner BH. The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int. 2010;105(3):300–308. | ||
Thorstenson A, Hagberg O, Ljungberg B, et al. Gender-related differences in urothelial carcinoma of the bladder: a population-based study from the Swedish National Registry of Urinary Bladder Cancer. Scand J Urol. 2016;50(4):292–297. | ||
Noon AP, Albertsen PC, Thomas F, Rosario DJ, Catto JW. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer. 2013;108(7):1534–1540. | ||
Donsky H, Coyle S, Scosyrev E, Messing EM. Sex differences in incidence and mortality of bladder and kidney cancers: national estimates from 49 countries. Urol Oncol. 2014;32(1):23–31. | ||
Soave A, Dahlem R, Hansen J, et al. Gender-specific outcomes of bladder cancer patients: a stage-specific analysis in a contemporary, homogenous radical cystectomy cohort. Eur J Surg Oncol. 2015;41(3):368–377. | ||
Henning A, Wehrberger M, Madersbacher S, et al. Do differences in clinical symptoms and referral patterns contribute to the gender gap in bladder cancer? BJU Int. 2013;112(1):68–73. | ||
Horstmann M, Witthuhn R, Falk M, Stenzl A. Gender-specific differences in bladder cancer: a retrospective analysis. Gend Med. 2008;5(4):385–394. | ||
Rieken M, Shariat SF, Kluth L, et al. Comparison of the EORTC tables and the EAU categories for risk stratification of patients with nonmuscle-invasive bladder cancer. Urol Oncol. 2018;36(1):8.e17-18.e24. | ||
Xylinas E, Kent M, Kluth L, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer. 2013;109(6):1460–1466. | ||
Walczak R, Bar K, Walczak J. The value of EORTC risk tables in evaluating recurrent non–muscle–invasive bladder cancer in everyday practice. Cent European J Urol. 2013;66(4):418–422. | ||
Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–477. | ||
Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56(2):247–256. | ||
Solsona E, Iborra I, Ricós JV, Monrós JL, Casanova J, Dumont R. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term followup. J Urol. 1999;161(4):1120–1123. | ||
Perlis N, Zlotta AR, Beyene J, Finelli A, Fleshner NE, Kulkarni GS. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. Eur Urol. 2013;64(3):421–430. | ||
O’Brien T, Ray E, Chatterton K, Khan MS, Chandra A, Thomas K. Prospective randomized trial of hexylaminolevulinate photodynamic-assisted transurethral resection of bladder tumour (TURBT) plus single-shot intravesical mitomycin C vs conventional white-light TURBT plus mitomycin C in newly presenting non-muscle-invasive bladder cancer. BJU Int. 2013;112(8):1096–1104. | ||
Rink M, Babjuk M, Catto JW, et al. Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: a critical review of the current literature. Eur Urol. 2013;64(4):624–638. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.