Back to Journals » International Journal of Women's Health » Volume 14
Sex-Related Differences in the Incidence and Development of Carotid Plaques in a Low-Income Chinese Population—A Prospective Cohort Study
Authors Lin Y, Li Y , Li Z, Zhang Z, Liu J , Sun J, Tu J, Wang J , Zhang W , Li J, Ning X
Received 6 March 2022
Accepted for publication 9 June 2022
Published 16 June 2022 Volume 2022:14 Pages 787—795
DOI https://doi.org/10.2147/IJWH.S365242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Yunpeng Lin,1,* Yan Li,2,3,* Zhiying Li,4,* Zhen Zhang,5 Jie Liu,3,6– 8 Jiayi Sun,5 Jun Tu,3,6– 8 Jinghua Wang,3,6– 8 Wenjuan Zhang,5 Jidong Li,2,9 Xianjia Ning3,6– 8
1Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China; 2Department of Anesthesiology, Tianjin Jizhou People’s Hospital, Tianjin, 301900, People’s Republic of China; 3Center of Clinical Epidemiology & Evidence-Based Medicine, Tianjin Jizhou People’s Hospital, Tianjin, 301900, People’s Republic of China; 4Department of Acupuncture, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine & National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, 300193, People’s Republic of China; 5Department of Cardiology, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China; 6Department of Neurology, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China; 7Laboratory of Epidemiology, Tianjin Neurological Institute, Tianjin, 300052, People’s Republic of China; 8Tianjin Neurological Institute, Key Laboratory of Post-Neuroinjury Neuro-Repair and Regeneration in Central Nervous System, Ministry of Education and Tianjin City, Tianjin, 300052, People’s Republic of China; 9Department of Neurosurgery, Tianjin Jizhou People’s Hospital, Tianjin, 301900, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xianjia Ning, Department of Neurology, Tianjin Medical University General Hospital, 154 Anshan Road, Heping District, Tianjin, 300052, People’s Republic of China, Tel +86-22-60817505, Fax +86-22-60817448, Email [email protected] Jidong Li, Department of Neurosurgery, Tianjin Jizhou People’s Hospital, 18 Nanhuan Road, Jizhou District, Tianjin, 301900, People’s Republic of China, Tel/Fax +86-22- 60733586, Email [email protected]
Purpose: More than 150 million people are estimated to have been examined for the presence of carotid plaques (CPs) in China; a sex-related imbalance in the prevalence exists. However, the relationship between sex and the incidence of CP development is unclear, especially in low-income areas of China. Hence, this study aimed to identify the sex differences in CP development and CP burden in both sexes in this population.
Methods: The study population included individuals aged ≥ 45 years in a rural area of Tianjin, China. Carotid ultrasonography was performed in the 2014 and 2019 cohorts, and information on carotid ultrasonography, including the formation and number of CPs, was collected twice. Logistic analyses were used to investigate the predictors of CP formation and numbers of plaques.
Results: A total of 1479 participants were analyzed. The incidence of CP was 20.3% and 29.0% in women and men, respectively. In women, high low-density lipoprotein cholesterol (LDL-C) levels was independent predictors of CP formation (RR: 1.217, 95%CI: 1.010, 1.461; P=0.039). For men, the corresponding predictors were hypertension, alcohol consumption, and low high-density lipoprotein cholesterol (HDL-C) levels (all P< 0.05); none of the examined factors were associated with plaque numbers.
Conclusion: In the study population, men had a higher incidence of plaque than women. Predictors of CP are different in men and women. LDL-C control is critical for moderating atherosclerosis in women; in men, managing blood pressure, stopping alcohol consumption, and controlling HDL-C levels are important.
Keywords: atherosclerosis, carotid plaque, sex differences, predictors, prospective study
Introduction
Carotid atherosclerosis (CAS) is a progressive inflammatory disease that is independently associated with cardiovascular events.1,2 The presence of carotid plaques (CPs), identified using ultrasound measurements, is a surrogate marker of atherosclerotic disease that is closely related in epidemiological studies to cardiovascular events.3,4 Globally, in 2020, the number of people with CPs was estimated to be 815.76 million, approximately 60% higher than the estimate in 2000.5 Moreover, the prevalence of CP differs by sex (25.2% in men vs 17.1% in women).5 The burden of CP is heavy in China, where more than 150 million people are estimated to be affected; the prevalence is also higher in men than in women.6
Previous studies have demonstrated sex-related differences in the risk factors for CAS. The Tromsø Study showed that triglyceride (TC) levels were associated with CAS in women, whereas physical activity and smoking were predictors of CAS in men.7 The Gutenberg-Heart Study reported that diabetes was significantly associated with CAS in men, while low-density lipoprotein cholesterol (LDL-C) levels were significantly associated with CAS in women.8 Satoko et al reported that impaired fasting glucose levels, obesity, and smoking were CAS risk factors in men and that the corresponding factors in women were obesity, impaired fasting glucose levels, and dyslipidemia.9 These differences indicate that the burden of CAS varies between the sexes and that the study of CAS requires sex-specific approaches. Moreover, our previous cross-sectional study showed that the prevalence of CP was significantly higher in men than in women;10,11 however, whether a sex difference exists in the incidence of CP and the related determinants of developing CP in this low-income population was unclear. Strangely, the carotid intima-media thickness (CIMT) of the population in this region was significantly lower than that reported in developed countries.12 Therefore, this study prospectively investigated sex-related differences in CP incidence and predictors stratified by CP numbers.
Methods
Study Design
This was a population-based, prospective cohort study conducted in Tianjin, China, between May 2014 and May 2019. Participants were recruited from the previously described Tianjin Brain Study.11 The Tianjin Brain Study began in 1985 and included all residents from 18 administrative villages in the Ji county of Tianjin, China; 95% of these participants were low-income farmers with annual per capita incomes of < 100 US dollars (USD) in 1991 and < 2500 USD in 2018.13
In May 2014, individuals aged ≥ 45 years were recruited. Individuals were excluded from participation if they were known to have CP or cardiovascular disease (CVD) or if they died or were otherwise lost during follow-up. Baseline (May 2014) information was collected from participants upon recruitment, including carotid ultrasound examination data. Participants with normal carotid ultrasound at baseline were followed up until 2019, at which time they underwent a second carotid ultrasound examination. Among the data collected were CIMT, plaque number, location, size, stenosis and degree of stenosis from the participants’ carotid artery ultrasounds. Those who had baseline and follow-up data were included in the analysis for the present study.
This study was approved by the ethics committee of Tianjin Medical University General Hospital, and a written informed consent was obtained from each participant. This study complies with the Declaration of Helsinki.
Carotid Ultrasound Measurements
Participants were examined using high-resolution ultrasonography (Terason 3000, Burlington, MA, USA), with a 5–12 MHz linear array transducer. The bilateral extracranial carotid artery trees (including the common carotid artery, carotid bifurcation, internal carotid arteries, and external carotid arteries) were screened for the presence of plaques, which were defined as follows: (1) local CIMT >1.5 mm; (2) localized CIMT bulge protruding > 0.5 mm into the lumen; or (3) local CIMT that was > 50% of the adjacent CIMT.14 Plaque numbers were defined as the total number of plaques in the bilateral extracranial carotid artery trees. Though stratified by site into two sites, segmental plaques extending from the common carotid artery bifurcation to the internal carotid artery were considered to be one plaque. Total plaque numbers were stratified as < 2 or ≥ 2, and ≥ 2 plaques were considered multiple CPs. A sonographer, blinded to the participant information, performed the color Doppler ultrasound examinations.
Other Information Collected
Baseline characteristics, including sex, age, education level, presence of hypertension and/or diabetes mellitus, smoking status, and alcohol consumption habits, were collected through self-administered questionnaires. Participants were classified into three age groups: 45–54, 55–64, and ≥ 65 years. Education level was stratified into three groups: illiteracy (0 years), 1–6 years, and > 6 years of formal education. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg or use of anti-hypertension drugs.15 Diabetes mellitus was defined as fasting plasma glucose levels ≥ 7.0 mmol/L or use of antidiabetic medication.16 Smoking and drinking statuses were classified as never, ever, or current.
All participants underwent physical examinations that included measurements of blood pressure, height, weight, waist circumference, and hip circumference. Body mass indexes (BMIs) were calculated as weight (kg) divided by the square of height (m2) and classified as normal (≤ 23.9 kg/m2), overweight (24.0–27.9 kg/m2), or obese (≥ 28.0 kg/m2).17 Waist-hip circumference ratios (WHRs) were calculated as the waist circumference divided by the hip circumference. Biochemical determinations of each patient’s levels of fasting blood glucose (FBG), TC, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and LDL-C were measured according to the standard procedures at Jizhou People’s Hospital (Tianjin, China).
Study Process
The outcome in this study included the incidence of CP and the determinants of its development. Participants without CP at baseline in 2014 were followed for 5 years, monitoring the occurrence of CP and analyzing possible contributory factors from the start such as demographic information, previous disease history, lifestyle, and levels of glucose and lipids.
Statistical Analysis
Continuous variables (age, WHR, FBG, TC, TG, HDL-C, and LDL-C) are presented as means and standard deviations (SDs); between-sex comparisons of these values were performed using Student’s t-tests. Categorical variables (age group, education level, BMI group, presence of hypertension, presence of diabetes, smoking history, and alcohol consumption history) are presented as numbers and frequencies; between-sex comparisons were performed using chi-squared tests. Logistic regression analyses were used to explore the predictors of CP formation and the numbers of CPs; the results are expressed as adjusted relative risks (RRs) and 95% confidence intervals (CIs). The independent variables were chosen from variables exhibiting a P-value < 0.1 in the univariate analysis. All analyses were conducted using SPSS for Windows (version 22.0; SPSS, Chicago, IL, USA); P < 0.05 was considered statistically significant.
Results
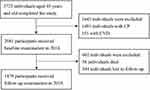
A total of 3723 participants aged ≥ 45 years were recruited in the study. After rejecting 151 with CVD and 1491 with CPs at baseline, we identified 2081 individuals for the 2014 baseline examinations. Following further exclusion of 58 participants who died and 544 who were lost to follow-up, 1479 individuals (513 men and 966 women) were ultimately included in the final analysis (Figure 1).
Participant Baseline Characteristics
At baseline, the average age was 57.89 years (men, 58.97 years; women, 57.31 years). The proportion of participants with more than 6 years of formal education was < 50%. Approximately 70% of the participants had abnormal BMI, including 45.4% who were overweight and 24.1% who were obese. The prevalence of hypertension and diabetes was 30.8% and 5.7%, respectively. Smoking and alcohol consumption were reported by 21.6% (50.9% of men) and 14.8% (40.5% of men), respectively (Table 1).
 |
Table 1 Baseline Characteristics of All Participants in This Study |
Univariable Analysis of Factors Associated with CP Formation and Numbers in Women
As shown in Table 2, the incidence of CP formation was 20.3% in women. The incidence of CP formation was significantly higher in patients with hypertension than in normotensive individuals (P = 0.034). Moreover, participants demonstrating CP formation had higher LDL-C levels than those without CPs (P = 0.019). In the univariable analysis, age group was associated with the number of CPs (P = 0.026).
 |
Table 2 Univariable Analysis of Associated Factors of CP Formation and Plaque Numbers in Women |
Univariable Analysis of Factors Associated with CP Formation and Numbers in Men
As shown in Table 3, the incidence of CP formation was 29.0% in men. Hypertension, alcohol consumption, elevated LDL-C levels, and low HDL-C levels were associated with a greater incidence of CP formation (P < 0.1). However, none of the factors were found to be associated with the presence of ≥ 2 plaques.
 |
Table 3 Univariable Analysis of Associated Factors of CP Formation and Plaque Numbers in Men |
Sex-Related Differences in Predictors of CP Formation and Numbers
In women, the multivariable analysis revealed that advanced age, hypertension, and high LDL-C levels were independent predictors of CP formation. Individuals with hypertension revealed a 35.9% greater risk of CP formation than normotensive individuals (RR = 1.359; 95% CI, 0.963–1.919), but there was no statistical difference (P = 0.081). Moreover, the risk of CP formation increased by 21.7% for each one-SD increment of the LDL-C concentration (RR = 1.217; 95% CI, 1.010–1.461; P = 0.039).
For male participants, the multivariable analysis revealed that hypertension, alcohol consumption, and low HDL-C levels were predictors of CP formation; no factors were associated with plaque numbers. Male participants with hypertension had a 79.7% increased risk of CP formation compared to their normotensive counterparts (RR = 1.797; 95% CI, 1.174–2.751; P = 0.007). Participants who consumed alcohol demonstrated a 66.1% greater risk of CP formation than non-drinkers (RR = 1.661; 95% CI, 1.112–2.480; P = 0.013). Moreover, each one-SD increase in HDL-C levels decreased the risk of CP formation by 23.1% (RR = 0.769; 95% CI, 0.607–0.976; P = 0.031) (Table 4).
 |
Table 4 Sex Differences of Predictors for CP Formation and Plaque Numbers in Multivariable Analysis |
Discussion
This is the first prospective cohort study that investigated sex-related differences in CP incidence and predictors in a low-income Chinese population. The incidence of CP was 20.3% and 29.0% in women and in men, respectively. In women, high LDL-C levels were independent predictors of CP formation; while in men, the predictors of CP formation were hypertension, alcohol consumption, and low HDL-C levels.
Previous studies have reported that women have more stenosis but less plaque than men.18,19 Consistent with previous studies, this study found that there was a higher incidence in men than in women. Estrogens play a fundamental role in most aspects of plaque growth, conferring a cardiovascular advantage in women.20 Moreover, atherosclerosis in men exhibits inflammatory and histological features that are distinctly different from those in women.21 In addition, relative to the external carotid artery and the common carotid artery, the internal carotid artery is larger in women than in men, and women also have a larger outflow than inflow area.22 Bifurcation anatomy has been implicated in the development of plaque, and sex differences in bifurcation anatomy could partly account for the sex differences in the incidence of CP.
Age is an important risk factor for CP formation, and the relationship between CP formation and age has been previously reported. The Rotterdam Study reported that age is a strong and independent predictor of CP formation, with each one-SD increase in age yielding an increased risk of CP formation of 42%.23 The Gutenberg-Heart Study reported that, in women, each one-SD increase in age increased the risk of CP 1.9-fold; the corresponding increase in men was 2.16-fold.8 However, this trend was not evident in this study for either men or women.
Serum lipids, especially LDL-C and HDL-C, are well-known to have an important influence on CP formation. The Multi-Ethnic Study of Atherosclerosis (MESA) study reported that HDL-C protected against new plaque formation (OR = 0.97, P =0.021).24 A Chinese cross-sectional study revealed that high LDL-C levels were associated with a higher risk of CP, but there was no association between HDL-C levels and CP formation.25 Another Chinese study reported that CP formation is independently predicted by LDL-C and HDL-C levels (OR = 1.32 and OR = 0.093, respectively).26 A study focused on patients with familial hypercholesterolemia showed that in women but not men, HDL-C was associated with the presence of CPs; each one-SD increase in concentration was associated with a 55% decrease in the risk of CP formation.27 In this study, serum lipid levels showed a sex-related difference in their ability to predict CP formation; high levels of LDL-C in women and low HDL-C levels in men were associated with higher odds of CP formation.
Hypertension is an important, modifiable risk factor for atherosclerosis. A meta-analysis of eight pooled studies, including 12,474 individuals, reported that hypertension increased the risk of CP formation by 81%.28 A similar association was found in another meta-analysis that reported that hypertension increased the odds of CP formation by 82% in a Chinese population.6 Several longitudinal studies, including the MESA and Rotterdam studies, also confirmed that hypertension was a strong and independent predictor of CP formation.23,24,29 Consistent with previous studies, the present study showed that hypertension was an independent predictor of CP formation, increasing the odds of CP formation by 79.7% in men.
Many studies have shown a J-shaped relationship between alcohol consumption and CIMT; however, the relationship between alcohol consumption and CP formation has not been sufficiently investigated.30–32 A Korean study reported a significant positive correlation between alcohol consumption and CP formation, with the odds of CP formation increasing 80% in men who consumed alcohol when compared with that in non-drinking men; the correlation was not observed in women.33 Another study reported a dose-dependent effect of alcohol consumption on CP formation. A protective effect was found in men consuming < 50 g/day of alcohol, whereas there was a 2.55-fold higher risk of CP formation associated with alcohol consumption of > 100 g/day.34 In this study, although we did not collect information on the amount of alcohol consumed, sex-related differences were observed between drinking and CP formation: men who consumed alcohol demonstrated a 66.1% greater risk of CP formation than non-drinking men; the association was not observed in women.
There are some limitations in this study. First, the population represented a low-income area of China; thus, the results may not be generalizable to other areas. Second, only participants aged ≥ 45 years were included; therefore, these findings cannot be extended to broader age groups. Third, information regarding drug usage, including lipid-lowering drugs, was not collected. However, due to the low socioeconomic status and lack of secondary prevention education, the frequency of medicine use within this population would have been low. Therefore, even though medication use information was not collected, such information would have had limited impact on our results. Finally, plaque severity was assessed as plaque number in this study, making the assessment incomplete. In actual practice, consideration of severity should take into account the location of the plaque, whether it causes lumen stenosis, the degree of stenosis, and the nature of the plaque. In follow-up studies, the plaque property index will be analyzed.
Conclusions
Significant differences were found between men and women regarding the risk of CP formation, suggesting that associated preventive efforts in rural China will best be tailored by sex. In women, the predictors of CP formation were high LDL-C levels. In men, the corresponding predictors were hypertension, alcohol consumption, and low levels of HDL-C. In women, the preventive measures should focus on controlling LDL-C levels, while in men, preventive measures should more appropriately focus on cessation of alcohol consumption and on control of HDL-C levels.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kawai T, Ohishi M, Takeya Y, et al. Carotid plaque score and intima media thickness as predictors of stroke and mortality in hypertensive patients. Hypertens Res. 2013;36(10):902–909. doi:10.1038/hr.2013.61
2. Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2(2):e000087. doi:10.1161/JAHA.113.000087
3. Selwaness M, Bos D, van den Bouwhuijsen Q, et al. Carotid atherosclerotic plaque characteristics on magnetic resonance imaging relate with history of stroke and coronary heart disease. Stroke. 2016;47(6):1542–1547. doi:10.1161/STROKEAHA.116.012923
4. Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220(1):128–133. doi:10.1016/j.atherosclerosis.2011.06.044
5. Song P, Fang Z, Wang H, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8(5):e721–e729. doi:10.1016/S2214-109X(20)30117-0
6. Song P, Xia W, Zhu Y, et al. Prevalence of carotid atherosclerosis and carotid plaque in Chinese adults: a systematic review and meta-regression analysis. Atherosclerosis. 2018;276:67–73. doi:10.1016/j.atherosclerosis.2018.07.020
7. Stensland-Bugge E, Bønaa KH, Joakimsen O, Njølstad I. Sex differences in the relationship of risk factors to subclinical carotid atherosclerosis measured 15 years later: the Tromsø study. Stroke. 2000;31(3):574–581. doi:10.1161/01.str.31.3.574
8. Sinning C, Wild PS, Echevarria FMO, Sinning C, Wild PS, Echevarria FM, et al. Sex differences in early carotid atherosclerosis (from the community-based Gutenberg-Heart Study). Am J Cardiol. 2011;107(12):1841–1847. doi:10.1016/j.amjcard.2011.02.318
9. Ojima S, Kubozono T, Kawasoe S, et al. Gender differences in the risk factors associated with atherosclerosis by carotid intima-media thickness, plaque score, and pulse wave velocity. Heart Vessels. 2021;36(7):934–944. doi:10.1007/s00380-021-01775-5
10. Wang J, Bai L, Shi M, et al. Trends in age of first-ever stroke following increased incidence and life expectancy in a low-income Chinese population. Stroke. 2016;47(4):929–935. doi:10.1161/STROKEAHA.115.012466
11. Wang J, An Z, Li B, et al. Increasing stroke incidence and prevalence of risk factors in a low-income Chinese population. Neurology. 2015;84(4):374–381. doi:10.1212/WNL.0000000000001175
12. Liu B, Ni J, Shi M, et al. Carotid intima-media thickness and its association with conventional risk factors in low-income adults: a population-based cross-sectional study in China. Sci Rep. 2017;7(1):41500. doi:10.1038/srep41500
13. China statistical yearbook 2019; 2019. Available from: http://www.stats.gov.cn/tjsj/ndsj/.
14. Lutsey PL, Diez Roux AV, Jacobs DR, et al. Associations of acculturation and socioeconomic status with subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Am J Public Health. 2008;98(11):1963–1970. doi:10.2105/AJPH.2007.123844
15. Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi:10.1161/HYPERTENSIONAHA.120.15026
16. American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–S31. doi:10.2337/dc20-S002
17. Zhou BF. Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases–report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed Environ Sci. 2002;15(3):245–252.
18. Iemolo F, Martiniuk A, Steinman DA, Spence JD. Sex differences in carotid plaque and stenosis. Stroke. 2004;35(2):477–481. doi:10.1161/01.STR.0000110981.96204.64
19. Ota H, Reeves MJ, Zhu DC, et al. Sex differences in patients with asymptomatic carotid atherosclerotic plaque: in vivo 3.0-T magnetic resonance study. Stroke. 2010;41(8):1630–1635. doi:10.1161/STROKEAHA.110.581306
20. Jacobs AK. Women, ischemic heart disease, revascularization, and the gender gap: what are we missing. J Am Coll Cardiol. 2006;47(3 Suppl):S63–65. doi:10.1016/j.jacc.2004.12.085
21. Frink RJ. Gender gap, inflammation and acute coronary disease: are women resistant to atheroma growth? Observations at autopsy. J Invasive Cardiol. 2009;21(6):270–277.
22. Schulz UG, Rothwell PM. Sex differences in carotid bifurcation anatomy and the distribution of atherosclerotic plaque. Stroke. 2001;32(7):1525–1531. doi:10.1161/01.str.32.7.1525
23. van der Meer IM, Iglesias Del Sol A, Hak AE, Bots ML, Hofman A, Witteman JC. Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree: the Rotterdam Study. Stroke. 2003;34(10):2374–2379. doi:10.1161/01.STR.0000088643.07108.19
24. Tattersall MC, Gassett A, Korcarz CE, et al. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45(11):3257–3262. doi:10.1161/STROKEAHA.114.005669
25. Wang C, Lv G, Zang D. Risk factors of carotid plaque and carotid common artery intima-media thickening in a high-stroke-risk population. Brain Behav. 2017;7(11):e00847. doi:10.1002/brb3.847
26. Yang C, Sun Z, Li Y, Ai J, Sun Q, Tian Y. The correlation between serum lipid profile with carotid intima-media thickness and plaque. BMC Cardiovasc Disord. 2014;14(1):181. doi:10.1186/1471-2261-14-181
27. Waluś-Miarka M, Czarnecka D, Kloch-Badełek M, Wojciechowska W, Kapusta M, Malecki MT. Carotid artery plaques - Are risk factors the same in men and women with familial hypercholesterolemia. Int J Cardiol. 2017;244:290–295. doi:10.1016/j.ijcard.2017.06.076
28. Ji X, Leng XY, Dong Y, et al. Modifiable risk factors for carotid atherosclerosis: a meta-analysis and systematic review. Ann Transl Med. 2019;7(22):632. doi:10.21037/atm.2019.10.115
29. Vigen T, Ihle-Hansen H, Lyngbakken MN, et al. Blood pressure at age 40 predicts carotid atherosclerosis two decades later: data from the Akershus Cardiac Examination 1950 Study. J Hypertens. 2019;37(10):1982–1990. doi:10.1097/HJH.0000000000002131
30. Schminke U, Luedemann J, Berger K, et al. Association between alcohol consumption and subclinical carotid atherosclerosis: the Study of Health in Pomerania. Stroke. 2005;36(8):1746–1752. doi:10.1161/01.STR.0000173159.65228.68
31. Mukamal KJ, Kronmal RA, Mittleman MA, et al. Alcohol consumption and carotid atherosclerosis in older adults: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2003;23(12):2252–2259. doi:10.1161/01.ATV.0000101183.58453.39
32. Damiani IT, Gagliardi RJ, Scaff M. The influence of ethanol in alcoholic beverages in extracranial carotid arteries atherosclerosis. Arq Neuropsiquiatr. 2004;62(4):1022–1026. Article in Portuguese. doi:10.1590/s0004-282x2004000600017
33. Lee YH, Shin MH, Kweon SS, et al. Alcohol consumption and carotid artery structure in Korean adults aged 50 years and older. BMC Public Health. 2009;9(1):358. doi:10.1186/1471-2458-9-358
34. Kiechl S, Willeit J, Rungger G, Egger G, Oberhollenzer F, Bonora E. Alcohol consumption and atherosclerosis: what is the relation? Prospective results from the Bruneck Study. Stroke. 1998;29(5):900–907. doi:10.1161/01.str.29.5.900
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.