Back to Journals » Cancer Management and Research » Volume 11
Sevoflurane Inhibited Osteosarcoma Cell Proliferation And Invasion Via Targeting miR-203/WNT2B/Wnt/β-Catenin Axis
Authors Chen M, Zhou L, Liao Z, Ye X, Xuan X, Gu B, Lu F
Received 3 August 2019
Accepted for publication 15 October 2019
Published 11 November 2019 Volume 2019:11 Pages 9505—9515
DOI https://doi.org/10.2147/CMAR.S225911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Meixian Chen,1,* Lisheng Zhou,1,* Zhaoxia Liao,1 Xijiu Ye,1 Xujun Xuan,2 Beibei Gu,1 Fuding Lu1
1Department of Anesthesiology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China; 2Department of Andrology, The Seventh Affiliated Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fuding Lu
Department of Anesthesiology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China
Tel +86-13560371081
Email [email protected]
Background: Osteosarcoma is one of the most common primary bone cancers with predominant occurrence in children and adolescents. This study aimed to determine the effects of sevoflurane treatment on the osteosarcoma progression and to explore the underlying molecular mechanisms.
Materials and methods: The mRNA and protein expression levels were determined by qPCR and Western blot, respectively. Osteosarcoma cell proliferation, apoptosis and invasion were determined by MTT, caspase-3 activity, colony formation and Transwell invasion assays, respectively. The interaction between miR-203 and WNT2B 3ʹ untranslated region was confirmed by luciferase reporter assay.
Results: Sevoflurane treatment for 6 hrs concentration-dependently suppressed cell viability, increased caspase-3 activity and up-regulated miR-203 expression in both U2OS and MG63 cells. MiR-203 overexpression suppressed cell viability, increased caspase-3 activity and suppressed cell growth and invasion of osteosarcoma cells. In addition, miR-203 knockdown attenuated the tumor-suppressive effects of sevoflurane treatment on osteosarcoma cells. Mechanistic studies showed that miR-203 repressed the expression of WNT2B in U2OS cells, and inhibition of miR-203 attenuated the suppressive effects of sevoflurane on WNT2B expression. More importantly, WNT2B overexpression attenuated the effects of sevoflurane treatment on cell viability, caspase-3 activity, cell growth and invasion of U2OS cells. MiR-203 overexpression suppressed Wnt/β-catenin signalling. Similarly, sevoflurane suppressed the activity of Wnt/β-catenin signalling, which was partially reversed by miR-203 knockdown and WTN2B overexpression.
Conclusion: Our data showed the tumor-suppressive effects of sevoflurane on osteosarcoma cells, and mechanistic studies revealed that sevoflurane inhibited osteosarcoma cell proliferation and invasion partly via targeting the miR-203/WNT2B/Wnt/β-catenin axis.
Keywords: osteosarcoma, proliferation, invasion, sevoflurane, miR-203, WNT2B, Wnt/β-catenin
Introduction
Osteosarcoma is one of the most common primary bone cancers with predominant occurrence in children and adolescents.1,2 Due to the improvement of therapeutic strategies for osteosarcoma, the 5-year survival rate of patients with non-metastatic osteosarcoma has increased to more than 60%.3 However, due to the aggressiveness of osteosarcoma, around half of the patients will develop metastases, which largely affected the long-term survival of the osteosarcoma patients.4 Thus, it is imperative to further decipher the mechanisms associated with osteosarcoma metastasis, which is crucial for developing new therapeutics for osteosarcoma and improving treatment outcomes.
There is growing evidence showing that anaesthesia may impact on the tumor growth and metastases after surgery possibly via regulating the neuroendocrine stress response and immune system of the cancer patients.5 Recently, the volatile anaesthetics including sevoflurane, desflurane and isoflurane have been suggested to regulate cancer cell proliferation and metastases.6–8 For examples, sevoflurane was found to inhibit the malignant potential of head and neck squamous cell carcinoma via regulating hypoxia-inducible factor-1 alpha signalling.9 Sevoflurane could inhibit glioma cell proliferation and metastasis via up-regulating miR-124-3p and down-regulating ROCK1 signalling pathway.10 In addition, sevoflurane reduced invasion of colorectal cancer cells via down-regulation of matrix metalloproteinase-9.11 Recent evidence implied that sevoflurane exerted anti-proliferative and anti-invasive actions on osteosarcoma cells via inactivating PI3K/AKT pathway.12
MicroRNAs (miRNAs) belong to a class of small non-coding RNAs with 21–23 nucleotides in length and represses gene expression via forming imperfect bindings with 3ʹ untranslated regions (3ʹUTRs) of the targeted genes.13 MiRNAs have been extensively explored in cancer studies due to the diverse functions in regulating cancer cell proliferation and metastasis.14 Recently, miRNAs were also found to involve in the sevoflurane-mediated cancer progression. Sevoflurane up-regulated miR-637 expression and repressed glioma cell migration and invasion.15 More importantly, sevoflurane was found to suppress both colorectal cancer and breast cancer proliferation via up-regulating miR-203.16,17 However, whether sevoflurane exerted its anti-cancer effects via modulating miRNAs expression in osteosarcoma is largely unknown.
In the present study, we aimed to determine the effects of sevoflurane on the osteosarcoma cell proliferation and invasion in vitro. Further mechanistic studies revealed that sevoflurane-mediated processes in osteosarcoma cells may involve the modulation of miR-203 expression as well as WNT2B/Wnt/β-catenin signalling pathways in osteosarcoma cells.
Materials And Methods
Cell Culture
The osteosarcoma cell lines (U2OS and MG63) were purchased from ATCC company (Manassas, USA), and U2OS and MG63 cells were cultured in DMEM medium (Thermo Fisher Scientific, Waltham, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific), 100 μg/mL streptomycin (Sigma, St. Louis, USA) and 100 U/mL penicillin (Sigma). Cells were maintained in a humidified incubator with 5% CO2 at 37°C.
Sevoflurane Treatment, Oligonucleotides Synthesis And Cell Transfections
For the sevoflurane (Sigma) treatment, the cell culture plates were placed in the airtight incubator connected to an anesthesia machine (R540; RWD Life Sciences, Shenzhen, China) that was used to supply sevoflurane into the incubator. The concentrations of sevoflurane in the incubator were detected using a gas monitor (CAPNOTURE; MEDACX, Hampshire, UK); U2OS and MG63 cells were exposed to different concentrations of sevoflurane (0%, 1%, 2%, 5% and 10%), respectively, for 6 hrs before further in vitro assays. The miR-203 mimics and inhibitors (named as miR mimics and miR inhibitors, respectively) and their corresponding negative controls (NC; named as mimics NC and inhibitors NC, respectively) were synthesized by RiboBio company (Guangzhou, China). The pcDNA3.1 constructs with WNT2B overexpression (pcDNA3.1-WNT2B) were designed and synthesized by GenePharma Company (Shanghai, China), and pcDNA3.1 was served as the NC. The transfections of miRNAs or pcDNA3.1 constructs were performed in U2OS and MG63 cells using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, USA) by following the manufacturer’s instructions. At 24 hrs following transfections, osteosarcoma cells were processed for further in vitro assays.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA from osteosarcoma cells were isolated using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. For the detection of miR-203 expression, cDNA was synthesized using TaqMan microRNA Reverse Transcription Kit (Applied Biosystems, Foster City, USA); for the detection of WNT2B mRNA expression, cDNA was synthesized using M-MLV Reverse Transcription kit (Thermo Fisher Scientific). The synthesized cDNA was then subjected to real-time PCR reactions on an HT7900 Fast Real-time PCR systems (Applied Biosystems) using SYBR Green Master Mix Kit (Takara, Dalian, China). The expression of miR-203 and WNT2B mRNA was normalized to U6 and β-actin, respectively, and was analysed using 2−ΔΔCt method.
Western Blot Analysis
The U2OS and MG63 cells after different treatments were lysed in RIPA buffer supplemented with 1% protease inhibitors (Sigma). Protein concentrations were measured by BCA assay kit, and the protein samples were then denatured at 98°C for 10 min. Equal amounts of the denatured proteins were then subjected to electrophoresis on a 10% SDS-PAGE gel followed by transferring to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, USA). The PVDF membranes were incubated with 5% skimmed milk for 1 hrs at room temperature. Subsequently, the PVDF membranes were incubated with different primary antibodies against WNT2B (Abcam, Cambridge, USA), active β-catenin (Abcam), total β-catenin (Abcam), cyclin D1 (Abcam), c-myc (Abcam) and β-actin (Abcam) at 4°C overnight. After washing with Tris-buffered saline containing 0.1% Tween 20 for 3 times, the PVDF membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Abcam) for 2 hrs at room temperature. The protein bands were detected using an enhanced chemiluminescence kit (Thermo Fisher Scientific) and β-actin was used as the internal control.
MTT Assay
MTT assay was used to detect cell viability of U2OS and MG63 cells. Briefly, U2OS and MG63 cells were seeded onto the 96-well plates and cells were received different treatments for the indicated time duration. Then, the treated cells were incubated with 20 μl MTT solution for 4 hrs at 37°C. After that, the culture medium was discarded and 150 μl dimethyl sulfoxide was added into each well to dissolve formazan crystal. Cell viability was determined by measuring the absorbance at 570 nm wavelength on a Bio-Tek microplate reader (Bio-Tek, Winooski, USA).
Caspase-3 Activity
The caspase-3 activity of the treated U2OS and MG63 cells were measured using the Caspase-3 activity assay kit (Abcam) following the manufacturer’s protocol.
Colony Formation Assay
Colony formation assay was used to detect cell growth of U2OS and MG63 cells. Briefly, U2OS and MG63 cells after different treatments were seeded onto 24-well plates at a density of 500 cells/well. The cells were cultured for 14 d with the medium being refreshed every 3 d. After that, cells were washed with phosphate-buffered saline for 3 times followed by 4% paraformaldehyde fixation for 10 min. The cells were stained with 0.5% crystal violet at room temperature for 10 min. The number of colonies was counted under a light microscope by randomly selecting three fields.
Transwell Invasion Assay
Transwell invasion assay was used to determine cell invasive potential of U2OS and MG63 cells. Briefly, the transwell inserts (8 µm pore size) were pre-coated with Matrigel (Sigma), and the treated U2OS and MG63 cells were re-suspended in the transwell inserts of the upper chamber containing FBS-free medium, while the lower chamber was filled with DMEM medium containing 10% FBS. Following incubation at 37°C for 24 hrs. The non-invaded cells on the upper surface of the inserts were removed by a sterilized cotton swab, and the invaded cells in the lower surface were fixed with 4% paraformaldehyde for 10 min and were stained with 0.5% crystal violet for 10 min at room temperature. The number of stained cells was counted under a light microscope by randomly selecting three fields.
Luciferase Reporter Assay
Using the TargetScan computational algorithm, we found the putative miR-203 binding site in the 3ʹUTR of WNT2B. The 3ʹUTR of WNT2B containing the miR-203 putative binding site was amplified from genomic DNA and subcloned into the pGL3 luciferase reporter vector (Promega, Madison, USA) and named as 3ʹUTR of WNT2B (wt). For the mutated reporter vector, point mutations were generated into seed region of the miR-203 binding sites and were named as 3ʹUTR of WNT2B (mut). For the luciferase reporter assay, U2OS cells were seeded onto the 24-well plates and were co-transfected with the luciferase reporter vectors along with mimics NC, miR mimics, inhibitors NC or miR inhibitors using Lipofectamine 2000 reagent (Invitrogen). At 48 h following co-transfections, luciferase activities were determined using the Dual-Luciferase Reporter Assay systems (Promega).
Statistical Analysis
All the data analysis was performed using GraphPad Prism 6.0 (GraphPad software, La Jolla, USA). Data of the multiple experiments were presented as mean ± standard deviation. P values between different treatment groups were determined using unpaired Student’s t-test or one-way ANOVA followed by Bonferroni’s multiple comparison test, as appropriate. P < 0.05 was considered to show a statistically significant difference.
Results
Sevoflurane Suppresses Cell Viability, Increases Caspase-3 Activity And Up-Regulates miR-203 Expression In Osteosarcoma Cells
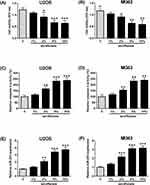
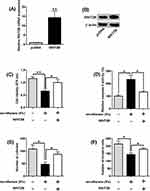
MTT assay and caspase-3 activity assay kit were performed to determine the effects of sevoflurane treatment on the osteosarcoma cell viability and caspase-3 activity, and Figure 1A and B showed that sevoflurane at 2% significantly suppressed cell viability of U2OS and MG63 cells in comparison with the control group; and 5% and 10% sevoflurane caused a further inhibition of cell viability. On the other hand, the increase of caspase-3 activity was observed in cells after being treated with 2%, 5% and 10% sevoflurane, and the enhanced effects of sevoflurane on caspase-3 activity were concentration-dependent (Figure 1C and D). Further qRT-PCR results showed that sevoflurane concentration-dependently up-regulated miR-203 expression with the significant increase being observed at 2%, 5% and 10% (Figure 1E and F).
MiR-203 Overexpression Suppresses Cell Viability, Increases Caspase-3 Activity And Inhibits Cell Invasion Of Osteosarcoma Cells
As miR-203 has been demonstrated for its tumor-suppressive role in osteosarcoma, we further confirmed this using in vitro functional assays. Overexpression of miR-203 was seen in U2OS and MG63 cells after being transfected with miR mimics (Figure 2A and B). MTT assay and caspase-3 activity assay showed that miR-203 overexpression suppressed cell viability and increased the caspase-3 activity of U2OS and MG63 cells in comparison with the control group (Figure 2C and D). Furthermore, miR-203 overexpression also reduced the number of colonies and invasive cells as determined by colony formation assay and Transwell invasion assay (Figure 2E and F).
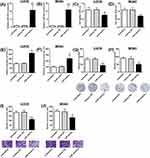
MiR-203 Knockdown Attenuates The Effects Of Sevoflurane Treatment On Cell Viability, Caspase-3 Activity And Cell Invasion Of Osteosarcoma Cells
Whether miR-203 could attenuate the treatment effects of sevoflurane was determined using in vitro functional assays. A significant down-regulation of miR-203 was observed in osteosarcoma cells with miR inhibitors' transfection (Figure 3A and B). For the in vitro functional assays, U2OS and MG63 cells were exposed to 5% sevoflurane for 6 hrs and were then transfected with inhibitors NC or miR inhibitors for 24 hrs. The MTT results showed that miR-203 knockdown partially reversed the inhibitory effects of sevoflurane on the cell viability of U2OS and MG63 cells (Figure 3C and D). The caspase-3 activity was significantly reduced after miR-203 knockdown in sevoflurane-treated osteosarcoma cells (Figure 3E and F). Consistently, sevoflurane treatment suppressed the cell growth and cell invasion, which was attenuated by the presence of miR-203 inhibitors in U2OS and MG63 cells (Figure 3G–J).
MiR-203 Targets 3ʹUTR Of WNT2B And Inversely Regulates WNT2B Expression In U2OS Cells
To further gain into mechanistic actions of miR-203, we used TargetScan tool for bioinformatics analysis and found that WNT2B was a direct target of miR-203 (Figure 4A). The luciferase reporter assay showed that miR-203 overexpression suppressed the luciferase activity of wild type reporter vector, while miR-203 knockdown increased the luciferase activity of wild type reporter vector (Figure 4B). The luciferase activity of the mutated reporter vector was unaffected in U2OS cells with miRNAs transfection (Figure 4C). Further qRT-PCR and Western blot assays consistently showed that miR-203 inversely regulated the expression of WNT2B in both mRNA and protein levels (Figure 4D). In addition, 5% sevoflurane treatment down-regulated WNT2B expression, which was partially restored by miR-203 knockdown in U2OS cells (Figure 4E).
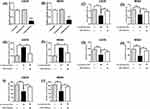
WNT2B Overexpression Attenuates The Effects Of Sevoflurane Treatment On Cell Viability, Caspase-3 Activity And Cell Invasion Of Osteosarcoma Cells
Whether WNT2B overexpression could attenuate the treatment effects of sevoflurane was also determined using in vitro functional assays. The WNT2B overexpression was achieved by transfecting U2OS cells with pcDNA-WNT2B (Figure 5A and B). As expected, 5% sevoflurane caused a decrease in cell viability, cell growth and cell invasion, but increased caspase-3 activity, which was partially reversed by WNT2B overexpression in U2OS cells (Figure 5C–F).
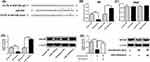
Sevoflurane Regulates Wnt/Β-Catenin Signalling Via miR-203/WNT2B Axis
Furthermore, the mechanistic actions of sevoflurane on Wnt/β-catenin signalling were determined by Western blot assay. MiR-203 overexpression suppressed the protein expression levels of active β-catenin, c-myc and cyclin D1, but not total β-catenin in U2OS cells (Figure 6A). More importantly, 5% sevoflurane also imposed the suppressive effects on active β-catenin, c-myc and cyclin D1 protein expression, while total β-catenin protein level was unaffected by sevoflurane (Figure 6B). MiR-203 knockdown and WNT2B overexpression both significantly attenuated the inhibitory effects of sevoflurane on these protein expression levels in U2OS cells (Figure 6B).
Discussion
Cancer cell invasion and migration largely contributed to the disease recurrence.18 Recently, anaesthetics have been shown to affect the clinical outcome of cancer patients who underwent surgical resection.19 Sevoflurane is one of the most commonly used volatile anaesthetics and has been demonstrated to suppress cancer cell proliferation and metastasis in various types of cancers including osteosarcoma.10,12,16,17,20,21 Unfortunately, the molecular mechanisms underlying sevoflurane-mediated osteosarcoma progression remain under-explored. Hence, in this study, we focused on the anti-proliferative and anti-invasive effects of sevoflurane in osteosarcoma cells and explored its potential for regulation miR-203 expression. Here, we found that sevoflurane exerted tumor-suppressive effects in osteosarcoma cells. Evidence from mechanistic studies implied that sevoflurane inhibited osteosarcoma cell proliferation and invasion partly via regulating miR-203/WNT2B/Wnt/β-catenin signalling axis.
Sevoflurane was demonstrated to inhibit cancer cell proliferation, invasion and migration in various types of cancers including colorectal cancer, glioma, breast cancer, cervical cancer and osteosarcoma.10,12,16,17,20,21 Hurmath et la. showed that 2.5% sevoflurane treatment for 1.5 hrs decreased migration and matrix metalloproteinase-2 activity in glioma cells.22 In addition, 2.5% sevoflurane treatment for 4 hrs suppressed hypoxia-induced lung cancer cell growth and metastasis.23 Fan et al showed that 4% sevoflurane treatment for 6 hrs was effective to inhibit colorectal cell migration but not proliferation.16 In a recent study, sevoflurane ranges from 2.5% to 10% (6 hrs treatment) significantly inhibited osteosarcoma cell proliferation and migration.12 Consistently, our data showed that sevoflurane (2–10%) treatment for 6 hrs suppressed cell viability, cell growth and cell invasion, but increased caspase-3 activity in both U2OS and MG63 cells, suggesting the tumor-suppressive role of sevoflurane in osteosarcoma cell proliferation and invasion.
Studies have shown that sevoflurane exerted its actions on cancer cells via regulating miRNAs expression. Wang et al showed that 3% sevoflurane induced cell apoptosis in A549 cells and also affected the expression of miRNAs that regulate apoptosis.21 In glioma cells, miR-737 was up-regulated upon sevoflurane treatment, which subsequently suppressed glioma cell migration and invasion.15 In a more recent study, sevoflurane inhibited glioma cell proliferation and metastasis via miR-124-3p/ROCK1 axis.10 In colorectal and breast cancers, miR-203 could be significantly induced by sevoflurane, and the up-regulated miR-203 further contributed to tumor-suppressive effects of sevoflurane.16,17 In osteosarcoma, miR-203 was found to function as a tumor suppressor and decreased miR-203 expression level was associated with poor prognosis of osteosarcoma patients.24–27 In our study, we found that sevoflurane consistently induced miR-203 up-regulation and miR-203 overexpression suppressed osteosarcoma cell proliferation and invasion. More importantly, miR-203 inhibition attenuated the tumor-suppressive effects of sevoflurane on osteosarcoma cells. Collectively, these findings may imply that sevoflurane exerted its anti-proliferative and anti-invasive effects via up-regulation of miR-203 in osteosarcoma cells.
Furthermore, the mechanistic actions of miR-203 were explored using bioinformatics analysis, and WNT2B was found to be negatively regulated by miR-203 in U2OS cells. Overexpression of WNT2B attenuated the tumor-suppressive effects of sevoflurane. The Western blot assays revealed that miR-203 overexpression suppressed Wnt/β-catenin signalling. Similarly, sevoflurane suppressed the activity of Wnt/β-catenin signalling, which was partially reversed by miR-203 knockdown and WTN2B overexpression. So far, the effects of sevoflurane on the Wnt/β-catenin signalling have been explored in several studies. Ruan et al showed that sevoflurane inhibited the proliferation of leukemia cells via inhibition of Wnt/β-catenin.28 Hu et al further showed that treatment with 3.6% sevoflurane for 6 hrs inhibited the Wnt/β-catenin signalling pathway, increasing GSK-3β and decreasing β-catenin, which down-regulates the expression of Annexin A1 in microvascular endothelial cells.29 Recently, sevoflurane in pregnant rats was found to inhibit Wnt/β-catenin signalling to affect nerve in offspring.30 Collectively, these findings may imply that sevoflurane exerted the tumor-suppressive effects on osteosarcoma cells via up-regulating miR-203 and inhibition of Wnt/β-catenin signalling.
In the present study, we demonstrated the tumor-suppressive effects of sevoflurane on osteosarcoma cells, and mechanistic studies revealed that sevoflurane inhibited osteosarcoma cell proliferation and invasion partly via targeting the miR-203/WNT2B/Wnt/β-catenin axis. This study provides novel insights into the role of sevoflurane in the osteosarcoma progression.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgements
This study was supported by Sun Yat-sen Memorial Hospital, Sun Yat-sen University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Benjamin RS. Osteosarcoma: better treatment through better trial design. Lancet Oncol. 2015;16(1):12–13. doi:10.1016/S1470-2045(14)71186-6
2. Heymann D. Metastatic osteosarcoma challenged by regorafenib. Lancet Oncol. 2019;20(1):12–14. doi:10.1016/S1470-2045(18)30821-0
3. Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18(1):39–50. doi:10.1080/14737140.2018.1413939
4. Friebele JC, Peck J, Pan X, Abdel-Rasoul M, Mayerson JL. Osteosarcoma: a meta-analysis and review of the literature. Am J Orthop. 2015;44(12):547–553.
5. Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastasis. 2018;35(4):347–358. doi:10.1007/s10585-017-9862-x
6. Ghoneim AA, Azer MS, Ghobrial HZ, El Beltagy MA. Awakening properties of isoflurane, sevoflurane, and desflurane in pediatric patients after craniotomy for supratentorial tumours. J Neurosurg Anesthesiol. 2015;27(1):1–6. doi:10.1097/ANA.0000000000000058
7. Jiao B, Yang C, Huang NN, Yang N, Wei J, Xu H. Relationship between volatile anesthetics and tumor progression: unveiling the mystery. Curr Med Sci. 2018;38(6):962–967. doi:10.1007/s11596-018-1970-6
8. Kim HC, Hong WP, Lim YJ, Park HP. The effect of sevoflurane versus desflurane on postoperative catheter-related bladder discomfort in patients undergoing transurethral excision of a bladder tumour: a randomized controlled trial. Can J Anaesth. 2016;63(5):596–602. doi:10.1007/s12630-016-0600-7
9. Yang Y, Hu R, Yan J, et al. Sevoflurane inhibits the malignant potential of head and neck squamous cell carcinoma via activating the hypoxiainducible factor-1alpha signaling pathway in vitro. Int J Mol Med. 2018;41(2):995–1002. doi:10.3892/ijmm.2017.3306
10. Gao C, Shen J, Meng ZX, He XF. Sevoflurane inhibits glioma cells proliferation and metastasis through miRNA-124-3p/ROCK1 axis. Pathol Oncol Res. 2019. doi:10.1007/s12253-019-00597-1
11. Muller-Edenborn B, Roth-Z’graggen B, Bartnicka K, et al. Volatile anesthetics reduce invasion of colorectal cancer cells through down-regulation of matrix metalloproteinase-9. Anesthesiology. 2012;117(2):293–301. doi:10.1097/ALN.0b013e3182605df1
12. Gao K, Su Z, Liu H, Liu Y. Anti-proliferation and anti-metastatic effects of sevoflurane on human osteosarcoma U2OS and Saos-2 cells. Exp Mol Pathol. 2019;108:121–130. doi:10.1016/j.yexmp.2019.04.005
13. Palmini G, Marini F, Brandi ML. What is new in the miRNA world regarding osteosarcoma and chondrosarcoma? Molecules. 2017;22(3):417. doi:10.3390/molecules22030417
14. Sasaki R, Osaki M, Okada F. MicroRNA-based diagnosis and treatment of metastatic human osteosarcoma. Cancers. 2019;11(4):553. doi:10.3390/cancers11040553
15. Yi W, Li D, Guo Y, Zhang Y, Huang B, Li X. Sevoflurane inhibits the migration and invasion of glioma cells by upregulating microRNA-637. Int J Mol Med. 2016;38(6):1857–1863. doi:10.3892/ijmm.2016.2797
16. Fan L, Wu Y, Wang J, He J, Han X. Sevoflurane inhibits the migration and invasion of colorectal cancer cells through regulating ERK/MMP-9 pathway by up-regulating miR-203. Eur J Pharmacol. 2019;850:43–52. doi:10.1016/j.ejphar.2019.01.025
17. Liu J, Yang L, Guo X, et al. Sevoflurane suppresses proliferation by upregulating microRNA-203 in breast cancer cells. Mol Med Rep. 2018;18(1):455–460. doi:10.3892/mmr.2018.8949
18. Zhou W, Hao M, Du X, Chen K, Wang G, Yang J. Advances in targeted therapy for osteosarcoma. Discov Med. 2014;17(96):301–307.
19. Soltanizadeh S, Degett TH, Gogenur I. Outcomes of cancer surgery after inhalational and intravenous anesthesia: a systematic review. J Clin Anesth. 2017;42:19–25. doi:10.1016/j.jclinane.2017.08.001
20. Ding J, Zhang L, Zeng S, Feng T. Clinically relevant concentration of sevoflurane suppresses cervical cancer growth and migration through targeting multiple oncogenic pathways. Biochem Biophys Res Commun. 2019;514:1179–1184. doi:10.1016/j.bbrc.2019.05.082
21. Wang L, Wang T, Gu JQ, Su HB. Volatile anesthetic sevoflurane suppresses lung cancer cells and miRNA interference in lung cancer cells. Onco Targets Ther. 2018;11:5689–5693. doi:10.2147/OTT.S171672
22. Hurmath FK, Mittal M, Ramaswamy P, Umamaheswara Rao GS, Dalavaikodihalli Nanjaiah N. Sevoflurane and thiopental preconditioning attenuates the migration and activity of MMP-2 in U87MG glioma cells. Neurochem Int. 2016;94:32–38. doi:10.1016/j.neuint.2016.02.003
23. Liang H, Yang CX, Zhang B, et al. Sevoflurane suppresses hypoxia-induced growth and metastasis of lung cancer cells via inhibiting hypoxia-inducible factor-1alpha. J Anesth. 2015;29(6):821–830. doi:10.1007/s00540-015-2035-7
24. Chen X, Chen XG, Hu X, et al. MiR-34a and miR-203 inhibit survivin expression to control cell proliferation and survival in human osteosarcoma cells. J Cancer. 2016;7(9):1057–1065. doi:10.7150/jca.15061
25. Lin W, Zhu X, Yang S, et al. MicroRNA-203 inhibits proliferation and invasion, and promotes apoptosis of osteosarcoma cells by targeting Runt-related transcription factor 2. Biomed Pharmacother. 2017;91:1075–1084. doi:10.1016/j.biopha.2017.05.034
26. Liu S, Feng P. MiR-203 determines poor outcome and suppresses tumor growth by targeting TBK1 in osteosarcoma. Cell Physiol Biochem. 2015;37(5):1956–1966. doi:10.1159/000438556
27. Yang D, Liu G, Wang K. miR-203 acts as a tumor suppressor gene in osteosarcoma by regulating RAB22A. PLoS One. 2015;10(9):e0132225. doi:10.1371/journal.pone.0132225
28. Ruan X, Jiang W, Cheng P, et al. Volatile anesthetics sevoflurane targets leukemia stem/progenitor cells via Wnt/beta-catenin inhibition. Biomed Pharmacother. 2018;107:1294–1301. doi:10.1016/j.biopha.2018.08.063
29. Hu N, Wang C, Zheng Y, et al. The role of the Wnt/beta-catenin-Annexin A1 pathway in the process of sevoflurane-induced cognitive dysfunction. J Neurochem. 2016;137(2):240–252. doi:10.1111/jnc.13569
30. Wang Y, Li Y, Xing Q, et al. Sevoflurane anesthesia in pregnant rats negatively affects nerve function in offspring potentially via inhibition of the Wnt/beta-catenin pathway. Mol Med Rep. 2017;15(5):2753–2759. doi:10.3892/mmr.2017.6316
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.