Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 17
Severe Vincristine-Induced Peripheral Neuropathic Weakness in Both Lower Limbs in an Asian Adolescent with CYP3A4 rs2740574 TT Genotype
Received 22 January 2024
Accepted for publication 9 April 2024
Published 16 April 2024 Volume 2024:17 Pages 125—131
DOI https://doi.org/10.2147/PGPM.S460878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Dongdong Zhang,1 Jie Bai2
1Department of Oncology, Xiangyang No. 1 People’s Hospital, Hubei University of Medicine, Xiangyang, 441000, People’s Republic of China; 2Department of Neurology, Xiangyang No. 1 People’s Hospital, Hubei University of Medicine, Xiangyang, People’s Republic of China
Correspondence: Dongdong Zhang, Department of Oncology, Xiangyang No. 1 People’s Hospital, Hubei University of Medicine, Jiefang Road No. 15, Xiangyang, Hubei, 441000, People’s Republic of China, Tel +8615072278600, Email [email protected]
Background: Vincristine (VCR)-induced peripheral neuropathy (VIPN) is a common adverse reaction during cancer treatment, typically characterized by numbness and paresthesias. This study aimed to report a rare case of VIPN with an atypical genotype, manifesting as grade 3 weakness of the lower limbs.
Case Presentation: A 19-year-old man, diagnosed with alveolar rhabdomyosarcoma for 8 months, was transferred to our hospital for further treatment after the failure of first-line treatment. He developed severe long-standing weakness in both lower limbs and could not walk after four sessions of second-line chemotherapy. The diagnosis of VIPN was confirmed based on the patient’s physical examination, imaging studies, electromyogram results, and treatment history. Furthermore, the pharmacogenetic analysis indicated that the patient harbored CYP3A4 rs2740574 TT genotypes.
Conclusion: We have reported for the first time a VIPN patient whose main clinical manifestation is severe weakness in both lower limbs, accompanied by the CYP3A4 rs2740574 TT phenotype. This case may provide new information on the phenotypic features of VIPN, and may help to better understand the disease pathogenesis and contributing factors.
Keywords: vincristine, vincristine-induced peripheral neuropathy, CYP3A rs2740574 TT genotype
Introduction
Vinca alkaloids (VAs) are the earliest and classic microtubule-targeting agents that can interfere with continuous mitotic division and disrupt cancer cell growth.1 VCR is one of the most important VAs incorporated into several polychemotherapy regimens for the treatment of hematological malignancies, lymphoma, sarcomas and pediatric cancers. However, the dose-limiting side effects caused by VCR can potentially lead to treatment discontinuations and interruptions, and even impact the patients’ quality of life.
VIPN is the most common adverse reaction in clinical practice. As VCR can poorly penetrate across the blood–brain barrier, the occurrence of central nervous system toxicity is relatively low. The VIPN etiology is mainly associated with the disruption of microtubule structures, axonal dysfunction, and inflammation.2 VIPN can be categorized into sensory neuropathy, autonomic neuropathy, and motor neuropathy based on clinical symptoms.2 Sensory neuropathy is more common, with paresthesia, numbness, neuropathic pain or jaw pain, and sensory abnormalities as the main clinical manifestations. Autonomic neuropathy can manifest as symptoms such as urinary retention, constipation, and so on.3 However, manifestations of autonomic neurotoxicity are difficult to identify because symptoms are easily confused with other treatment-related side effects. Motor neuropathy can be presented as extremity weakness, walking difficulties, and impaired balance, and its incidence is relatively high in pediatric patients with cancer receiving long-time VCR-based treatment. Further, these symptoms usually develop early during treatment and persist for a relatively long time.4
Currently, the Common Terminology Criteria for Adverse Events and Total Neuropathy Score-Pediatric Vincristine tools are commonly used for evaluating VIPN.5,6 Previous studies have shown that multiple factors contribute to the development and severity of VIPN, including age, genetic ancestry, dose, impaired liver function, combination medicine and single-nucleotide polymorphisms (SNPs).7–9 Previous studies indicated that CYP3A4 was not prevalent in Asian. However, CYP3A4 rs2740574 genotype commonly associated with a severe peripheral neurotoxicity associated with taxanes.10 This study reported a rare case of VIPN presenting as severe weakness in both lower limbs in an Asian adolescent harboring an uncommon CYP3A4 rs2740574 TT genotype.
Case Presentation
A 19-year-old obese male, complaining of protruding eyeballs, accompanied with decreased and blurred vision for 1 day, was admitted to Guangzhou People’s Hospital. Magnetic resonance imaging (MRI) suggested a mass in the left ethmoid sinus and orbit, with compressed left medial rectus muscle, leading to the eyeball protrusion. He then underwent endoscopic excision of the nasal mass. Postoperative pathology indicated alveolar rhabdomyosarcoma (RMS) of the left ethmoid sinus (Intergroup Rhabdomyosarcoma Study group III, an intermediate risk group). The patient then received postoperative adjuvant chemotherapy according to the Chinese Children Cancer Group Rhabdomyosarcoma 2016 regimen.11 Unfortunately, he experienced persistent headaches for 10 days after the completion of the 10th chemotherapy session. The patient visited our hospital seeking further examination and treatment. MRI indicated a hyperintense lesion on the T1- and T2-weighted image on the lateral wall of the orbit, extensive meningeal metastasis, multiple intracranial metastases, and bilateral multiple cervical lymphadenopathy, suggesting tumor recurrence and progression (Figure S1). The patient received concurrent chemoradiotherapy, and intrathecal chemotherapy drugs were administered before each chemotherapy session simultaneously. The patient’s symptoms relieved after the completion of the first session of chemotherapy. The brain metastases significantly reduced in size after the completion of four sessions of chemotherapy (Figure 1); however, the patient experienced weakness in both lower limbs and walking difficulty, the weakness spreads gradually from the distal end to the proximal end within 5 days. The physical examination suggested normal muscle strength in both upper limbs, but the muscle strength in both lower limbs was graded as 3 in the proximal muscles. The pathological reflex was negative. The cranial MRI, vertebral MRI, and cerebrospinal fluid analysis did not reveal any significant abnormality. The electromyogram (EMG) revealed a reduction in the motor conduction velocity, and the evoked potentials indicated prolonged latency of peripheral nerve action potentials, suggesting peripheral neuropathy (Figure 2).
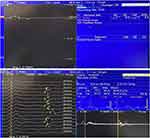 |
Figure 2 EMG indicated reduced motor conduction velocity and prolonged latency of F-wave. |
The patient received a cumulative dose of 28 mg of VCR during both first-line and second-line treatments, we confirmed the diagnosis of VIPN based on the patient’s long-term history of VCR treatment and the results of EMG. Furthermore, we performed a gene polymorphism analysis to investigate potential factors that could influence the occurrence of VIPN. The pharmacogenetic results revealed the presence of a complex pattern of polymorphisms, including the following genotypes: CYP3A4 rs2740574 TT and CEP72 rs924607 CC genotypes (Figure 3). The patient’s symptoms relieved gradually after oral administration of pyridoxine (vitamin B6), glutamine, and pyridostigmine.
 |
Figure 3 Sanger sequencing indicates the presence of both CYP3A4 rs2740574 TT (A) and CEP72 rs924607 CC genotypes (B). |
Discussion
Since the introduction of VCR as a chemotherapeutic agent for treating cancer in 1962, its most significant neurotoxic side effect, especially symmetric sensory-motor neuropathy, has been widely studied and closely monitored in clinical settings.12 This study reported an adolescent with refractory rhabdomyosarcoma developing dose-limiting VIPN whose complex and atypical pattern of genetic polymorphisms might be the cause of severe extremity weakness.
Multiple factors are involved in the development and severity of VIPN, and they are often interrelated. VIPN is a dose-limiting side effect, and one of these factors is the dose of administration, including the cumulative dose and maximum single dose. When the cumulative dose of VCR exceeds 2–6 mg/m2, approximately 35–45% of patients are at risk of developing VIPN.13 Previous studies have shown that higher single doses of VCR are associated with an increased occurrence and severity of VIPN.14 This validated the recommended VCR dose administration: 0.05–0.065 mg/kg in infants and 1.3 mg/m2 in children and adults, with a maximum single dose of 2 mg.8 The next factor was the administration frequency of VCR. Rosca et al discovered that when the administration of VCR was more closely spaced, the incidence of VIPN also increased accordingly.15 This finding suggested that VCR should be administered with a minimum of 1-week interval between each dose. Furthermore, the method of administration is also related to the risk of developing VIPN. The standard route of administration for VCR is bolus push injection, and continuous infusion seems to increase systemic exposure.16 Prolonged infusion can increase the systemic exposure of VCR. One systemic review showed that VCR bolus injections over 1–5 min, which may increase inter-compartmental clearance and high peak-plasma concentrations, induced a higher incidence of VIPN compared with prolonged 1-h infusion.8 However, a randomized study indicated no significant difference in the incidence of VIPN between the 1-h infusion group and the VCR push injection group.17 Notably, VCR was metabolized in the liver through the CYP3A4 enzymes. CYP3A4 inhibitors, such as azole antifungals, could slow down the clearance of VCR, thereby increasing the risk of developing VIPN.18 However, 1-h infusions were advantageous in reducing the risk of VIPN, especially in patients who received concurrent azole therapy.17
Patient-related factors influence the risk of developing VIPN. Although a significant inter-individual variability in VCR pharmacokinetics has been observed, previous studies have indicated that children have a higher plasma clearance than adults; this diminished drug exposure may lower the risk of developing VIPN.16 Certain genetic variations can affect the metabolism and clearance of VCR, thereby impacting the risk of developing VIPN. The aforementioned CYP3A enzyme, including CYP3A4 and CYP3A5, is primarily responsible for drug metabolism. CYP3A4 and CYP3A5 genes are present in all ethnicities, while CYP3A4 is prevalent in Caucasians, the Chinese population accounts for only 30% of the total population;19 CYP3A5 is commonly found in 45% of Africans-Americans, only 7% of East Asian.20 CYP3A5 has been identified as a more selective and efficient metabolizer of VCR than CYP3A4.21 Individuals with the CYP3A5*3/*3 genotype are typically considered to have very low or no expression of the CYP3A5. These patients have a lower predicted VCR clearance rate compared to individuals who express CYP3A5. Some studies indicated that expression of the CYP3A5*3/*3 genotype was typically associated with an increased risk of VIPN.20 However, some other studies and a recent meta-analysis demonstrated that CYP3A5 expression status had no significant impact on the development of VIPN.22–24 Studies have identified that patients with the CYP3A4 loss-of-function variants and missense variants have a higher risk of neurotoxicity.25 In this study, the Asian adolescent developed severe neurotoxicity, which may be associated with the CYP3A4 TT genotype. Due to limited testing panel and the patient’s poor economic condition, we did not perform a single test for CYP3A5 polymorphism.
CEP72 promoter polymorphism is closely associated with the occurrence of VIPN.26 Further, studies have demonstrated that the CEP72 rs924607 TT genotype induces a higher rate of severe VIPN than the CC or CT genotypes.27 However, some other studies have indicated no association between the early occurrence of VIPN and the CEP72 rs924607 TT genotype.28 Gutierrez et al found that patients with the CEP72 CT genotype had a higher risk and severity of VIPN occurrence compared with those with the TT or CC genotype in a Spanish population; and patients with CEP72 CC genotype had the lowest risk of developing grade 2–4 neurotoxicity in their results.28 Although rare, we presented a case with the CEP72 CC genotype developing severe VIPN in an Asian individual.
Other reported genes related to VIPN, such as ABCC1 and ABCB1, SLC5A7 and TTPA, do not directly contribute to the occurrence of VIPN. Nevertheless, the polymorphisms of these genes may be related to the susceptibility of VIPN.29,30 Therefore, there are no highly selective and widely accepted biomarkers available for predicting the occurrence of VIPN. However, our study indicated that SNP testing was of certain significance in predicting VIPN.
Obesity is one of the nongenetic factors correlated with VIPN.31 This can be explained by the release of pro-inflammatory cytokines and the storage of VCR in adipose tissue, which may enhance the neurotoxicity of VCR. Besides previously mentioned azole antifungals, some antiemetic drugs, such as aprepitant and fosaprepitant, can also lead to the occurrence of VIPN as they have moderate inhibition on CYP3A4.32 The patient in our study is moderately obese and has a history of using fluconazole and aripiprazole, both of which may be factors contributing to the occurrence of VIPN. Thus, the choice of antiemetic drugs should take into consideration the risk of developing VIPN during VCR-containing regimens treatment.
Currently, no unified standard exists for preventing and treating VIPN. It is recommended to avoid long-term and repetitive use of VCR. When VIPN occurs, the dosage of VCR should be reduced or discontinued depending on the patient’s condition. Pyridoxine has neuroprotective effects and can be used for treating VIPN. Pyridoxine plus pyridostigmine therapy has been confirmed to be an effective treatment for VIPN.33 The recommended doses for pyridoxine and pyridostigmine are 150 mg/(m2·day) and 3 mg/(kg·day), respectively. Glutamate, as an excitatory neurotransmitter, also possesses some neuroprotective properties. Glutamine supplements can enhance sensory function and overall quality of life, with a recommended dose of 6 g/m2 administered twice daily for 21 days.34 Gabapentin can be used to treat neuropathic pain at an initial dosage of 5–10 mg/(kg·day) [with a maximum of 50–70 mg/(kg·day)]. Opioid drugs can be introduced if the pain control is not satisfactory. Additionally, capsaicin can also be used for topical treatment of peripheral neuropathic pain.35
The limitation of this study is that we did not conduct whole exome sequencing, so we cannot definitively determine the status of all other genes related to peripheral neuropathy. Additionally, this study is a case report, and we can only speculate that VIPN may be related to CYP3A4. Further large-scale clinical trials are still needed to validate this hypothesis.
In conclusion, we presented a case of VIPN in a patient presenting mainly with severe bilateral lower-limb weakness harboring CYP3A4 rs2740574 TT genotypes. We also reviewed the risk factors and treatment strategies for VIPN. Currently, no widely accepted genetic biomarkers have been reported for predicting VIPN. Further studies are needed to develop a comprehensive risk assessment system to better understand the safe and individualized use of VCR in patients.
Data Sharing Statement
The clinical data supporting the conclusions of this manuscript will be made available by the corresponding author.
Ethics Approval
This study was approved by the Ethics and Scientific Committee of Hubei University of Medicine with approval number 2022PR-H002. Written informed consent was obtained from the individual for the publication of any potentially identifiable images included in this article. Institutional approval was also obtained to publish the case details.
Funding
This study was supported Platform Special Fund for Scientific Research of Xiangyang No.1 People’s Hospital (Grants number: XYY2022P05), and Innovative Research Program of Xiangyang No.1 People’s Hospital (Grants number: XYY2023SD06 and XYY2023QB07).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Martino E, Casamassima G, Castiglione S, et al. Vinca alkaloids and analogues as anti-cancer agents: looking back, peering ahead. Bioorg Med Chem Lett. 2018;28(17):2816–2826. doi:10.1016/j.bmcl.2018.06.044
2. Triarico S, Romano A, Attinà G, et al. Vincristine-Induced Peripheral Neuropathy (VIPN) in pediatric tumors: mechanisms, risk factors, strategies of prevention and treatment. Int J Mol Sci. 2021;22(8):4112. doi:10.3390/ijms22084112
3. Tay CG, Lee VWM, Ong LC, Goh KJ, Ariffin H, Fong CY. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. 2017;64(8). doi:10.1002/pbc.26471
4. Li T, Kandula T, Cohn RJ, Kiernan MC, Park SB, Farrar MA. Prospective assessment of vincristine-induced peripheral neuropathy in paediatric acute lymphoblastic leukemia. Clin Neurophysiol. 2023;154:157–168. doi:10.1016/j.clinph.2023.08.002
5. Gilbert A, Piccinin C, Velikova G, et al. Linking the European organisation for research and treatment of cancer item library to the common terminology criteria for adverse events. J Clin Oncol. 2022;40(32):3770–3780. doi:10.1200/JCO.21.02017
6. Lavoie Smith EM, Li L, Hutchinson RJ, et al. Measuring vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. Cancer Nurs. 2013;36(5):E49–E60. doi:10.1097/NCC.0b013e318299ad23
7. Yang QY, Hu YH, Guo HL, et al. Vincristine-induced peripheral neuropathy in childhood acute lymphoblastic leukemia: genetic variation as a potential risk factor. Front Pharmacol. 2021;12:771487. doi:10.3389/fphar.2021.771487
8. van de Velde ME, Kaspers GL, Abbink FCH, Wilhelm AJ, Ket JCF, van den Berg MH. Vincristine-induced peripheral neuropathy in children with cancer: a systematic review. Crit Rev Oncol/Hematol. 2017;114:114–130. doi:10.1016/j.critrevonc.2017.04.004
9. Tunjungsari DA, Gunawan PI, Ugrasena IDG. Risk factors of vincristine-induced peripheral neuropathy in acute lymphoblastic leukaemia children. J Med Invest. 2021;68(3–4):232–237. doi:10.2152/jmi.68.232
10. Kus T, Aktas G, Kalender ME, et al. Polymorphism of CYP3A4 and ABCB1 genes increase the risk of neuropathy in breast cancer patients treated with paclitaxel and docetaxel. Onco Targets Ther. 2016;9:5073–5080. doi:10.2147/OTT.S106574
11. Ma XL, Tang JY. 中国儿童及青少年横纹肌肉瘤诊疗建议(cccg-rms-2016)解读 [Interpretation of recommendations for the diagnosis and treatment of rhabdomyosarcoma in Chinese children and adolescents (CCCG-RMS-2016)]. Zhonghua er ke za zhi. 2017;55(10):735–737. Chinese.
12. Jain P, Gulati S, Seth R, Bakhshi S, Toteja GS, Pandey RM. Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: prevalence and electrophysiological characteristics. J Child Neurol. 2014;29(7):932–937. doi:10.1177/0883073813491829
13. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419–437. doi:10.3322/caac.21204
14. Madsen ML, Due H, Ejskjær N, Jensen P, Madsen J, Dybkær K. Aspects of vincristine-induced neuropathy in hematologic malignancies: a systematic review. Cancer Chemother Pharmacol. 2019;84(3):471–485. doi:10.1007/s00280-019-03884-5
15. Rosca L, Robert-Boire V, Delisle JF, Samson Y, Perreault S. Carboplatin and vincristine neurotoxicity in the treatment of pediatric low-grade gliomas. Pediatr Blood Cancer. 2018;65(11):e27351. doi:10.1002/pbc.27351
16. Kellie SJ, Koopmans P, Earl J, et al. Increasing the dosage of vincristine: a clinical and pharmacokinetic study of continuous-infusion vincristine in children with central nervous system tumors. Cancer. 2004;100(12):2637–2643. doi:10.1002/cncr.20220
17. van de Velde ME, Kaspers GJL, Abbink FCH, et al. Vincristine-induced peripheral neuropathy in pediatric oncology: a randomized controlled trial comparing push injections with one-hour infusions (The VINCA Trial). Cancers. 2020;12(12):3745. doi:10.3390/cancers12123745
18. Ruggiero A, Arena R, Battista A, Rizzo D, Attinà G, Riccardi R. Azole interactions with multidrug therapy in pediatric oncology. Eur J Clin Pharmacol. 2013;69(1):1–10. doi:10.1007/s00228-012-1310-x
19. Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nature Genet. 2001;27(4):383–391. doi:10.1038/86882
20. Sims RP. The effect of race on the CYP3A-mediated metabolism of vincristine in pediatric patients with acute lymphoblastic leukemia. J Oncol Pharm Pract. 2016;22(1):76–81. doi:10.1177/1078155214553143
21. Dennison JB, Kulanthaivel P, Barbuch RJ, Renbarger JL, Ehlhardt WJ, Hall SD. Selective metabolism of vincristine in vitro by CYP3A5. Drug Metab Dispos. 2006;34(8):1317–1327. doi:10.1124/dmd.106.009902
22. Uittenboogaard A, Neutel CLG, Ket JCF, et al. Pharmacogenomics of vincristine-induced peripheral neuropathy in children with cancer: a systematic review and meta-analysis. Cancers. 2022;14(3):612. doi:10.3390/cancers14030612
23. Ceppi F, Langlois-Pelletier C, Gagné V, et al. Polymorphisms of the vincristine pathway and response to treatment in children with childhood acute lymphoblastic leukemia. Pharmacogenomics. 2014;15(8):1105–1116. doi:10.2217/pgs.14.68
24. Guilhaumou R, Solas C, Bourgarel-Rey V, et al. Impact of plasma and intracellular exposure and CYP3A4, CYP3A5, and ABCB1 genetic polymorphisms on vincristine-induced neurotoxicity. Cancer Chemother Pharmacol. 2011;68(6):1633–1638. doi:10.1007/s00280-011-1745-2
25. Apellániz-Ruiz M, Lee MY, Sánchez-Barroso L, et al. Whole-exome sequencing reveals defective CYP3A4 variants predictive of paclitaxel dose-limiting neuropathy. Clin Cancer Res. 2015;21(2):322–328. doi:10.1158/1078-0432.CCR-14-1758
26. Stock W, Diouf B, Crews KR, et al. An inherited genetic variant in CEP72 promoter predisposes to vincristine-induced peripheral neuropathy in adults with acute lymphoblastic leukemia. Clin Pharmacol Ther. 2017;101(3):391–395. doi:10.1002/cpt.506
27. Diouf B, Crews KR, Lew G, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313(8):815–823. doi:10.1001/jama.2015.0894
28. Gutierrez-Camino A, Martin-Guerrero I, Lopez-Lopez E, et al. Lack of association of the CEP72 rs924607 TT genotype with vincristine-related peripheral neuropathy during the early phase of pediatric acute lymphoblastic leukemia treatment in a Spanish population. Pharm Genom. 2016;26(2):100–102. doi:10.1097/FPC.0000000000000191
29. Diouf B, Evans WE. Pharmacogenomics of vincristine-induced peripheral neuropathy: progress continues. Clin Pharmacol Ther. 2019;105(2):315–317. doi:10.1002/cpt.1209
30. Wright GEB, Amstutz U, Drögemöller BI, et al. Pharmacogenomics of vincristine-induced peripheral neuropathy implicates pharmacokinetic and inherited neuropathy genes. Clin Pharmacol Ther. 2019;105(2):402–410. doi:10.1002/cpt.1179
31. Sajdyk TJ, Boyle FA, Foran KS, et al. Obesity as a potential risk factor for vincristine-induced peripheral neuropathy. J Pediat Hematol Oncol. 2020;42(7):e637–e640. doi:10.1097/MPH.0000000000001604
32. Edwards JK, Bossaer JB, Lewis PO, Sant A. Peripheral neuropathy in non-Hodgkin’s lymphoma patients receiving vincristine with and without aprepitant/fosaprepitant. J Oncol Pharm Pract. 2020;26(4):809–813. doi:10.1177/1078155219870840
33. Aydin Köker S, Gözmen S, Demirağ B, et al. Effect of pyridoxine plus pyridostigmine treatment on vincristine-induced peripheral neuropathy in pediatric patients with acute lymphoblastic leukemia: a single-center experience. Neurol Sci. 2021;42(9):3681–3686. doi:10.1007/s10072-020-04970-w
34. Sands S, Ladas EJ, Kelly KM, et al. Glutamine for the treatment of vincristine-induced neuropathy in children and adolescents with cancer. Support Care Cancer. 2017;25(3):701–708. doi:10.1007/s00520-016-3441-6
35. Maihöfner C, Diel I, Tesch H, Quandel T, Baron R. Chemotherapy-induced peripheral neuropathy (CIPN): current therapies and topical treatment option with high-concentration capsaicin. Support Care Cancer. 2021;29(8):4223–4238. doi:10.1007/s00520-021-06042-x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.