Back to Journals » Orthopedic Research and Reviews » Volume 15
Severe Untreated Scoliosis and Early Onset Breast Cancer in a Patient with Neurofibromatosis Associated with a Nonsense Variant of NF1 Gene
Authors Reinhold V , Saarinen A, Suominen E, Syrjänen S , Kankuri-Tammilehto M
Received 5 April 2023
Accepted for publication 30 June 2023
Published 26 September 2023 Volume 2023:15 Pages 183—189
DOI https://doi.org/10.2147/ORR.S415978
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Vivian Reinhold,1 Antti Saarinen,2 Eetu Suominen,2 Stina Syrjänen,3,4 Minna Kankuri-Tammilehto1,5
1Institute of Biomedicine, University of Turku, Turku, Finland; 2Department of Paediatric Orthopaedic Surgery, University of Turku and Turku University, Turku, Finland; 3Department of Oral Pathology and Radiology, Institute of Dentistry, Faculty of Medicine, University of Turku, Turku, Finland; 4Department of Pathology, University of Turku, Turku University Hospital, Turku, Finland; 5Department of Clinical Genetics, Turku University Hospital and University of Turku, Turku, Finland
Correspondence: Vivian Reinhold, Institute of Biomedicine, Department of Clinical Genetics, Turku University Hospital and University of Turku, Kiinamyllynkatu 10, Turku, 20520, Finland, Tel +358503453948, Email [email protected]
Background: Neurofibromatosis 1 (NF1) is a relatively common genetic disorder linked to skeletal abnormalities and elevated risk of cancer. Early onset scoliosis is common in patients with NF1 although severe scoliosis is rare. Scoliosis complicates the normal development and growth and may lead to thoracic insufficiency syndrome. The increased risk for breast cancer in young NF1 female patients has been recently identified.
Case Presentation: We describe a NF1 patient with dystrophic scoliosis symptoms emerged at childhood. At 37 years of age major scoliosis curve in the thoracolumbar region was 80 degrees. The patient was diagnosed with breast cancer at the age of 37 years, histologically the breast cancer was ductal, hormone receptor positive and Her2-positive.
Results: A novel pathogenic variant in NF1 p.(Trp2348*) was identified by next-generation sequencing method. The patient did not have pathogenic variants in BRCA genes or in other currently known hereditary breast cancer genes.
Conclusion: Here, we describe a novel pathogenic variant in NF1 named p.(Trp2348*) which may cause severe dystrophic scoliosis and deteriorate the quality of life and physical function, as well as Her-2 positive breast cancer. Untreated dystrophic scoliosis in patients with NF1 may result in significant spinal deformity and deteriorate the quality of life and physical function. Genetic counseling is recommended in all patients with NF1. Patients need routine follow-up throughout life. Multidisciplinary consulting is warranted in patients with neurofibromatosis 1.
Keywords: spinal deformity, neurofibromatosis, breast cancer
Introduction
Type 1 neurofibromatosis (NF1) is an autosomal dominant disorder caused by a pathogenic variant in the NF1 gene in chromosome 17q11.2.1 NF1 gene works as tumor suppressor gene by producing neurofibromin protein which controls cellular growth and differentiation. Abnormal function caused by a pathogenic variant of the NF1 gene leads to abnormal cell proliferation, which leads to multisystemic manifestation including abnormalities of the nervous tissue, soft tissue, skin, and bone. Café au lait spots and plexiform neurofibromas are common clinical manifestations (Box 1). NF1 is one of the most common genetic disorders, with prevalence approximately 1:2500–3000.1
NF1 patients may have skeletal manifestations such as short stature, osteopenia or osteoporosis, tibial bowing, congenital pseudoarthrosis (false joint), and scoliosis.1 Spinal deformities are common in patients with NF1.2,3 Often NF1 patients have mild scoliosis. However, some NF1 patients have dystrophic scoliosis, a more severe type of scoliosis. Dystrophic scoliosis is a rapidly progressing early onset scoliosis with characteristic spinal changes.2 Dystrophic scoliosis often presents a striking curvature (short and angular) in the thoracolumbar spine and is often associated with extensive kyphosis.
Spinal tumors are common in dystrophic scoliosis.3,4 Severe dystrophic scoliosis may lead to neurologic injury. Dystrophic scoliosis does not typically respond to conservative treatment. Surgical treatment is warranted with growth-friendly instrumentation in infantile patients and spinal fusion in adolescent patients. Untreated scoliosis may lead to decreased quality of life and pulmonary compromise. Surgical treatment in patients with NF1 is complicated due to poor bone quality, sharp angular curvatures, vertebral rotation, and dural ectasia.4 Male-to-female ratio of 4:1 was reported for surgically treated dystrophic scoliosis in NF1 child patients.4
Case Presentation
A 38-year-old woman originally from the Middle East received referral to the Department of Clinical Genetics after moving to Finland at the age of 37 years. She had a clinical diagnosis of neurofibromatosis 1. Skin freckles were observed in the upper body along with six café au lait spots, large plexiform neurofibroma was observed in the right sided chest area which was surgically removed at the age of 37 years. Neurological status was normal. Patient is 142 cm tall.
Patient was evaluated with native radiographs, computer tomography, and magnetic resonance imaging (MRI). Severe dystrophic kyphoscoliosis was present in the plain radiographs. Imaging revealed severe kyphoscoliosis with major scoliosis curve in the thoracolumbar region of 80 degrees and a marked kyphosis. Thoracic height (T1-T12) was 185 mm. MRI scan revealed three masses in the abdominal cavity which were suspected as neurofibromas. Anomaly in T12 vertebra and severe lumbar vertebra rotation was observed in CT and MRI scans. Patient had no spinal tumors and no compression of the spinal cord.
Patient had worn an orthopedic cast at the age 3 to 4 for early onset scoliosis. Operative treatment was suggested but the family had refused the treatment. Currently, the patient is able to walk for 10 minutes before muscle cramps forces her to rest. Patient has pain in the lower extremities and experienced shortness of breath during minor physical performance. Patient does not experience back pain at rest or when moving. Patient manages light physical activities and work but lifting of heavy objects is not possible and rotational movements are not recommended. The patient was recently evaluated by an orthopedic surgeon in our university hospital. Despite the marked deformity, surgical treatment was not recommended to the patient as it would be unlikely to improve the symptoms. Patient is being monitored for progression of the deformity. Patient did not either want surgical treatment. Physiotherapy was organized for the patient. Spinal deformity has not progressed during the 6-year follow-up in Finland (Figures 1–3).
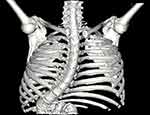 |
Figure 1 3-D reconstruction of the patient. Sternum was removed from the reconstruction for illustrative purposes. Severe dystrophic scoliosis is seen. |
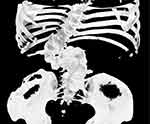 |
Figure 2 3-D reconstruction of the patient. Severe kyphosis is seen. |
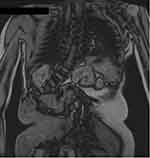 |
Figure 3 MRI of the patient’s thorax. Paraspinal neurofibromas are seen. |
At the age of 37 patient was diagnosed with ductal breast carcinoma. The carcinoma was estrogen, progesterone receptor, and HER2 positive by immunohistochemistry. Patient was treated with surgery (mastectomy and reconstruction) combined with radiotherapy and adjuvant therapy. Patient has remained disease free.
Family
The patient’s mother and aunt have reportedly similar clinical signs and symptoms suggesting NF1. None of the family members had severe scoliosis. There were no other breast cancer cases in the near family. Family members did not participate in NF1 genetic investigations.
Result of Genetic Studies
Patient’s DNA from peripheral blood was analyzed using neurofibromatosis next-generation sequencing gene panel which presented a heterozygous nonsense variant in the NF1 gene c.7044G>A in exon 47, GenBank reference sequence (NM_000267.3(NF1). Variant is also named c.7107G>A, p.Trp2369Ter according to GenBank reference sequence NM_001042492.3(NF1). Variant was confirmed by bi-directional Sanger sequencing. This variant has not been observed in the large reference population cohort in Genome Aggregation Database (gnomAD) or in Sequencing Initiative Suomi (SISu) database which refers to the pathogenicity of the variant. This variant changes the amino acid from a tryptophan to a premature stop codon [p.(Trp2348*)], and is expected to result in an absent or disrupted protein product and is predicted to cause loss of normal protein function either through protein truncation or nonsense-mediated mRNA decay. Loss-of-function variants in NF1 are known to be pathogenic. The variant described here has been previously reported twice in NF1 patients by laboratory of Invitae (classified as pathogenic, ClinVar database, ID: 404475) and by laboratory of Ambry Genetics (classified as pathogenic, ClinVar database, ID: 404475). In silico MUTTASTER analysis evaluated the pathogenicity of the variant as disease causing. The same variant has been recently associated also with somatic variant in brain tumors.5 So far, there is no functional evidence in ClinVar for this genetic NF1 variation. The American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) classification system is an important interpretation standardization system for variants and, according to this system the variant is classified as likely pathogenic.6 We suggest here, that the NF1 variant identified here should be classified too pathogenic. This is because the variant presents a nonsense mutation, and it is rare in control populations and has been found earlier in two individuals with a NF1 phenotype. No other pathogenic variants were found in BRCA genes or in currently known other hereditary breast cancer genes.
Discussion
Skeletal deformities are common in NF1. However, there are fewer published reports about severe scoliosis in NF1.2 Here, we describe an adult NF1 patient with dystrophic early onset scoliosis, her performance status and found pathogenic variant in NF1. The presently described patient had poor physical performance and shortness of breath during normal ambulation which is likely to partly be caused by the spinal deformity. Despite of severe thoracolumbar deformity the patient’s physical performance is good. She manages light physical activities and work. Surgical treatment for early onset scoliosis during adult age is unlikely to significantly improve the pulmonary symptoms. Patient als did not want surgical treatment.
Approximately 10% of NF1 children have early onset scoliosis.4,7 Dystrophic scoliosis often progresses even after skeletal maturity to a severe deformity thus requiring surgical treatment. Growth-friendly treatment is used to prevent the progression of the deformity while allowing further spinal growth in young patients with early onset scoliosis.4,8,9 Thoracic height of 18 cm is considered satisfactory to prevent thoracic insufficiency syndrome.10 Spinal fusion is used in adolescent and adult patients. Severe early onset scoliosis may complicate normal cardiopulmonary development and lead to decreased pulmonary function.11 Most cases of NF1 associated dystrophic scoliosis are progressive. Due to the risk of severe progression surgical treatment in childhood is often recommended, although there is no clear consensus on the indications of the surgical treatment.4 Operative treatment of dystrophic scoliosis remains a significant challenge. Complications and need of revision surgery are common in patients with dystrophic scoliosis. NF1 patients have an increased risk for osteoporosis12 and risk increases with age. Bisphosphonate and asfotase alfa in combination have been used after spinal surgery in NF1 patient at her Middle Ages after to enhance bone healing.13
The patient described here had neurofibromas typical to NF1 and ductal breast carcinoma at relatively young age similarly as recently observed in NF1 patients without familial breast cancer risk.14 NF1 gene functions as tumor suppressor. Patients with NF1 have increased prevalence of both benign and malignant tumors. Recently it has been reported that ductal breast carcinoma in patients under 50 years is significantly more common in female NF1 patients than in the general population.15,16 Elevated risk of breast cancer should be assessed in patients with NF1.16 Our patient has been organized annual mammography and breast MRI for the evaluation of the collateral breast until the age of 50 years.
Our study emphases that the identified NF1 variant is pathogenic 1) because this variant is found in our patient having a typical NF1 phenotype, 2) the same variant has previously reported in two other patients with NF1 phenotype, 3) the variant is exceptionally rare and not found in control populations, 4) the variant is a nonsense variant, and 5) it is in silico MUTTASTER analysis a disease-causing variant. Previously, nearly 700 nonsense mutations have been reported in NF1. Genotype-phenotype correlation in NF1 is not well understood.3,17 Previously, only a few clear genotype-phenotype correlations have been observed.18 In a recent study skeletal abnormalities were associated with frameshift variants.19 The variant in our patient changes the amino acid from a tryptophan to a premature stop codon [p.(Trp2348*)], and is expected to result in an absent or disrupted protein product and is predicted to cause loss of normal protein function either through protein truncation or nonsense-mediated mRNA decay. Our study provided a new potential finding on the genotype-phenotype correlation in NF1. Thus, further studies on this topic are urgently warranted.
In a recent study it was shown that in mice that NF1 expression in bone marrow osteoprogenitors is required for the maintenance of the adult skeleton.20 Recent studies suggest that a somatic NF1 second hit mutation contributes to dystrophic scoliosis.21,22
Based on the ClinVar database the other of the two previously reported NF1 patients had a cardiovascular phenotype. In some NF1 patients the hypertrophic cardiomyopathy has been seen23. Cardiac ultrasound was also performed with our patient because of the Herceptin treatment due to her Her-2 positive ductal breast carcinoma. No abnormalities were found in the ultrasound. Currently, cardiac ultrasound screening is not a routine in NF1 patients.
Conclusion
We described a novel pathogenic variant in NF1 named p.(Trp2348*) which may cause severe dystrophic scoliosis and deteriorate the quality of life and physical function, as well as Her-2 positive ductal breast carcinoma. Untreated dystrophic scoliosis in patients with NF1 may result in significant spinal deformity and deteriorate the quality of life and physical function. Genetic counseling is recommended in all patients with NF1. Patients need routine follow-up throughout life to detect possible associated diseases at early stage. Multidisciplinary consulting is warranted in management of patients with neurofibromatosis 1.
Consent for Publication
This study is a case report and informed consent was obtained from the patient regarding the use of information obtained during clinical treatment included consent for case details and accompanying images to be published. The patient had been treated at the hospital. As no new samples were required a separate ethics board permit was not required.
Author Contributions
All authors made a significant contribution to the work reported including the conception, study design, execution, acquisition of data, analysis and interpretation, and participated in drafting, revising or critically reviewing the manuscript. All gave final approval of the last version of the manuscript to be submitted and agreed on the journal for submission; and agreed to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13(8):834–843. doi:10.1016/S1474-4422(14)70063-8
2. Crawford AH, Herrera-Soto J. Scoliosis associated with neurofibromatosis. Orthop Clin North Am. 2007;38(4):553–562. doi:10.1016/j.ocl.2007.03.008
3. Well L, Careddu A, Stark M, et al. Phenotyping spinal abnormalities in patients with Neurofibromatosis type 1 using whole-body MRI. Sci Rep. 2021;11(1):1–13. doi:10.1038/s41598-021-96310-x
4. Neifert SN, Khan HA, Kurland DB, et al. Management and surgical outcomes of dystrophic scoliosis in neurofibromatosis type 1: a systematic review. Neurosurg Focus. 2022;52(5):E7. doi:10.3171/2022.2.FOCUS21790
5. Kim H, Lim KY, Park JW, et al. Sporadic and Lynch syndrome associated mismatch repair-deficient brain tumors. Lab Investigat. 2022;102(16171):160–171. doi:10.1038/s41374-021-00694-3
6. Richards S, Aziz N, Bale S, et al., ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics andGenomics and the Association for Molecular Pathology. Genet Med. 2015;175(5):405–424. doi:10.1038/gim.2015.30
7. Toro G, Santoro C, Ambrosio D, et al. Natural History of Scoliosis in Children with NF1: an Observation Study. Healthcare. 2021;9(7):881. doi:10.3390/healthcare9070881
8. Haapala H, Saarinen AJ, Salonen A, Helenius I. Shilla growth guidance compared with magnetically controlled growing rods in the treatment of neuromuscular and syndromic early onset scoliosis. Spine. 2020;45(23):E1604–E1614. doi:10.1097/BRS.0000000000003654
9. Jain V, Berry CA, Crawford AH, Emans JB, Sponseller PD. Growing rods are an effective fusionless method of controlling early-onset scoliosis associated with neurofibromatosis type 1 (NF1): a multicenter retrospective case series. J Pediatr Orthop. 2017;37(8):e612–e618. doi:10.1097/BPO.0000000000000963
10. Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. JBJS. 2008;90(6):1272–1281. doi:10.2106/JBJS.G.00184
11. Pehrsson K, Larsson S, Oden A, Nachemson A. Long-term follow-up of patients with untreated scoliosis. A study of mortality causes of death, and symptoms. Spine. 1992;17(9):1091–1096. doi:10.1097/00007632-199209000-00014
12. Poyrazoğlu HG, Baş VN, Arslan A, et al. Bone mineral density and bone metabolic markers’ status in children with neurofibromatosis type 1. J Pediatr Endocrinol Metabol. 2017;30(2):175–180. doi:10.1515/jpem-2016-0092
13. Harindhanavudhi T, Takahashi T, Petryk A, Polly DW. An Adjunctive use of Asfotase alfa and Zoledronic acid after spinal surgery in neurofibromatosis type I related dystrophic scoliosis. AACE Clin Case Rep. 2020;6(6):305–310. doi:10.4158/ACCR-2020-0222
14. Evans DGR, Kallionpää RA, Clementi M, et al. Breast cancer in neurofibromatosis 1: survival and risk of contralateral breast cancer in a five country cohort study. Genet Med. 2020;22(2):398–406. doi:10.1038/s41436-019-0651-6
15. Walker L, Thompson D, Easton D, et al. A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br J Cancer. 2006;95(2):233–238. doi:10.1038/sj.bjc.6603227
16. Uusitalo E, Kallionpää RA, Kurki S, et al. Breast cancer in neurofibromatosis type 1: overrepresentation of unfavourable prognostic factors. Br J Cancer. 2017;116(2):211–217. doi:10.1038/bjc.2016.403
17. Li H, Zhang W, Yao Z, Guo R, Hao C, Zhang X. Genotypes and clinical intervention of patients with neurofibromatosis type 1 associated dystrophic scoliosis. Front Pediatr. 2022;10. doi:10.3389/fped.2022.918136
18. Shofty B, Mauda-Havakuk M, Weizman L, et al. The effect of chemotherapy on optic pathway gliomas and their sub-components: a volumetric MR analysis study. Pediatr Blood Cancer. 2015;62(8):1353–1359. doi:10.1002/pbc.25480
19. Scala M, Schiavetti I, Madia F, et al. Genotype-phenotype correlations in neurofibromatosis type 1: a single-center cohort study. Cancers. 2021;13(8):1879. doi:10.3390/cancers13081879
20. Paria N, Khalid A, Shen B, et al. Molecular dissection of somatic skeletal disease in neurofibromatosis type 1. J Bone Miner Res. 2023;38(2):288–299. doi:10.1002/jbmr.4755
21. Chelleri C, Scala M, De Marco P, et al. Somatic double inactivation of NF1 associated with NF1-related pectus excavatum deformity. Hum Mutat. 2023;2023–2030. doi:10.1155/2023/3160653
22. Margraf RL, VanSant-Webb C, Mao R, et al. NF1 somatic mutation in dystrophic scoliosis. J Mol Neurosci. 2019;68(1):11–18. doi:10.1007/s12031-019-01277-0
23. Incecik F, Hergüner ÖM, Alınç Erdem S, Altunbaşak Ş. Neurofibromatosis type 1 and cardiac manifestations. Turk Kardiyol Dern Ars. 2015;43(8):714–716. doi:10.5543/tkda.2015.27557
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

