Back to Journals » Cancer Management and Research » Volume 14
Severe Hypercalcemia as an Initial Presentation of Advanced Hepatocellular Carcinoma: A Case Report
Authors Bashir AM , Mohamed AH , Mohamed HN, Ibrahim IG
Received 9 March 2022
Accepted for publication 21 April 2022
Published 28 April 2022 Volume 2022:14 Pages 1577—1580
DOI https://doi.org/10.2147/CMAR.S364996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Ahmed Muhammad Bashir,1 Abdirashid Hashi Mohamed,1 Hawa Nuradin Mohamed,1 Ismail Gedi Ibrahim2
1Internal Medicine Department, Mogadishu Somali Turkey, Recep Tayyip Erdogan, Training and Research Hospital, Mogadishu, Somalia; 2Radiology Department, Mogadishu Somali Turkey, Recep Tayyip Erdogan, Training and Research Hospital, Mogadishu, Somalia
Correspondence: Ahmed Muhammad Bashir, Internal Medicine Department, Mogadishu Somali Turkey, Recep Tayyip Erdogan, Training and Research Hospital, Mogadishu, Somalia, Email [email protected]
Background: It is extremely rare for hypercalcemia to appear as the first symptom of hepatocellular carcinoma. Instead, it occurs primarily as a paraneoplastic manifestation after the disease is already diagnosed.
Methods: In this report, we describe a 55-year-old woman who presented with symptoms of acute severe hypercalcemia and was negative for hepatitis B surface antigen and hepatitis C virus antibodies.
Results: Laboratory tests confirmed hypercalcemia (serum calcium 16.2 mg/dL) with intact parathyroid hormone (2 pg/mL). Alpha-fetoprotein serum level was 3031.14 ng/mL. Abdominal ultrasonography and computed tomography revealed a big vascularized mass of 7 × 5.5cm in diameter, occupying most of the right lobe of the liver.
Conclusion: Based on these findings, hepatocellular carcinoma may present late in disease progression with isolated hypercalcemia; therefore, HCC should be considered in the differential diagnosis in a hypercalcemic patient.
Keywords: hypercalcemia, hepatocellular carcinoma, Somalia
Introduction
Globally, hepatocellular carcinoma (HCC) is the fourth most common cancer.1 In Somalia, it’s estimated to be the third in the most common cancers in the general population and 2nd most among male patients.2,3 80–90% of HCC cases are the result of underlying cirrhosis caused by chronic hepatitis B or C, alcoholism, alpha-1 antitrypsin deficiency, or nonalcoholic steatohepatitis (NASH).4 The number of non-cirrhotic HCC cases is still unknown despite having a significantly lower prevalence. According to one large multicenter study in Italy, only 52 of 3000 cases of HCC were caused by non-cirrhotic HCC, or <2%.5 The pathogenesis of HCC in patients with cirrhotic disease results from stepwise mutations, while the disease progression of non-cirrhotic disease is only obscure and may be due to de novo carcinogenesis.6 Hepatocellular carcinoma (HCC) can cause paraneoplastic syndromes, and 4–7% of patients have been documented to have hypercalcemia. Hypercalcemia without bone metastases is rare in HCC patients.7
HCC is occasionally, but infrequently, associated with paraneoplastic syndromes. Hypoglycemia,8 demyelination, pemphigus vulgaris,9 thrombocytosis,10 hypercalcemia, hypercholesterolemia, and erythrocytosis11 have all been associated with HCC.
The presence of intrinsic hypercalcemia, caused by a humoral secretion from cancer cells and manifesting as the initial symptom of hepatocellular carcinoma, is extremely rare.
Here we present a case from Somalia, a country of a low income and fragile healthcare system, where our patient initially presented with clinical features of hypercalcemia and later was diagnosed as hepatocellular carcinoma with hypercalcemia as paraneoplastic syndrome.
Case
A 55-year-old woman with a known case of diabetes for 5 years, presented to our hospital with a 1-week history of abdominal pain, generalized weakness, loss of appetite and constipation. The patient had no history of smoking or alcohol use and no known family history of cancer. The physical exam was unremarkable. Laboratory results revealed elevated calcium 16.5 mg/dL (normal range 8.3–10.6 mg/dL), aspartate aminotransferase (AST) 102 U/L (0–31), albumin 3.1 g/dl (3.5–5.5). Total bilirubin and Alanine transaminase were normal. The patient denied any excess intake of antacids or vitamins A and D.
Renal sonography requested to detect a renal origin of the hypercalcemia incidentally revealed multiple hypoechoic lesions in the liver parenchyma, prompting computed tomography (CT) of the abdomen which demonstrated multiple hypodense lesions that showed peripheral contrast enhancement the largest one measured 7×5.5cm, consistent with multifocal hepatocellular carcinoma (Figure 1), computed tomography of the chest revealed metastatic nodular lesions in all lobes of both lungs. CT images did not show any sign of bony metastasis.
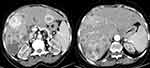 |
Figure 1 Contrast abdominal Computed tomography shows multifocal heterogeneous contrast-enhancing lesion in both liver lobes. |
Additional laboratory testing showed Alpha-fetoprotein (AFP) 3031.14 ng/mL (0–8.78), parathyroid hormone (PTH) 2 pg/mL (15–68), Vitamin D 38.4 ng/mL (20–30), Carcinoembryonic antigen (CEA) 4 ng/mL (<2.5), CA-125 45.8 U/mL (0–35), CA-15-3 19.3 U/mL (0–31,4).
The patient was given zoledronic acid 4 mg/dL as an infusion in a single dose, a bisphosphonate that is effective in lessening hypercalcemia, in addition to hydration with 3 L of normal saline. Two weeks later, her calcium was 7 mg/dL.
Discussion
Patients with non-cirrhotic HCC, in fact, have an insidious course of disease due to the lack of symptoms, resulting in delayed detection. Because of the delay in diagnosis, these patients frequently have greater tumor burdens at first presentation, with an average of 12 cm, compared to cirrhotic HCC, which has a range of sizes but is often smaller.12 Resection is the first step in treatment for patients with preserved hepatic function (Childs-Pugh score A) and a solitary mass, according to current clinical evidence.13
Hypercalcemia in cancer patients has four primary causes. PTHrP secretion by tumor cells, also known as humoral hypercalcemia of malignancy, accounts for 80% of cases and is most common in squamous cell malignancies. Another 20% of cases are caused directly by osteolytic activity at bone metastasis sites. Hypercalcemia is typically caused by this mechanism in breast cancer, multiple myeloma, and lymphomas. Rarely, hypercalcemia can be caused by tumor secretion of vitamin D, which has been linked to some lymphomas, or by ectopic tumor secretion of PTH.14
Despite the lack of sufficient resources to measure the PHTrP, our patient had hypercalcemia, low PTH and underlying malignancy, that can be interpreted as humoral hypercalcemia of malignancy (HHM). Non-cirrhotic HCC and HHM were both discovered in this patient, which is unique. The majority of HHM patients have severe and visible symptoms, the majority of which are neurological.15
In the presence of serious hypercalcemia, the patient is given isotonic saline, calcitonin, and a bisphosphonate all at the same time (typically, zoledronic acid [ZA]). Within 12 to 48 hours, calcitonin with saline hydration should result in a significant drop in blood calcium concentrations. By the second to fourth day, the bisphosphonate will be effective and have a longer-lasting impact, allowing the hypercalcemia to be controlled.16
Although there is a lack of access to proper healthcare and screening in Somalia and most of the patients present in advanced stage, this atypical presentation of HCC makes it more difficult to for physicians to reach diagnosis.
Conclusion
The patient’s elevated calcium level without accompanying symptoms reveals HHM’s possibly shady character. Although rare, non-cirrhotic HCC might present late in disease progression with isolated hypercalcemia, and so HCC should be considered in a hypercalcemic patient’s differential diagnosis. Primary cancer screening is beneficial in low-income countries whose patients’ cannot afford the advanced cancer therapy.
Consent
Written and informed consent for publication of the case was taken from the patient.
Ethical Approval
In our institution, case reports do not need institutional review board (IRB) approval.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Newman NB, Jabbour SK, Hon JDC, et al. Hepatocellular carcinoma without cirrhosis presenting with hypercalcemia: case report and literature review. J Clin Exp Hepatol. 2015;5(2):163–166. doi:10.1016/j.jceh.2015.04.001
2. Baş Y, Hassan HA, Adıgüzel C, Bulur O, Ibrahim İA, Soydan S. The distribution of cancer cases in Somalia. Semin Oncol. 2017;44(3):178–186. doi:10.1053/j.seminoncol.2017.10.007
3. Şahiner F, Tanoğlu A, Hoşbul T, et al. Seroprevalence and genotype distribution of hepatitis c virus in Mogadishu, Somalia: a comprehensive study. J Mol Virol Immunol. 2021;2(3):115–122. doi:10.46683/jmvi.2021.38
4. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;265(12):1118–1127. doi:10.1056/NEJMra1001683
5. Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36(6):1349–1354. doi:10.1002/hep.1840360609
6. Gaddikeri S, McNeeley MF, Wang CL, et al. Hepatocellular carcinoma in the noncirrhotic liver. Am J Roentgenol. 2014;203(1):34–47. doi:10.2214/AJR.13.11511
7. Das L, Viji M, Mahalakshmi honasoge ES. Hepatocellular carcinoma presenting as hypercalcemia. Endocr Pract. 2018;24:139–140.
8. Sorlini M, Benini F, Cravarezza P, Romanelli G. Hypoglycemia, an atypical early sign of hepatocellular carcinoma. J Gastrointest Cancer. 2010;41(3):209–211. doi:10.1007/s12029-010-9137-0
9. Hinterhuber G, Drach J, Riedl E, et al. Paraneoplastic pemphigus in association with hepatocellular carcinoma. J Am Acad Dermatol. 2003;49(3):538–540. doi:10.1067/S0190-9622(03)01581-0
10. Hwang SJ, Luo JC, Li CP, et al. Thrombocytosis: a paraneoplastic syndrome in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10(17):2472–2477. doi:10.3748/wjg.v10.i17.2472
11. Chang PE, Ong WC, Lui HF, Tan CK. Epidemiology and prognosis of paraneoplastic syndromes in hepatocellular carcinoma. ISRN Oncol. 2013;2013:1–8. doi:10.1155/2013/684026
12. Giannini EG, Marenco S, Bruzzone L, et al. Hepatocellular carcinoma in patients without cirrhosis in Italy. Dig Liver Dis. 2013;45(2):164–169. doi:10.1016/j.dld.2012.08.018
13. Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona clinic liver cancer staging system. J Hepatol. 2006;44(4):723–731. doi:10.1016/j.jhep.2005.12.015
14. Stewart AF. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373–379. doi:10.1056/NEJMcp042806
15. Stroffolini T, Andreone P, Andriulli A, et al. Characteristics of hepatocellular carcinoma in Italy. J Hepatol. 1998;29(6):944–952. doi:10.1016/S0168-8278(98)80122-0
16. Shane E, Berenson JR. Treatment of hypercalcemia; 2022.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
