Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Serum Pigment Epithelium-Derived Factor Levels are Associated with Estradiol and Decrease After Adjusting for Alanine Aminotransferase in Chinese Women Based on Multiple Linear Regression Analysis
Received 22 June 2022
Accepted for publication 13 September 2022
Published 23 September 2022 Volume 2022:15 Pages 2901—2909
DOI https://doi.org/10.2147/DMSO.S378561
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Cuiliu Li, Yunna Zhang, Fang Gao
The Second Department of Endocrinology, Cangzhou Central Hospital, Cangzhou, 061001, People’s Republic of China
Correspondence: Cuiliu Li, Tel +8603172075935, Email [email protected]
Purpose: To assess changes in pigment epithelium-derived factor (PEDF) levels in patients with metabolic syndrome (MetS) and to investigate sexual dimorphism in serum PEDF levels and their relationships with estradiol.
Methods: A total of 318 individuals (145 men, 173 women) who underwent health examinations in our department were selected. Serum PEDF, estradiol and other metabolic parameters were determined. Homeostasis model assessment of insulin resistance (HOMA- IR) and homeostasis model assessment of β-cell function (HOMA-β) were calculated to evaluate insulin resistance and β-cell function, respectively. Multiple linear regression analysis was used to analyse the factors influencing serum PEDF.
Results: Serum PEDF levels were significantly higher in subjects with MetS in both men and women (12.09± 2.75 vs 8.97± 3.19 μg/mL in men and 11.31± 2.79 vs 8.40± 2.32 μg/mL in women, MetS vs non-MetS, P< 0.001). Correlation analysis showed that serum PEDF levels were significantly correlated with body mass index (BMI), waist circumference, waist-to-hip ratio, diastolic blood pressure (DBP), fasting and 2-h postprandial glucose, fasting and 2-h postprandial insulin, HOMA-β, HOMA-IR, hemoglobin A1c (HbA1c), alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid (UA), triacylglycerol (TG) and high-density lipoprotein cholesterol (HDL-C). Elevated ALT, HOMA-IR and TG were significant predictors of increased PEDF concentrations. In women, estradiol was inversely correlated with PEDF levels (r=− 0.25, P=0.011), and the association was no longer significant after adjustment for ALT.
Conclusion: PEDF could be used as a biomarker of MetS in both men and women. This study reported for the first time that circulating PEDF displays sexual dimorphism, which could be related to estrogen. The association between estrogen and circulating PEDF levels was attenuated after adjusting for ALT.
Keywords: pigment epithelium-derived factor, metabolic syndrome, sexual dimorphism, estradiol, alanine aminotransferase
Introduction
With the rapid development of society and the economy, people’s lifestyles have also undergone great changes, and an increasing number of people tend to live sedentary lifestyles and eat too much high-calorie food. As a result, the prevalence of metabolic syndrome (MetS) and its complications is rapidly growing worldwide and becoming an increasingly serious health problem. The latest research shows that the MetS global prevalence ranged from 12.5% to 31.4%.1
As a secreted glycoprotein that belongs to the superfamily of serine protease inhibitors, pigment epithelium-derived factor (PEDF) has a broad range of physiological functions, such as neuroprotection, anti-angiogenesis, anti-inflammatory, antitumourigenesis, antifibrosis and anti-ageing.2–7 PEDF might improve metabolic abnormalities partly by inhibiting oxidative stress generation and/or inflammatory reactions in the adipose tissue and liver and act as a negative regulator of adipogenesis.8,9 Studies have shown that circulating PEDF levels are significantly increased in subjects with MetS and its related complications, such as coronary heart disease, diabetes and nonalcoholic fatty liver disease (NAFLD).10–12 PEDF is strongly linked to obesity-related insulin resistance,13,14 and insulin resistance is the central link and common pathogenesis of various metabolic abnormalities in MetS. Studies from different populations have shown that the circulating PEDF levels of patients with MetS are significantly higher than those of patients with non-MetS, and researchers believe that PEDF could be used as a new biomarker and predictor of metabolic syndrome.15–17
In recent years, sexual dimorphism in the prevalence of MetS and its components has become apparent.18 As a potential biomarker and therapeutic agent for MetS,8 sex differences were also found in PEDF, with PEDF levels in men being significantly higher than those in women.11,15,17,19,20 However, the evidence for the sex differences in PEDF has been limited and inconsistent, and there have also been studies showing no sexual dimorphism in PEDF concentrations.10,16,21,22 Estradiol is the predominant circulating bioactive estrogen in premenopausal women,23,24 and it can downregulate the expression of PEDF in tissues such as the ovary, breast, and endometrium.25–28 A hypothesis has been suggested that the lower level of PEDF in women may be due to the suppressive effect of oestrogen,17 but there was no evidence to support this hypothesis. Therefore, this study aimed to assess the changes in PEDF levels in patients with MetS and investigate sexual dimorphism in serum PEDF levels and their relationships with estradiol.
Methods
Study Subjects
A total of 318 individuals aged between 20 and 75 years old who underwent a health check-up in our department from January 2019 to December 2020 were included in this cross-sectional study. All participants were free of known diabetes, tumours, cardiovascular disease, hypertension, acute and chronic kidney disease, liver disease or other severe metabolic abnormalities and were not using medications that would interfere with the study outcomes; there was also no history of excessive alcohol intake in the previous 12 months. The study was approved by the Ethics Committee of Cangzhou Central Hospital and was conducted in accordance with the Declaration of Helsinki. All subjects provided written informed consent.
Clinical Measurements
All patients underwent anthropometric measurements of their blood pressure, weight, height, waist circumference and hip circumference as described elsewhere.29 Body mass index (BMI) was calculated as the ratio of body weight in kilograms to height in metres squared. The waist-to-hip ratio was calculated as waist circumference divided by hip circumference.
Biochemical Measurements
All subjects underwent an oral glucose tolerance test (OGTT) with 75 g of glucose after overnight fasting. Blood samples for plasma glucose, insulin, hemoglobin A1c (HbA1c), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine (Cr), uric acid (UA), triacylglycerol (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), estradiol and PEDF were drawn in the fasting state, and then blood samples were collected at 120 min for measurement of plasma glucose and insulin. Blood samples were centrifuged, and separated serum was kept frozen at –80 °C until assayed for estradiol and PEDF. Serum PEDF concentration was determined with a PEDF sandwich enzyme-linked immunosorbent assay kit (BioVendor, Brno, Czech Republic) according to the manufacturer’s instructions, as described previously by Stejskal et al.16 This test method has proven to be sensitive and stable in Asian, European and American populations with different metabolic profiles and in different age groups.14,19–21,30–32 The serum estradiol level was measured by electrochemiluminescence (Immulite2000, Diagnostic Products Corp, USA). The plasma glucose concentration was tested by a hexokinase method (TBA-200FR, Tokyo, Japan). Insulin was determined by electrochemiluminescent immunoassay (Roche, USA). HbA1c was measured by high-performance liquid chromatography (HLC-73G8, Tosoh, Japan). ALT, AST, BUN, Cr, UA, and serum lipids were detected by an automatic biochemical instrument (Hitachi LABOSPECT 008AS, Tokyo, Japan); ALT and AST were detected by the kinetic method; BUN, Cr and UA were tested by the enzymatic method; TC was measured by the cholesterol oxidase (CHOD-PAP) method; TG was measured by the glycerol lipase oxidase (GPO-PAP) method; and precipitation and direct methods were used to detect HDL-C and LDL-C.
MetS
The diagnostic criteria for MetS are based on the diagnostic criteria for MetS of the Chinese Medical Association Diabetes Society:33 (1) abdominal obesity (central obesity): waist circumference ≥ 90 cm for men and ≥ 85 cm for women; (2) hyperglycemia: fasting plasma glucose ≥ 6.1 mmol/L or 2-hour postload plasma glucose ≥ 7.8 mmol/l and/or those who have been diagnosed with diabetes and treated; (3) hypertension: blood pressure ≥ 130/85 mmHg (1 mmHg = 0.133 kPa) and/or those who have been confirmed as having hypertension and treated; (4) fasting triglyceride (TG) ≥ 1.70 mmol/L; and (5) Fasting HDL-C < 1.04 mmol/l. MetS was defined as having three or more of the above metabolic risk factors. Subjects were divided into a MetS group and a non-MetS group according to the diagnostic criteria for MetS.
Calculations
Insulin resistance was measured by the homeostasis model assessment of insulin resistance (HOMA-IR) index, and the homeostasis model assessment of insulin secretion (HOMA-β) was calculated as basal insulin release as described previously:34
Formula: HOMA-IR = Fasting Insulin (FINS)× Fasting Plasma Glucose (FPG)/22.5; HOMA-β = (20 × FINS)/(FPG − 3.5).
Statistical Analysis
All data were analysed using SPSS software, version 25.0. Measurement data are expressed as the mean ± SD. Variables with skewed distributions were logarithmically transformed before analysis. The independent samples t-test was used for comparisons between two groups. Spearman correlation test was used to determine the relations between serum PEDF and clinical indicators. Multiple linear regression analysis was used to analyse the factors influencing serum PEDF. Differences were considered to be significant at P < 0.05.
Results
Clinical Characteristics of Subjects According to the Presence or Absence of MetS
A total of 318 subjects aged 20–75 years old were included in this study, with an average age of 49.73±14.76 years old, including 145 men and 173 women. The study population was divided into a non-MetS group and a MetS group. As expected, the BMI, waist circumference, waist-to-hip ratio, SBP, DBP, fasting glucose, 2-h glucose, fasting insulin, 2-h insulin, HOMA-IR, HbA1c, ALT, AST, UA, TG, TC, and PEDF in the MetS group were significantly higher than those in the non-MetS group (all P<0.05). HDL-C and estradiol in the MetS group were significantly lower than those in the non-MetS group (all P<0.05). Age, HOMA-β, BUN, Cr and LDL-C showed no significant differences between the two groups (Table 1).
 |
Table 1 Clinical Parameters of Subjects According to the Presence or Absence of MetS |
The overall serum PEDF level was 10.43±3.14 μg/mL, of which the PEDF level in men was significantly higher than that in women (10.94±3.28 vs 10.01±2.96 μg/mL, P=0.006). The serum PEDF level was significantly higher in subjects with MetS in both men and women (12.09±2.75 vs 8.97±3.19 μg/mL in men and 11.31±2.79 vs 8.40±2.32 μg/mL in women, MetS vs non-MetS, P<0.001). The PEDF level in men with MetS was significantly higher than that in women with MetS, however, this difference was no longer evident in the non-MetS group (Table 1, Figure 1).
 |
Figure 1 Analysis of circulating pigment epithelium-derived factor (PEDF) levels in different groups. Data are shown as the means ± SEs. *P<0.05. |
Correlation Analysis of Serum PEDF Levels and Metabolic Factors
Correlation analysis showed that serum PEDF levels were positively correlated with BMI, waist circumference, waist-to-hip ratio, DBP, fasting glucose, 2-h glucose, fasting insulin, 2-h insulin, HOMA-IR, HOMA-β, HbA1c, ALT, AST, UA and TG (all P<0.01) and were negatively correlated with HDL-C (P<0.05). No obvious correlations were found between PEDF levels and the remaining metabolic parameters (Table 2).
 |
Table 2 Spearman Correlation Analysis of PEDF and Clinical Indices |
Factors Influencing Serum PEDF Levels
After adjusting for other metabolic indices (BMI, waist circumference, waist-to-hip ratio, DBP, fasting glucose, 2-h glucose, fasting insulin, 2-h insulin, HOMA-IR, HOMA-β, HbA1c, ALT, AST, UA, TG, HDL-C), multiple stepwise regression analysis showed that TG, ALT and HOMA-IR were independently correlated with serum PEDF (Table 3).
 |
Table 3 Stepwise Multivariate Regression Analysis of Serum PEDF Levels |
To assess whether estradiol is a potential independent contributor of serum PEDF, multiple linear regression analyses were performed among women (Table 4). In Model 1, women with higher estradiol levels had lower PEDF levels (standardized β=−0.25, P=0.011, Figure 2), and this correlation weakened but remained significant after adjusting for HOMIA-IR and TG levels (Model 2, standardized β= −0.179, P=0.037); however, after additional adjustment for ALT levels, the association between estradiol and PEDF levels was no longer obvious (Model 3, P=0.191).
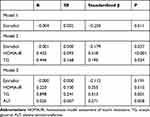 |
Table 4 Multivariate Regression Analysis of Serum PEDF Levels in Women |
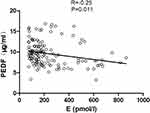 |
Figure 2 Correlation analysis of serum pigment epithelium-derived factor (PEDF) levels and estradiol (E) concentrations in women. |
Discussion
In this manuscript, we investigated the relationship between serum PEDF and MetS and explored the factors influencing circulating PEDF. Furthermore, we conducted a preliminary analysis of the causes of sex differences in PEDF. Our research revealed that serum PEDF levels were significantly increased in patients with MetS, and ALT, TG and HOMA-IR were independent risk factors for circulating PEDF and estrogens, which could be among the reasons for sex differences in serum PEDF levels.
Previous cell culture and animal model studies have shown controversial results about the role of PEDF in insulin resistance. PEDF therapy could improve insulin resistance in adipocytes and the liver but impair insulin sensitivity in skeletal muscles.8 Although the mechanisms underlying these distinct observations are not clear, many clinical studies have supported the use of PEDF as a biomarker of various metabolic disorders. Circulating PEDF levels in patients with MetS were significantly increased and were correlated with metabolic indicators, such as waist circumference, BMI, blood lipids, blood glucose, HOMO-IR and so on, consistent with the results of previous studies.10,11,15–17 Several biomarkers have already been proposed for MetS,35 and there is currently no single marker that can screen for or diagnose MetS. The test method of PEDF has proven to be sensitive and robust in different populations, as mentioned above. PEDF can be a marker of insulin resistance even in healthy young people without overt metabolic or vascular disease14 and could be useful in the prediction of MetS and cardiovascular outcomes,17,36 which might facilitate diagnosis of MetS earlier and proactively address MetS-related complications. However, there are still many problems, such as circulating PEDF levels, that can be affected by other conditions and pathologies. Different sexes, races, and ages should be considering when setting the diagnostic cut-off point, and there is still a long way to go to apply it to clinical practice.
In this study, ALT, TG and HOMA-IR were independent influencing factors of circulating PEDF levels. Unlike HOMA-IR and TG, which have been shown to be unequivocally associated with PEDF,10,11,37 a number of previous studies did not include ALT in their analysis and failed to find a correlation between PEDF and liver transaminases.10,15,17,22 Serum aminotransferase has been regarded as a marker of nonalcoholic fatty liver disease (NAFLD) for a long time, and the accuracy of ALT in identifying NAFLD is higher than that of AST.38 The liver is the main source of PEDF in the circulation,39 and clinical studies have shown that serum PEDF levels are significantly elevated in patients with biopsy-proven NAFLD, and the relationship between the two is independent of other metabolic factors.12 Some scholars believe that the reduction in PEDF levels can promote the uptake of fatty acids in the liver and the formation of lipid droplets, leading to the occurrence of NAFLD and indicating that PEDF plays an important role in the occurrence and development of hepatic steatosis.9 In light of the above findings, PEDF could become a potential biomarker and therapeutic agent for NAFLD, but it still requires further investigation.
Similar to MetS and its components, evidence has also shown sex-based differences in serum PEDF levels, which were significantly higher in men than in women;11,15,17,19,20 however, no sex difference was found in other studies.10,16,21,22 In the current research, our data indicated that men have higher PEDF levels than women, and this difference was no longer evident in subjects without MetS. The above findings suggested that the sex difference in PEDF could be related to the difference in the metabolic components of the study population.
When sex is factored into disease risk, it is well established that premenopausal women are relatively protected from MetS-related diseases compared with age-matched men.40 This “sex advantage” disappears after menopause, leading to the generally accepted conclusion that sex hormones, especially estrogens, protect against MetS. Therefore, it is not yet clear whether the reason for this sex dimorphism in PEDF is related to estrogen. In the present study, we found a significant correlation between estradiol and circulating PEDF, and this relationship persisted after adjusting for HOMA-IR and TG. However, the association was attenuated after adjusting for ALT. The prevalence and severity of NAFLD are higher in men than in women during the reproductive years; however, postmenopausal women have a higher incidence of NAFLD, and collective evidence has reflected that estrogen protects against NAFLD.41 In the present study, the effect of estradiol on serum PEDF could be corrected by ALT, indicating that the effect of estrogen on PEDF is likely achieved mainly through amelioration of hepatic steatosis. Consistent with our results, Moreno-Navarrete et al reported that circulating PEDF and liver PEDF gene expression significantly and positively correlated with ALT, and circulating PEDF levels might be derived from the liver rather than adipose tissue, in association with metabolically induced liver damage.39
There are still deficiencies of this study. First, the current study did not perform liver biopsy or even liver ultrasound to clarify the liver status of the research subjects. The indices of insulin resistance were not derived directly from glucose clamp techniques, which are the gold standard for the assessment of insulin sensitivity. The strengths of our study include its relatively large sample size and broad range of metabolic profiles. This research confirmed that the PEDF level in the blood is elevated in patients with MetS and might be useful as a biomarker for its diagnosis, and importantly, we expounded on the link among PEDF, estrogen and NAFLD for the first time. This finding provides a foundation for future research to investigate the regulation of estrogen regarding circulating PEDF, which might be able to explain the metabolic changes between pre- and postmenopausal women and the metabolic differences between men and women.
Conclusions
In the present study, we confirmed that serum PEDF concentrations could be used as a biomarker of MetS and its related complications. We reported for the first time that serum PEDF displays sexual dimorphism, which could be related to estrogen, and that the association between estrogen and circulating PEDF levels was attenuated after adjusting for ALT.
Abbreviations
PEDF, pigment epithelium-derived factor; MetS, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of insulin secretion; HbA1c, hemoglobin A1c; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; UA, uric acid; TG, triacylglycerol; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author.
Ethical Standard Statement
The study was approved by the Ethics Committee of Cangzhou Central Hospital and and was conducted in accordance with the Declaration of Helsinki. All subjects provided written informed consent.
Acknowledgments
We are very grateful to all participants for their support of this study.
Funding
This work was supported by the Key Research and Development Program Guidance Project of Cangzhou City (213106005).
Disclosure
The authors declare that they have no competing interests.
References
1. Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, et al. Geographic distribution of metabolic syndrome and its components in the general adult population: a meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. 2022;188:109924. doi:10.1016/j.diabres.2022.109924
2. Abooshahab R, Dass CR. The biological relevance of pigment epithelium-derived factor on the path from aging to age-related disease. Mech Ageing Dev. 2021;196:111478. doi:10.1016/j.mad.2021.111478
3. Michelis G, German OL, Villasmil R, et al. Pigment epithelium-derived factor (PEDF) and derived peptides promote survival and differentiation of photoreceptors and induce neurite-outgrowth in amacrine neurons. J Neurochem. 2021;159(5):840–856. doi:10.1111/jnc.15454
4. Zhao F, Fei W, Li Z, Yu H, Xi L. Pigment epithelium-derived factor-loaded PEGylated nanoparticles as a new antiangiogenic therapy for neovascularization. J Diabetes Res. 2022;2022:1193760. doi:10.1155/2022/1193760
5. Ma B, Zhou Y, Liu R, et al. Pigment epithelium-derived factor (PEDF) plays anti-inflammatory roles in the pathogenesis of dry eye disease. Ocul Surf. 2021;20:70–85. doi:10.1016/j.jtos.2020.12.007
6. Abooshahab R, Al-Salami H, Dass CR. The increasing role of pigment epithelium-derived factor in metastasis: from biological importance to a promising target. Biochem Pharmacol. 2021;193:114787. doi:10.1016/j.bcp.2021.114787
7. Qin X, Jia C, Liang J, et al. PEDF is an antifibrosis factor that inhibits the activation of fibroblasts in a bleomycin-induced pulmonary fibrosis rat model. Respir Res. 2022;23(1):100. doi:10.1186/s12931-022-02027-4
8. Yamagishi SI, Matsui T. Pigment epithelium-derived factor: a novel therapeutic target for cardiometabolic diseases and related complications. Curr Med Chem. 2018;25(13):1480–1500. doi:10.2174/0929867324666170608103140
9. Huang KT, Chen KD, Hsu LW, et al. Decreased PEDF promotes hepatic fatty acid uptake and lipid droplet formation in the pathogenesis of NAFLD. Nutrients. 2020;12(1):270. doi:10.3390/nu12010270
10. Wang F, Ma X, Zhou M, et al. Serum pigment epithelium-derived factor levels are independently correlated with the presence of coronary artery disease. Cardiovasc Diabetol. 2013;12:56. doi:10.1186/1475-2840-12-56
11. Choi KM, Hwang SY, Hong HC, et al. C1q/TNF-related protein-3 (CTRP-3) and pigment epithelium-derived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes. 2012;61(11):2932–2936. doi:10.2337/db12-0217
12. Yilmaz Y, Eren F, Ayyildiz T, et al. Serum pigment epithelium-derived factor levels are increased in patients with biopsy-proven nonalcoholic fatty liver disease and independently associated with liver steatosis. Clin Chimica Acta. 2011;412(23–24):2296–2299. doi:10.1016/j.cca.2011.08.025
13. Carnagarin R, Dharmarajan AM, Dass CR. PEDF-induced alteration of metabolism leading to insulin resistance. Mol Cell Endocrinol. 2015;401:98–104. doi:10.1016/j.mce.2014.11.006
14. Sunderland KL, Tryggestad JB, Wang JJ, et al. Pigment epithelium-derived factor (PEDF) varies with body composition and insulin resistance in healthy young people. J Clin Endocrinol Metab. 2012;97(11):E2114–2118. doi:10.1210/jc.2012-1894
15. Yamagishi S, Adachi H, Abe A, et al. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab. 2006;91(6):2447–2450. doi:10.1210/jc.2005-2654
16. Stejskal D, Karpísek M, Svesták M, Hejduk P, Sporová L, Kotolová H. Pigment epithelium-derived factor as a new marker of metabolic syndrome in Caucasian population. J Clin Lab Anal. 2010;24(1):17–19. doi:10.1002/jcla.20360
17. Chen C, Tso AW, Law LS, et al. Plasma level of pigment epithelium-derived factor is independently associated with the development of the metabolic syndrome in Chinese men: a 10-year prospective study. J Clin Endocrinol Metab. 2010;95(11):5074–5081. doi:10.1210/jc.2010-0727
18. Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6:14. doi:10.1186/s13293-015-0033-y
19. Hui E, Yeung CY, Lee PC, et al. Elevated circulating pigment epithelium-derived factor predicts the progression of diabetic nephropathy in patients with type 2 diabetes. J Clin Endocrinol Metab. 2014;99(11):E2169–E2177. doi:10.1210/jc.2014-2235
20. Toloza FJK, Pérez-Matos MC, Ricardo-Silgado ML, et al. Comparison of plasma pigment epithelium-derived factor (PEDF), retinol binding protein 4 (RBP-4), chitinase-3-like protein 1 (YKL-40) and brain-derived neurotrophic factor (BDNF) for the identification of insulin resistance. J Diabetes Complications. 2017;31(9):1423–1429. doi:10.1016/j.jdiacomp.2017.06.002
21. Höpfinger A, Berghoff M, Karrasch T, Schmid A, Schäffler A. Systematic quantification of neurotrophic adipokines RBP4, PEDF, and clusterin in human cerebrospinal fluid and serum. J Clin Endocrinol Metab. 2021;106(5):e2239–e2250. doi:10.1210/clinem/dgaa983
22. Fujikawa T, Ohara M, Kohata Y, et al. Glucose variability is independently correlated with serum level of pigment epithelium-derived factor in type 2 diabetes. Diabetes Ther. 2021;12(3):827–842. doi:10.1007/s13300-021-01008-y
23. Thomas MP, Potter BV. The structural biology of oestrogen metabolism. J Steroid Biochem Mol Biol. 2013;137:27–49. doi:10.1016/j.jsbmb.2012.12.014
24. Frederiksen H, Johannsen TH, Andersen SE, et al. Sex-specific estrogen levels and reference intervals from infancy to late adulthood determined by LC-MS/MS. J Clin Endocrinol Metab. 2020;105(3):754–768. doi:10.1210/clinem/dgz196
25. Cheung LW, Au SC, Cheung AN, et al. Pigment epithelium-derived factor is estrogen sensitive and inhibits the growth of human ovarian cancer and ovarian surface epithelial cells. Endocrinology. 2006;147(9):4179–4191. doi:10.1210/en.2006-0168
26. Chuderland D, Ben-Ami I, Kaplan-Kraicer R, et al. Hormonal regulation of pigment epithelium-derived factor (PEDF) in granulosa cells. Mol Hum Reprod. 2013;19(2):72–81. doi:10.1093/molehr/gas046
27. Chuderland D, Ben-Ami I, Friedler S, et al. Hormonal regulation of pigment epithelium-derived factor (PEDF) expression in the endometrium. Mol Cell Endocrinol. 2014;390(1–2):85–92. doi:10.1016/j.mce.2014.04.006
28. Jan R, Huang M, Lewis-Wambi J. Loss of pigment epithelium-derived factor: a novel mechanism for the development of endocrine resistance in breast cancer. Breast Cancer Res. 2012;14(6):R146. doi:10.1186/bcr3356
29. Li C, Yang H, Tong G, et al. Correlations between A1c, fasting glucose, 2h postload glucose, and β-cell function in the Chinese population. Acta Diabetol. 2014;51(4):601–608. doi:10.1007/s00592-014-0563-5
30. Low S, Pek S, Moh A, et al. Low muscle mass is associated with progression of chronic kidney disease and albuminuria - an 8-year longitudinal study in Asians with type 2 diabetes. Diabetes Res Clin Pract. 2021;174:108777. doi:10.1016/j.diabres.2021.108777
31. Sylvetsky AC, Issa NT, Chandran A, et al. Pigment epithelium-derived factor declines in response to an oral glucose load and is correlated with vitamin D and BMI but not diabetes status in children and young adults. Horm Res Paediatr. 2017;87(5):301–306. doi:10.1159/000466692
32. Karasek D, Spurna J, Kubickova V, et al. Association of pigment epithelium derived factor with von Willebrand factor and plasminogen activator inhibitor 1 in patients with type 2 diabetes. Physiol Res. 2019;68(3):409–418. doi:10.33549/physiolres.934013
33. Chinese Medical Association Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Diabetes Mellitus. 2021;13(4):315–409.
34. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi:10.1007/BF00280883
35. Hsiao YT, Shimizu I, Yoshida Y, Minamino T. Role of circulating molecules in age-related cardiovascular and metabolic disorders. Inflamm Regen. 2022;42(1):2. doi:10.1186/s41232-021-00187-2
36. Chen C, Tso AW, Cheung BM, et al. Plasma concentration of pigment epithelium-derived factor is closely associated with blood pressure and predicts incident hypertension in Chinese: a 10-year prospective study. Clin Endocrinol (Oxf). 2012;76(4):506–513. doi:10.1111/j.1365-2265.2011.04178.x
37. Gattu AK, Birkenfeld AL, Jornayvaz F, et al. Insulin resistance is associated with elevated serum pigment epithelium-derived factor (PEDF) levels in morbidly obese patients. Acta Diabetol. 2012;49(Suppl 1):S161–169. doi:10.1007/s00592-012-0397-y
38. Long MT, Pedley A, Colantonio LD, et al. Development and validation of the framingham steatosis index to identify persons with hepatic steatosis. Clin Gastroenterol Hepatol. 2016;14(8):1172–1180.e1172. doi:10.1016/j.cgh.2016.03.034
39. Moreno-Navarrete JM, Touskova V, Sabater M, et al. Liver, but not adipose tissue PEDF gene expression is associated with insulin resistance. Int J Obes. 2013;37(9):1230–1237. doi:10.1038/ijo.2012.223
40. Morselli E, Frank AP, Santos RS, Fátima LA, Palmer BF, Clegg DJ. Sex and gender: critical variables in pre-clinical and clinical medical research. Cell Metab. 2016;24(2):203–209. doi:10.1016/j.cmet.2016.07.017
41. Lonardo A, Nascimbeni F, Ballestri S, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):1457–1469. doi:10.1002/hep.30626
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
