Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Serum Pentosidine is Associated with Cardiac Dysfunction and Atherosclerosis in T2DM
Authors Cao Y, Ye X , Yuan X, Liu J, Zhang Q
Received 18 November 2022
Accepted for publication 17 January 2023
Published 26 January 2023 Volume 2023:16 Pages 237—244
DOI https://doi.org/10.2147/DMSO.S398119
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Yuyan Cao,1,* Xinhua Ye,1,* Xiaoqing Yuan,1 Juan Liu,1 Qing Zhang2
1Department of Endocrinology, The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University, Changzhou, People’s Republic of China; 2Changzhou Medical Center, The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University, Changzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Juan Liu; Qing Zhang, Email [email protected]; [email protected]
Aim: The purpose of this paper is to investigate the relationship between serum pentosidine levels and cardiac function and vascular disease in diabetic patients, and to provide a new reference indicator for the early detection of diabetic cardiovascular complications.
Materials and Methods: This was a cross-sectional study. One hundred and twenty-two patients with type 2 diabetes were grouped by LVEF quartiles to compare the differences between their clinical data and serum pentosidine levels. Also, the correlation between serum pentosidine and clinical indicators was assessed. The effect of serum pentosidine on cardiac function and vascular stiffness was analyzed by multiple stepwise regression.
Results: Serum pentosidine levels were higher in patients with LVEF ≤ 57%. Serum pentosidine levels were positively correlated with waist-to-hip ratio, hemoglobin, AIP, baPWV, LVESD, and ARD, and negatively correlated with LVEF. Low serum pentosidine was associated with increased LVESD; high pentosidine was significantly associated with increased ARD, high AIP and high baPWV.
Conclusion: The results suggest that serum pentosidine, a member of AGEs, may reflect cardiac remodeling and dysfunction as well as atherosclerosis.
Keywords: pentosidine, T2DM, cardiac dysfunction, atherosclerosis
Introduction
According to the 10th edition of IDF Diabetes Atlas released in 2021, it is not difficult to see that the overall number of diabetics is predicted to rise to 643 million (11.3%) by 2030 and 783 million (12.2%) by 2045.1 The clinical guidelines for the prevention and treatment of diabetes in the elderly in China released in January 2022 disclosed that there are approximately 260.4 million elderly people in China who are 60 years of age or older, with 78.13 million of them having diabetes.2 The active prevention and treatment of various complications in elderly diabetic patients and the improvement of their long-term survival quality are currently the focus of various studies. As one of the chronic complications of diabetes, the incidence rate of cardiovascular diseases (CVD) has increased significantly in recent years,3 but there are not many reliable markers to help with early detection.
Advanced glycation end-products (AGEs) are stable covalent adducts formed by the reaction of macromolecules such as proteins, lipids, nucleic acids with glucose, or other reducing monosaccharides under the action of non-enzymatic glycation reaction.4 In patients with diabetes, the accumulation of AGEs accelerates and participates in the occurrence of chronic complications of diabetes.5,6 It has also been shown that AGEs can contribute to cardiac and vascular dysfunction through a variety of pathways. However, because of the high heterogeneity of AGEs, there is no specific test for AGEs. Serum pentosidine, a member of AGEs, is significantly associated with AGEs in vivo and serum pentosidine levels can reflect AGEs levels.7 Therefore, we hypothesized that serum pentosidine concentrations may reflect the degree of cardiovascular disease in patients with Type 2 diabetes. In this study, we investigated the relationship between serum pentosidine and cardiac function and vascular disease in diabetic individuals, with the aim to provide a new reference indicator for the early detection of diabetic cardiovascular complications.
Materials and Methods
Subjects
Patients with Type 2 Diabetes Mellitus treated in the Department of Endocrinology of the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University from December 2020 to December 2021 were enrolled. These participants were hospitalized for complication screening and glucose-lowering regimen adjustment due to poor glycemic control. And finally, 122 patients were included according to the inclusion and exclusion criteria.
Inclusion Criteria
Patients meeting the diagnostic criteria for type 2 diabetes in the 2016 ADA guidelines; patients aging 45–65 years old.
Exclusion Criteria
Patients having a history of cardiovascular organic diseases such as heart valve disease; patients having previous macrovascular or microvascular complications of diabetes, including retinopathy, nephropathy, neuropathy, peripheral vascular disease, and stroke; pregnant women; patients suffering from malignant tumors, thyroid dysfunction, liver and kidney dysfunction, rheumatic immune diseases or major mental diseases and other serious complications.
All participants gave written informed consent in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of Changzhou Second People’s Hospital Affiliated with Nanjing Medical University (Approval date of Registry and the Registration No. of the study/trial: 1 July 2020, MR-32-21-013406).
Collection of Demographic and Laboratory Data
On the morning of the next day after admission, fasting peripheral blood samples were collected from each patient. The sex, age, body mass index, waist-hip ratio, and duration of diabetes were collected. We also collected the laboratory examination results of selected patients, including glucose 0 minutes (FBG), insulin 0 minutes (0 “INS), C-peptide 0 minutes (0” C-P), glycosylated hemoglobin (HbA1c), hemoglobin (HB), platelet count (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) γ- Glutamyl transpeptidase (γ- GT), serum albumin (ALB), serum urea nitrogen (BUN), serum creatinine (CR), serum uric acid (UA), serum triglyceride (TG), serum total cholesterol (TC), serum high-density lipoprotein (HDL-C), serum low-density lipoprotein (LDL-C), urine microalbumin/creatinine ratio (UA/CR), etc. Except for 2hPBG, all other indicators were obtained from blood samples collected after fasting for 8 hours and before taking hypoglycemic drugs.
The triglyceride-glucose (TyG) index=Ln(TG×FPG/2).8
Atherogenic index of plasma (AIP)=Lg(TG/HDL-C).9
HOMA-IR=(FINS×FPG)/22.5.
Detection of Serum Pentosidine Levels
We collected 2–3mL of patients’ fasting venous blood with a 5mL coagulation-promoting tube, centrifuged at 3000rpm at 4°C for 10 minutes, separated the upper serum, and stored it in the refrigerator at −80°C. The serum pentosidine level was measured with the ELISA Kit purchased by the Shanghai Tongwei company.
Evaluation of Cardiac Structure and Function
On the second day of admission, an experienced associate sonographer used the Y-video E9 system to perform an echocardiogram with the participants in the left lateral position and breathing quietly. The sonographer knew nothing else about the patient’s data. The observed parameters mainly include aortic root dimension (ARD), left atrial diameter (LAD), ventricular septal thickness (IVST), left ventricular end diastolic diameter (LVDD), left ventricular posterior wall thickness (LVPWT), left ventricular ejection fraction (LVEF) Left ventricular end systolic dimension (LVESD), etc.
Assessment of baPWV and ABI
After the subject was instructed to rest for 15 minutes, the examiner measured the blood pressure of the extremities using a fully automatic atherosclerometer (OMRON BP-203RPEIII). The instrument recorded the waveforms of the brachial and anterior tibial arteries and automatically calculated baPWV based on the transmission time and transmission distance and ABI based on the ratio of systolic pressure in the posterior tibial artery to that in the brachial artery.
Statistical Analysis
Patients were divided into four groups according to the quartile of the LVEF index. The measurement data are expressed by mean ± standard deviation; One-way analysis of variance (ANOVA) was used for comparison between groups; the Chi-square test was used to compare the count data between groups; Spearman correlation analysis was used to analyze the correlation between serum pentosidine and other clinical parameters. Multiple stepwise linear regression analysis was used to identify factors associated with echocardiographic parameters and arterial disease (AIP and baPWV). SPSS version 22.0 was used for statistical analysis. If the p-value <0.05, the difference is considered significant.
Results
Comparison of Clinical Characteristics in the Four Groups
One hundred and twenty-two participants were recruited and divided into four groups according to the quartile of the LVEF index, with 30, 30, 30, and 32 participants in each group. Table 1 shows the comparison of clinical characteristics between the study groups. The cut-off values of the quartile of LVEF index are LVEF > 63%, 61% < LVEF ≤ 63%, 57% < LVEF ≤ 61%, and LVEF ≤ 57%. The mean ± standard deviation of the LVEF index in the four groups are 65.80 ± 1.69, 62.23 ± 0.43, 59.30 ± 1.12, and 55.53 ± 1.67, respectively. Compared to quartiles 1 and 2, patients in quartile 4 had a greater waist-to-hip ratio, more visceral fat, higher triglyceride levels, higher Atherogenic index of plasma (AIP), higher triglyceride-glucose (TyG) index, and, of course, lower serum pentosidine levels. There were no differences in age, diabetes duration, medication, hepatic enzymes, creatinine, serum urea nitrogen, UA/CR, fT3, fT4, sTSH, and frequency of hypertension and hyperlipidemia among groups (Supplement Tables 1 and 2).
 |
Table 1 Comparison of Baseline Characteristics According to LVEF Quartiles |
Analysis of Serum Pentosidine and Related Clinical Parameters in Patients with T2DM
Serum pentosidine level was positively correlated with waist-hip ratio, hemoglobin, AIP, baPWV, LVESD, and ARD, and negatively correlated with LVEF (Table 2).
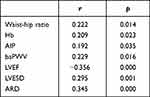 |
Table 2 Correlation Analysis of Serum Pentosidine and Clinical Test Results |
Influencing Factors of Echocardiographic Results in T2DM Patients
Table 3 summarizes the determinants of LVEF, LVESD, and ARD among the subjects. Stepwise regression analysis of age, BMI, waist-hip ratio, HOMA-IR, course of diabetes, fasting blood glucose, fasting insulin, serum pentosidine, Hb, PLT, ALT, AST, ALP, γ-GT, ALB, BUN, Cr, UA, TG, TC, HDL-C, LDL-C on cardiac ultrasound related parameters. Multivariable stepwise linear regression analysis showed that low serum pentosidine (p = 0.001), low BUN (p = 0.008), and low ALT (p = 0.038) were significantly correlated with high LVEF. High body weight (p = 0.000), high serum pentosidine (p = 0.000), low waist-hip ratio (p = 0.001), low triglycerides (p = 0.008) and low hemoglobin (p = 0.033) were associated with increased LVESD. High pentosidine (p = 0.009) and low 0’C-P (p = 0.047) were significantly correlated with the increase in ARD.
 |
Table 3 Determinants for Echocardiographic Parameters Using Multivariable Stepwise Linear Regression Analysis |
Influencing Factors of Atherosclerotic Indexes in Patients with T2DM
Table 4 lists the risk factors for atherosclerosis. In multiple stepwise linear regression analysis, high uric acid (p = 0.000), high FBG (p = 0.018), high serum pentosidine (p = 0.026), and high waist-hip ratio (p = 0.013) were significantly correlated with high AIP. High baPWV was significantly correlated with old age (p = 0.000), high diastolic blood pressure (p = 0.000), high pentosidine (p = 0.001), high uric acid (p = 0.003) and low alanine aminotransferase (p = 0.031).
 |
Table 4 Determinants for AIP, baPWV Using Multivariable Stepwise Linear Regression Analysis |
Conclusion
At present, heart failure (HF) has been considered a common complication of diabetes.10 The prevalence and incidence of diabetes is increasing. The data also show that diabetes patients without other basic diseases will also have heart failure, and heart failure may be the first manifestation of cardiovascular disease in many diabetes patients.11 The UK Prospective Diabetes Study (UKPDS) showed that during the 10-year follow-up period, the annual incidence rate was as high as 11.9/1000.12 So far, the evaluation of cardiac function in patients with type 2 diabetes mainly depends on echocardiography, and there is a lack of meaningful serological biomarkers. Therefore, starting with LVEF, which reflects cardiac function,13 this study found that serum pentosidine was significantly negatively correlated with LVEF, and proposed that serum pentosidine may become a serological indicator of early cardiac insufficiency in patients with diabetes, so as to further explore the correlation between serum pentosidine and cardiac function and arteriosclerosis in patients with type 2 diabetes.
AGEs are a kind of heterogeneous biological macromolecules produced by glucose, protein, lipid, and even nucleic acid through a series of non-enzymatic glycosylation reactions (ie, Maillard reaction).14,15 There are two sources of AGEs in human body: exogenous AGEs come from a daily high-calorie diet16 and endogenous AGEs come from Maillard reaction in the body.17 Diabetes patients with hyperglycemia are particularly prone to the formation of endogenous AGEs.18 After the generation of AGEs, it will damage the metabolism of lipids and proteins in cells.19 At the same time, AGEs interfere with cell signal transduction by promoting oxidative stress and producing pro-inflammatory factors and inflammatory mediators,20 which significantly affect the normal function of the body, especially insulin-mediated metabolic response.21 Serum pentosidine, a common glycosylation end-product, is positively correlated with AGEs and may represent serum accumulation of AGEs in vivo.
Previous studies have shown that AGEs may cause cardiac and vascular dysfunction in two ways: vascular stiffness and cardiac fibrosis through the cross-linking of elastin and collagen; inflammation and oxidative stress through AGER.22 Both in vivo and in vitro studies conducted by Zhong et al suggest that the endoplasmic reticulum stress induced by AGEs can mediate the activation of PERK/CaN/NFAT4c signal transduction in cardiac microvascular endothelial cells, and then trigger the transcription of targeted genes, leading to apoptosis, inflammation, and micro thrombosis, thereby exacerbating microvascular dysfunction in non-obstructive coronary artery disease.23 Liang B et al also found that the AGEs-RAGE axis mediates myocardial fibrosis by activating autophagy-induced cardiac fibroblasts in heart failure.24 Hoorn’s study showed that there was an association between the higher serum pentosidine level and serum total AGEs level and poor systolic function.25 A prospective study (community atherosclerosis risk study) explored the relationship between sRAGE and HF events, which concluded that lower levels of sRAGE were independently associated with the development of HF in the community population.26
It is reported that patients with metabolic syndrome without heart failure, myocardial infarction, and history of LVEF < 50% have subclinical left ventricular systolic dysfunction.27 In this study, we found that serum pentosidine levels were significantly negatively correlated with LVEF, suggesting that serum pentosidine accumulation in the body can cause subclinical left ventricular systolic dysfunction. At the same time, our results show that there is a significant positive correlation between serum pentosidine and LVESD, which proves that the increase of serum pentosidine can lead to ventricular remodeling, but other indicators of echocardiography, such as left atrial diameter and left ventricular end diastolic diameter, can be seen that Q1 group has no significant difference compared with Q3 and Q4 groups, but higher than Q2 group, so we further analyzed the reasons that may lead to this result, such as hypertension, hyperlipidemia, and smoking, We found that the proportion of smoking in Q1 group was significantly higher than that in other groups, which explained the reason for this result.28
Another important finding of this study is that serum pentosidine is positively correlated with AIP and baPWV. Previous studies have proved that AIP is a powerful and reliable predictor of coronary artery disease in patients with type 2 diabetes. High AIP levels are independently associated with the increased risk of rapid plaque progression.9,29,30 A 15-year cohort study shows that AIP is an independent factor in predicting cardiovascular events and related mortality.31 Similarly, as a powerful indicator of arteriosclerosis,32 baPWV has been proved to be closely related to the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) in the middle-aged and elderly population.33 Through multiple stepwise linear regression analysis, this study found that hemorrhagic pentosidine was significantly correlated with these two indicators. We speculated that the increase of serum pentosidine caused vascular inflammation and oxidative stress, affected the cross-linking of angioplasty protein and collagen, resulting in vascular sclerosis, and then caused cardiac insufficiency. This may become the research direction to reduce the occurrence of cardiovascular events in patients with type 2 diabetes in the future.
At present, there are few studies on the serum markers of cardiac function and vascular disease in patients with type 2 diabetes in the early stage. Therefore, we include patients with type 2 diabetes who have not undergone clinical changes in the early stage and introduce serum pentosidine, a member of AGEs, to explore its relationship with cardiac ultrasound parameters and indicators reflecting vascular hardness. However, this study is a single-center cross-sectional study, which may need to include more patients for prospective research in the future to further explore its relevance. At the same time, our current content is limited to clinical practice, and we have not yet discussed its in-depth mechanism, which will be our future research direction.
In conclusion, we proved the significant correlation between high serum pentosidine and high LVESD, low LVEF, high AIP, and high baPWV in this study. These results suggest that serum pentosidine, as a member of AGEs, may reflect cardiac remodeling, dysfunction, and atherosclerosis.
Data Sharing Statement
All datasets presented in this study are included in the article/Supplementary Material. Further enquiries can be directed to the corresponding author.
Ethics and Consent Statements
The study was approved by the ethics committee of Changzhou Second People’s Hospital Affiliated with Nanjing Medical University. Written informed consent was obtained from all the participants after a full explanation of the study. Approval date of Registry and the Registration No. of the study/trial: 1 July 2020, MR-32-21-013406.
Funding
This study was supported by grants from Changzhou Sci & Tech Program (No. CJ20210111), Young Talent Development Plan of Changzhou Health Commission (No. CZQM2020078) and “Mass Entrepreneurship and Innovation Plan” of Jiangsu Province (No. QT201919).
Disclosure
Yuyan Cao and Xinhua Ye are co-first authors for this study. The authors declare that they have no conflicts of interest for this work.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
2. Chinese Diabetes Society. 中国老年2型糖尿病防治临床指南(2022年版) [Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition)]. Zhonghua nei ke za zhi. 2022;61(1):12–50. Chinese. doi:10.3760/cma.j.cn112138-20211027-00751
3. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi:10.1016/j.jacc.2017.04.052
4. Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol. 2010;65(9):963–975. doi:10.1093/gerona/glq074
5. Bierhaus A, Hofmann MA, Ziegler R, et al. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovasc Res. 1998;37(3):586–600. doi:10.1016/S0008-6363(97)00233-2
6. Nin JW, Jorsal A, Ferreira I, et al. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes Care. 2011;34(2):442–447. doi:10.2337/dc10-1087
7. Kida Y, Saito M, Shinohara A, et al. Non-invasive skin autofluorescence, blood and urine assays of the advanced glycation end product (AGE) pentosidine as an indirect indicator of AGE content in human bone. BMC Musculoskelet Disord. 2019;20(1):627. doi:10.1186/s12891-019-3011-4
8. Alizargar J, Bai CH, Hsieh NC, et al. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19(1):8. doi:10.1186/s12933-019-0982-2
9. Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, et al. Atherogenic index of plasma: novel predictive biomarker for cardiovascular illnesses. Arch Med Res. 2019;50(5):285–294. doi:10.1016/j.arcmed.2019.08.009
10. Seferović PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(5):853–872. doi:10.1002/ejhf.1170
11. Klajda DM, Scott CG, Rodeheffer RJ, et al. Diabetes mellitus is an independent predictor for the development of heart failure. Mayo Clinic Proc. 2020;95(1):124–133. doi:10.1016/j.mayocp.2019.07.008
12. Hayes AJ, Leal J, Gray AM, et al. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–1933. doi:10.1007/s00125-013-2940-y
13. Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the heart failure society of America, heart failure association of the European Society of Cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure. J Card Fail. 2021;27:387–413. doi:10.1016/j.cardfail.2021.01.022
14. Vistoli G, De Maddis D, Cipak A, et al. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res. 2013;47:3–27. doi:10.3109/10715762.2013.815348
15. Shen CY, Lu CH, Wu CH, et al. The development of Maillard reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules. 2020;25(23). doi:10.3390/molecules25235591
16. Garay-Sevilla ME, Rojas A, Portero-Otin M, et al. Dietary AGEs as exogenous boosters of inflammation. Nutrients. 2021;13(8):2802. doi:10.3390/nu13082802
17. Sibbersen C, Johannsen M. Dicarbonyl derived post-translational modifications: chemistry bridging biology and aging-related disease. Essays Biochem. 2020;64(1):97–110. doi:10.1042/EBC20190057
18. Khalid M, Petroianu G, Adem A. Advanced glycation end products and diabetes mellitus: mechanisms and perspectives. Biomolecules. 2022;12(4):542. doi:10.3390/biom12040542
19. Menini S, Iacobini C, Ricci C, et al. The galectin-3/RAGE dyad modulates vascular osteogenesis in atherosclerosis. Cardiovasc Res. 2013;100(3):472–480. doi:10.1093/cvr/cvt206
20. Hu R, Wang MQ, Ni SH, et al. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-κB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur J Pharmacol. 2020;867:172797. doi:10.1016/j.ejphar.2019.172797
21. Chilelli NC, Faggian A, Favaretto F, et al. In vitro chronic glycation induces AGEs accumulation reducing insulin-stimulated glucose uptake and increasing GLP1R in adipocytes. American journal of physiology. Endocrinol Metab. 2021;320(5):E976–E988. doi:10.1152/ajpendo.00156.2020
22. Hirai T, Fujiyoshi K, Yamada S, et al. Advanced glycation end products are associated with diabetes status and physical functions in patients with cardiovascular disease. Nutrients. 2022;14(15):3032. doi:10.3390/nu14153032
23. Liu Z, Zhu H, Ma Y, et al. AGEs exacerbates coronary microvascular dysfunction in NoCAD by activating endoplasmic reticulum stress-mediated PERK signaling pathway. Metabolism. 2021;117:154710. doi:10.1016/j.metabol.2021.154710
24. Liang B, Zhou Z, Yang Z, et al. AGEs-RAGE axis mediates myocardial fibrosis via activation of cardiac fibroblasts induced by autophagy in heart failure. Exp Physiol. 2022;107(8):879–891. doi:10.1113/EP090042
25. Kremers S, Remmelzwaal S, Schalkwijk CG, et al. The role of serum and dietary advanced glycation endproducts in relation to cardiac function and structure: the Hoorn Study. NMCD. 2021;31(11):3167–3175. doi:10.1016/j.numecd.2021.07.020
26. Paradela-Dobarro B, Fernández-Trasancos Á, Bou-Teen D, et al. Evolution and bad prognostic value of advanced glycation end products after acute heart failure: relation with body composition. Cardiovasc Diabetol. 2017;16(1):115. doi:10.1186/s12933-017-0598-3
27. Bogdanović J, Ašanin M, Krljanac G, et al. Impact of acute hyperglycemia on layer-specific left ventricular strain in asymptomatic diabetic patients: an analysis based on two-dimensional speckle tracking echocardiography. Cardiovasc Diabetol. 2019;18(1):68. doi:10.1186/s12933-019-0876-3
28. Hu T, Gall SL, Widome R, et al. Childhood/adolescent smoking and adult smoking and cessation: the international childhood cardiovascular cohort (i3C) consortium. J Am Heart Assoc. 2020;9(7):e14381. doi:10.1161/JAHA.119.014381
29. Wang L, Chen F, Xiaoqi C, et al. Atherogenic index of plasma is an independent risk factor for coronary artery disease and a higher SYNTAX score. Angiology. 2021;72(2):181–186. doi:10.1177/0003319720949804
30. Won KB, Heo R, Park HB, et al. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis. 2021;324:46–51. doi:10.1016/j.atherosclerosis.2021.03.009
31. Sadeghi M, Heshmat-Ghahdarijani K, Talaei M, et al. The predictive value of atherogenic index of plasma in the prediction of cardiovascular events; a fifteen-year cohort study. Adv Med Sci. 2021;66(2):418–423. doi:10.1016/j.advms.2021.09.003
32. Zheng M, Zhang X, Chen S, et al. Arterial stiffness preceding diabetes: a longitudinal study. Circ Res. 2020;127(12):1491–1498. doi:10.1161/CIRCRESAHA.120.317950
33. Wang H, Wu X, Gu Y, et al. Relationship of noninvasive assessment of arterial stiffness with 10-year Atherosclerotic Cardiovascular Disease (ASCVD) risk in a general middle-age and elderly population. Int J Gen Med. 2021;14:6379–6387. doi:10.2147/IJGM.S330142
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
