Back to Journals » International Journal of General Medicine » Volume 15
Serum NOX4 as a Promising Prognostic Biomarker in Association with 90-Day Outcome of Severe Traumatic Brain Injury
Authors Jiang F, Chen Z, Hu J, Liu Q
Received 12 March 2022
Accepted for publication 24 May 2022
Published 30 May 2022 Volume 2022:15 Pages 5307—5317
DOI https://doi.org/10.2147/IJGM.S366170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Feng Jiang,1,2 Zhicheng Chen,1,2 Jiemiao Hu,1,2 Qianzhi Liu1,2
1Department of Neurosurgery, Ningbo Hangzhou Bay Hospital, Ningbo, 315336, People’s Republic of China; 2Department of Neurosurgery, Ningbo Branch, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Ningbo, 315336, People’s Republic of China
Correspondence: Jiemiao Hu, Department of Neurosurgery, Ningbo Hangzhou Bay Hospital, 1155 Binhai Second Road, Ningbo, 315336, People’s Republic of China, Tel +86 574 58981155, Email [email protected]
Purpose: Nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4) is related to brain oxidative stress. We attempted to examine the association between serum NOX4 levels, severity and prognosis of severe traumatic brain injury (sTBI).
Methods: We measured serum NOX4 levels in 105 patients with sTBI. Trauma severity was assessed using Glasgow coma scale (GCS) and Rotterdam computed tomography (CT) classification. Study outcome data on death and worst outcome (Glasgow outcome scale score of 1– 3) were collected at 90 days after trauma. Multivariate analyses were performed to determine independent factors for overall survival and worst outcome. Area under receiver operating characteristic curve (AUC) was estimated to assess prognostic predictive ability.
Results: Serum NOX4 levels were tightly correlated with GCS score (t=− 5.843, P < 0.001) and Rotterdam CT score (t = 4.231, P < 0.001). During 90 days of follow-up, 50 participants (47.6%) experienced a worse outcome, 28 (26.7%) died and the mean overall survival time was 71.9 days (95% confidence interval (CI), 65.7– 78.1 days). Serum NOX4 was independently associated with an increased risk of short overall survival (hazard ratio, 1.129; 95% CI, 1.039– 1.228) or worse outcome (odds ratio, 1.053; 95% CI, 1.014– 1.095). Serum NOX4 levels had a certain predictive value for the patient’s risk of mortality (AUC, 0.803; 95% CI, 0.714– 0.874) or worse outcome (AUC, 0.780; 95% CI, 0.689– 0.855). Moreover, its AUC was in the range of GCS score and Rotterdam CT score (both P > 0.05) and it significantly improved their AUCs (both P < 0.05).
Conclusion: Serum NOX4 levels in the acute phase of sTBI were associated with trauma severity, an increased risk of mortality and worse outcome, suggesting that serum NOX4 could be an important prognostic factor for sTBI.
Keywords: traumatic brain injury, biomarker, severity, NOX4, prognosis
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability globally.1 Secondary brain injury is a key player during the pathophysiological process after TBI.2 Oxidative stress, inflammation, excitatory amino acid toxicity and neuronal apoptosis play important roles in this complex cascade of secondary injury mechanisms and prominently contribute to neurovascular dysfunction.3,4 Glasgow Coma Scale (GCS) and Rotterdam computed tomography (CT) classification have been frequently used to assess the clinical and radiological severity of TBI.5,6 Over the past several decades, biomarkers have drawn researchers’ interests with respect to severity assessment and prognosis prediction, in order to improve the prognostic predictive ability of GCS or Rotterdam CT classification.7,8 Clearly, some biomarkers, such as S100B, glial fibrillary acidic protein, ubiquitin carboxyl-terminal hydrolase L1, myelin basic protein and neuron-specific enolase, have been confirmed to be highly related to trauma severity and clinical outcomes after TBI.9–12 However, circulating levels of such biomarkers have not been routinely measured for clinical service. Therefore, clinical investigation of different biochemical markers is still under way for severity and prognosis analysis of TBI.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) are a group of eukaryotic flavoenzymes that catalyze the reduction of dioxygen to the superoxide anion using electrons provided by NADPH.13 There are seven NOX isoenzymes, including NOX1, 2, 3, 4, 5 and dual oxidase 1, 2.14 NOX4 is a major subtype of NOX family and may be responsible for the majority of brain oxidative stress after acute brain injury.15 NOX4 exists in neurons and glial cells, as well as its expression was greatly enhanced in injured brain tissues.16 Clearly, experimental data have shown that NOX4 may exert a detrimental effect on acute brain injury and inhibition of NOX4 may lessen acute brain injury by attenuating oxidative stress.17 Alternatively, elevated brain NOX4 expressions were intimately correlated with increasing severity indicated by decreasing GCS score, but not with prognosis reflected by Glasgow outcome scale (GOS) score after human TBI.18 However, the insignificant association of brain NOX4 expression levels with prognosis may be caused by a small sample size (only 20 TBI patients). Of note, in a recent clinical study of 165 aneurysmal subarachnoid hemorrhage patients, elevated serum NOX4 levels were highly correlated with hemorrhagic severity, and were independently associated with delayed cerebral ischemia and long-term poor outcome.19 Hence, it has been postulated that NOX4 might be a potential prognostic biomarker for diseases with acute brain injury. In the current study, we intended to discern whether serum NOX4 levels were related to trauma severity and long-term clinical outcome among a cohort of patients with severe TBI (sTBI).
Methods
Patients
In this prospective cohort study performed at Ningbo Hangzhou Bay Hospital (Ningbo, China) from April 2017 to April 2020, we first assessed adult (age ≥18 years) patients with sTBI (post-resuscitation GCS score of 8 or less), who had injury severity score in non-cranial aspects below 9 and were hospitalized within post-traumatic twelve hours. Afterwards, we excluded those patients with known previous inflammatory or immunological diseases, severe heart, liver, lung or kidney diseases, prior neurological diseases (eg, hemorrhagic or ischemic stroke, brain tumors and sTBI) or malignant diseases. The study was done according to the principles of the Declaration of Helsinki, and approval for the study protocol was obtained from the ethics committee at Ningbo Hangzhou Bay Hospital (opinion number: 2017014). Informed consent was written by patients’ relatives.
Variables
We collected some information, including demographics (age and gender), adverse life habits (cigarette smoking and alcohol drinking), time parameters (time from trauma to admission and from trauma to blood-collection) and previous medical illnesses (hypertension, diabetes mellitus and hyperlipidemia). Clinical and radiological indicators of trauma severity were the GCS and Rotterdam CT classification, respectively. The recorded positive radiological appearances included skull-cap fracture, skull-base fracture, epidural hematoma, subdural hematoma, subarachnoid hemorrhage, intraventricular hemorrhage, cerebral hematoma, brain contusion, pneumocephalus, abnormal cistern and midline shift. By telephone, we obtained information regarding GOS score at 90 days after head trauma. The outcome parameters were death and worst outcome (GOS score of 1–3).
Immune Analysis
Peripheral venous blood was drawn once patients arrived at the emergency department. Blood glucose was measured using the hexokinase/glucose-6-phosphate dehydrogenase method. Serum C-reactive protein levels were quantified using a particle-enhanced immunonephelometry assay. For the determination of serum NOX4 levels, after blood centrifugation, the aliquots of the samples were immediately stored −80℃ until assayed. Serum NOX4 levels were determined in duplicate with a quantitative sandwich enzyme-linked immunosorbent assay kit (MyBiosource, USA) in accordance with the manufacturers' instructions. Two measurements were averaged for the final analysis. All determinations were performed by the same technician blinded to the clinical information.
Statistical Analysis
Data were analyzed using the SPSS 20.0 statistical package (SPSS Inc., Chicago, Illinois, USA), except receiver operating characteristic (ROC) curve analysis, where statistical analysis was done using MedCalc statistical software version 17.4 (MedCalc Software, Mariakerke, Belgium). Data were presented as means (standard deviations) for normally distributed quantitative variables, medians (upper – lower quartiles) for non-normally distributed quantitative variables and counts (percentages) for qualitative variables. Comparisons between the two groups were performed using the t-test, Mann–Whitney test, the Pearson’s chi-square test or Fisher’s exact test as appropriate. Comparisons among multiple groups were carried out using the Kruskal–Wallis H-test. The overall survival time between the two groups was compared using the Log rank test. The ROC curve was plotted to analyze the prognostic predictive value. The prognostic predictive ability was assessed using area under the ROC curve (AUC) with the corresponding 95% confidence interval (CI). A combined logistic regression model was configured to analyze the additive effect of serum NOX4 levels to GCS score and Rotterdam CT classification score for predicting prognosis. Comparisons of AUCs were done using Z test. Two multivariable models, namely the multivariate binary logistic regression model and multivariate Cox’s proportional hazard model, which included significant variables in univariate analyses, were built to determine independent predictors for 90-day worst outcomes and overall survival. The results were reported as odds ratio (95% CI) and hazard ratio (95% CI), respectively. Bivariate correlations were determined using the Spearman correlation coefficient test. Also, the multivariate linear regression model was established to find variables, which were independently correlated with serum NOX4 levels. For all tests, the two-sided p-values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
We initially assessed 146 patients with sTBI, who had isolated head trauma and were admitted within 12 hours after trauma. And then, we excluded 7 patients with known previous inflammatory, 4 with immunological diseases, 12 with severe heart, liver, lung or kidney diseases, 8 with prior neurological diseases (eg, hemorrhagic or ischemic stroke, brain tumors and sTBI), 4 with malignant diseases, 2 with loss to follow-up, 1 with refusal to participation and 3 with unavailable blood samples, and finally, a total of 41 patients were excluded and 105 eligible patients were included for further analysis. There were no significant differences in terms of age and gender between included patients and excluded patients (both P > 0.05).
A total of 105 sTBI patients were analyzed for their related data. There were 67 males and 38 females, and the ratio of male to female was 1.76. They had a mean age of 46.8 years (standard deviation, 14.6 years; range, 18–77 years). There were 50 cigarette smokers, 59 alcohol smokers, 18 cases with hypertension, 12 coexisting with diabetes mellitus and 22 suffering from hyperlipidemia. Patients were admitted from 0.8 to 11.5 hours after trauma (median, 4.6 hours; upper-lower quartiles, 3.6–5.9 hours). Blood samples were collected from 1.2 to 17.5 hours (median, 6.9 hours; upper-lower quartiles, 6.6–8.7 hours). In regards to traumatic causes, there were automobile/motorcycle (50 cases), fall/jump (41 cases) and others (14 cases). GCS score ranged from 3 to 8, with a median value of 5 and upper-lower quartiles of 4–6. Median Rotterdam CT classification was 5 (range, 3–6; percentiles 25th-75th, 4–6). The positive radiological appearances included skull-cap fracture (70 cases), skull-base fracture (54 cases), epidural hematoma (48 cases), subdural hematoma (65 cases), intraventricular hemorrhage (10 cases), intracerebral hematoma (62 cases), brain contusion (60 cases), pneumocephalus (32 cases), abnormal cisterns (80 cases), midline shift >5 mm (74 cases) and traumatic subarachnoid hemorrhage (71 cases). The mean systolic and diastolic arterial pressures were 118.1 mmHg (standard deviation, 29.1 mmHg; range, 71–178 mmHg) and 75.8 mmHg (standard deviation, 21.4 mmHg; range, 45–114 mmHg), respectively. Totally, 68 patients underwent surgery within 24 hours after head trauma. Within 90 days after head trauma, 28 patients were dead, 50 patients experienced a worse outcome (GOS score 1–3), and the mean overall survival time was 71.9 days (95% CI, 65.7–78.1 days).
Serum NOX4 Levels and Trauma Severity
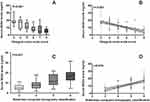
Serum NOX4 levels ranged from 1.4 to 31.5 ng/mL, and its median value was 10.8 ng/mL, as well as its 25th and 75th percentile values were 6.7 and 17.8, respectively. When patients were grouped based on GCS score, patients with GCS score 3 had the highest serum NOX4 levels and those with GCS score 8 showed the lowest serum NOX4 levels (Figure 1A). When GCS score was identified as a continuous variable, serum NOX4 levels were inversely correlated with GCS scores (Figure 1B). In addition, whether Rotterdam CT classification score was regarded as a categorical variable or as a continuous one, higher Rotterdam CT classification scores were, while higher serum NOX4 levels were (Figure 1C and D). In Table 1, besides GCS scores and Rotterdam CT classification score, blood glucose levels and serum C-reactive protein levels were tightly correlated with serum NOX4 levels. The above-mentioned four significantly correlated variables were forced into the multivariate linear regression model, and thereby it was revealed that serum NOX4 levels were independently correlated with GCS scores (t = −5.843, P < 0.001) and Rotterdam CT classification score (t = 4.231, P < 0.001).
 |
Table 1 Variables Correlated to Serum Nicotinamide Adenine Dinucleotide Phosphate Oxidase 4 Levels After Head Trauma |
Serum NOX4 Levels and 90-Day Worse Outcome
In Figure 2A and B, whether GOS score was identified as a categorical variable or a continuous variable, GOS score was highly correlated with serum NOX4 levels. In Figure 2C, serum NOX4 levels significantly differentiated between patients with a worse outcome and those not experiencing a worse outcome at 90 days after trauma; moreover, using maximum Youden’s index, an optimal threshold was selected, which predicted a worse outcome with medium–high sensitivity and specificity values. In addition, its prognostic predictive ability was similar to those of GCS score (AUC, 0.839; 95% CI, 0.754–0.903; P=0.202), and Rotterdam CT classification score (AUC, 0.761; 95% CI, 0.668–0.839; P = 0.707). Furthermore, serum NOX4 levels significantly improved AUCs of GCS score and Rotterdam CT classification to 0.879 (95% CI, 0.800–0.934; P = 0.040) and 0.826 (95% CI, 0.740–0.893; P = 0.030) respectively. In Table 2, some variables, namely age, GCS score, Rotterdam CT classification score, serum NOX4 levels, serum C-reactive protein levels and blood glucose levels, were tightly associated with a worse 90-day outcome after trauma. When the preceding variables were entered into the binary logistic regression model, it was found that GCS score (OR, 0.346; 95% CI, 0.177–0.677; P = 0.002), Rotterdam CT classification score (OR, 2.220; 95% CI, 1.229–4.010; P = 0.004) and serum NOX4 levels (OR, 1.053; 95% CI, 1.014–1.095; P = 0.008) were independently associated with a 90-day poor outcome after trauma.
 |
Table 2 Comparisons of Variables with Worse Outcome Within 90 Days Following Head Trauma |
Serum NOX4 Levels and 90-Day Overall Survival
Figure 2D displays that serum NOX4 levels were substantially higher in non-survivors than in survivors. Under the ROC curve (Figure 2E), serum NOX4 levels efficiently discriminated 90-day mortality. Alternatively, serum NOX4 levels >9.6 ng/mL predicted 90-day mortality with 92.9% sensitivity and 63.6% specificity. In addition, its death predictive ability was in range of GCS score (AUC, 0.819; 95% CI, 0.732–0.888; P = 0.722) and Rotterdam CT classification score (AUC, 0.762; 95% CI, 0.669–0.839; P = 0.402). Furthermore, serum NOX4 levels significantly enhanced AUCs of GCS score and Rotterdam CT classification to 0.865 (95% CI, 0.785–0.924; P = 0.034) and 0.836 (95% CI, 0.751–0.901; P = 0.016) respectively. Figure 2F shows that patients with serum NOX4 levels ≥9.6 ng/mL had significantly shorter overall survival time than the other remainders. In Table 3, age, GCS score, Rotterdam CT classification score, serum C-reactive protein levels, serum NOX4 levels and blood glucose levels were substantially related to 90-day overall survival using univariate Cox’s proportional hazard model. Furthermore, these significant variables were contained in multivariate Cox’s proportional hazard model and then, it was revealed that GCS score, Rotterdam CT classification score and serum NOX4 levels independently predicted 90-day overall survival with HR values of 0.584 (95% CI, 0.418–0.816), 1.825 (95% CI, 1.151–2.892) and 1.129 (95% CI, 1.039–1.228), respectively.
 |
Table 3 Factors Associated with Overall Survival During 90-Day Follow-Up Among Head Trauma Patients |
Discussion
TBI, especially sTBI, is the leading cause of death and disability in young adults worldwide.20 A complex cascade of secondary injury mechanisms follows the primary mechanical injury.21 These secondary pathological mechanisms involve edema, ischemia, neuroinflammation and hypoxia.22 Clearly, oxidative stress sufficiently contributes to secondary injury in TBI pathology.23 Oxidative stress poses a paramount role in the development of cerebral edema, inflammation, blood–brain barrier damage and secondary neuronal death after TBI.24 Reactive oxygen species (ROS) have a great detrimental effect on acute brain injury after TBI.25 Of the many enzymes that produce ROS in the cell, NOX is the only family of enzymes with the sole purpose of generating ROS.26 Thus, NOX may be detrimental and can even exacerbate the primary injury after TBI.
NOX4ʹs involvement in TBI has been recently reported. NOX4 has been revealed in neurons, astrocytes, and microglia following controlled cortical impact injury in mice.27 NOX4 mRNA was predominantly located in neurons in the injured cerebral cortex of adult male mice with TBI using Western blot analysis and double immunohistochemistry;28 Moreover, elevated NOX4 expression in cerebral cortex after TBI was correlated with increased oxidative stress damage to DNA, protein and lipids.28 Similarly, in blast-injured rats, elevated NOX4 expression was correlated with increased superoxide production.29 In addition, in the cerebral cortex from post-mortem human brains, NOX4 expression was found in neurons.18 Furthermore, the higher expression of NOX4 in the brain was correlated with the increasing severity of human TBI as determined by the GCS.18 In humans with an aneurysmal subarachnoid hemorrhage, serum NOX4 levels were highly correlated with hemorrhagic severity as indicated by the World Federation of Neurological Surgeons scale and modified Fisher grading scale.19 In our study, serum NOX4 levels were also closely correlated with trauma severity as reflected by GCS and Rotterdam CT classification. Hence, on the one hand, it is assumed that NOX4 may be implicated in secondary brain injury after TBI; on the other hand, it is presumed that serum NOX4 may be a potential biochemical variable that can aid in the assessment of trauma severity after sTBI.
GKT137831 (a specific inhibitor of Nox4) could substantially reduce neuronal death in rats with subarachnoid hemorrhage.30 Consistently, siRNA-mediated silencing of NOX4 suppressed neuronal apoptosis induced by oxygenate hemoglobin.30 After NOX4 knockdown, level of oxidative stress in the brain decreased considerably, neurobehavioral scores improved, levels of neuronal apoptosis reduced markedly, and impairment of blood–brain barrier function was significantly ameliorated in rats with intracerebral hemorrhage.31 Mice deficient in NOX4 were largely protected from oxidative stress, blood-brain-barrier leakage and neuronal apoptosis after both transient and permanent cerebral ischemia.32 In TBI, examination of NOX4−/− mice revealed a reduced number of apoptotic and degenerating cells in the perilesional cortex, as well as a smaller lesion size compared with the wild-type group.28 In a word, NOX4 may be a new therapeutic target in acute brain injury diseases, including TBI.
To the best of our knowledge, this study, for the first time, explored the relationship between serum NOX4 levels and disease severity plus long-term clinical outcome after sTBI. Our main findings are that the ascending serum NOX4 levels, in close correlation with declining GCS scores and increasing Rotterdam CT classification, were independently associated with 90-day death and 90-day poor outcome after sTBI. However, in a clinical study of only 20 TBI patients, there was an insignificant association of brain NOX4 expression levels with prognosis indicated by GOS score.18 This sort of insignificant prognosis relation may be caused by a small patient size. Notably, in a recent study of 165 patients with aneurysmal subarachnoid hemorrhage, serum NOX4 levels were revealed to be highly correlated with hemorrhagic severity, delayed cerebral ischemia and long-term functional outcome.19 Both our study and the preceding study19 showed that serum NOX4 levels had a marked prognostic predictive capability for long-term outcome after acute brain injury. Nevertheless, in that study regarding the relationship between serum NOX4 levels and prognosis after aneurysmal subarachnoid hemorrhage, AUCs were not compared between serum NOX4 and the World Federation of Neurological Surgeons scale plus modified Fisher grading scale for predicting prognosis after hemorrhagic stroke. In our study, we not only found that the prognostic predictive ability of serum NOX4 levels was equivalent to those of GCS score and Rotterdam CT classification score but also serum NOX4 levels substantially improved the prognostic predictive capabilities of GCS score and Rotterdam CT classification score under ROC curve. Such results may be further supportive of the notion that serum NOX4 could serve as a promising prognostic biomarker for sTBI.
sTBI is a fatal event with a poor outcome.1 Hence, having a way to predict outcome will be helpful for making decisions and necessary adjustments to care for the recovering patient. GOS and Rotterdam CT classification currently serve as the two most common prognostic predictors.5,6 Our study found a reproducible marker that would improve the prognostic predictive ability of GOS and Rotterdam CT classification. However, currently, the average turnaround time for laboratory results is between 30 and 40 minutes. Although measurement of serum NOX4 may currently not add much to the armamentarium of the clinician, it holds great potential to become more time efficient.
There are several limitations in this study. First, the current study only analyzed serum NOX4 for its relation to severity and prognosis after sTBI. However, whether other NOXs are associated with severity and prognosis after sTBI is unclear and, hence, further studies may be of clinical significance for analyzing other NOXs with respect to relations to severity and prognosis after sTBI. Second, TBI is a type of acute brain injury disease, and to the best of our knowledge, there is a great amount of literature available regarding circulating levels of other biomarkers at the acute time after TBI.7–12 Because a large portion of TBI patients often die within 1–2 weeks after head trauma,1–6 determination of circulating levels of biomarkers at the chronic time is not of large clinical value. Maybe, it is necessary to investigate serum NOX4 levels at the chronic time after TBI and further to analyze its association with clinical outcome. Third, the current study was designed to determine the relationship between serum NOX4 levels and trauma severity plus clinical outcome. Our data is supportive of the notion that serum NOX4 may serve as a promising prognostic biomarker for sTBI. A next study is warranted to set the control group for ascertaining dynamic changes of serum NOX4 levels after TBI. Finally, S100B, glial fibrillary acidic protein, ubiquitin carboxyl-terminal hydrolase L1, myelin basic protein and neuron-specific enolase are demonstrated to be potential prognostic biomarkers of TBI.9–12 The interesting finding in this study is that serum NOX4 levels combined with GCS score or Rotterdam CT score significantly improve their prognostic predictive ability alone. Therefore, the next work is to select a potential biomarker from the aforementioned biochemical markers, which can efficiently improve the prognostic ability when it is combined into the current model (namely, serum NOX4 levels combined with GCS score or Rotterdam CT score).
Conclusions
This is the first series for investigating the relationship between serum NOX4 levels and trauma severity, and long-term death plus functional outcome of sTBI. It is demonstrated that serum NOX4 levels are tightly correlated with trauma severity as indicated by GCS scores and Rotterdam CT classification, as well as are independently associated with poor long-term prognosis as reflected by 90-day mortality and poor functional outcome. Alternatively, the prognostic predictive ability of serum NOX4 levels is in the range of GCS score and Rotterdam CT score as well as, it also significantly improves the prognostic ability of GCS score and Rotterdam CT score for predicting 90-day death and poor outcome. Overall, serum NOX4 may represent a potential biomarker for assessing trauma severity and predicting long-term prognosis after sTBI.
Abbreviations
CT, computerized tomography; GCS, Glasgow Coma Scale; ROC, receiver operating characteristic; sTBI, severe traumatic brain injury; AUC, area under receiver operating characteristic curve; OR, odds ratio; HR, hazard ratio; NOX4, nicotinamide adenine dinucleotide phosphate oxidase 4; 95% CI, 95% confidence interval.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethical Review Approval
The study was done according to the principles of the Declaration of Helsinki, and approval for the study protocol was obtained from the ethics committee at Ningbo Hangzhou Bay Hospital (opinion number: 2017014).
Informed Consent
The written informed consent was acquired from relatives of patients.
Acknowledgments
The authors thank all participants for providing us with their clinical information and blood samples and for their willingness to participate in this study.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol. 2015;127:3–13.
2. Dixon KJ. Pathophysiology of traumatic brain injury. Phys Med Rehabil Clin N Am. 2017;28:215–225. doi:10.1016/j.pmr.2016.12.001
3. Khatri N, Thakur M, Pareek V, Kumar S, Sharma S, Datusalia AK. Oxidative stress: major threat in traumatic brain injury. CNS Neurol Disord Drug Targets. 2018;17(9):689–695. doi:10.2174/1871527317666180627120501
4. McGinn MJ, Povlishock JT. Pathophysiology of traumatic brain injury. Neurosurg Clin N Am. 2016;27(4):397–407. doi:10.1016/j.nec.2016.06.002
5. Reith FCM, Lingsma HF, Gabbe BJ, Lecky FE, Roberts I, Maas AIR. Differential effects of the Glasgow Coma Scale Score and its components: an analysis of 54,069 patients with traumatic brain injury. Injury. 2017;48:1932–1943. doi:10.1016/j.injury.2017.05.038
6. Munakomi S. A comparative study between Marshall and Rotterdam CT scores in predicting early deaths in patients with traumatic brain injury in a major tertiary care hospital in Nepal. Chin J Traumatol. 2016;19(1):25–27. doi:10.1016/j.cjtee.2015.12.005
7. Dong XQ, Yu WH, Du Q, et al. Serum periostin concentrations and outcomes after severe traumatic brain injury. Clin Chim Acta. 2017;471:298–303. doi:10.1016/j.cca.2017.06.020
8. Yan XJ, Li YB, Liu W, Wu HY, Yu GF. Elevated serum complement C1q levels after traumatic brain injury and its association with poor prognosis. Neuropsychiatr Dis Treat. 2022;18:47–55. doi:10.2147/NDT.S348682
9. Golden N, Mahadewa TGB, Aryanti C, Widyadharma IPE. S100B serum level as a mortality predictor for traumatic brain injury: a meta-analysis. Open Access Maced J Med Sci. 2018;6:2239–2244. doi:10.3889/oamjms.2018.432
10. Shemilt M, Boutin A, Lauzier F, et al. Prognostic value of glial fibrillary acidic protein in patients with moderate and severe traumatic brain injury: a systematic review and meta-analysis. Crit Care Med. 2019;47(6):e522–e529. doi:10.1097/CCM.0000000000003728
11. Anderson TN, Hwang J, Munar M, et al. Blood-based biomarkers for prediction of intracranial hemorrhage and outcome in patients with moderate or severe traumatic brain injury. J Trauma Acute Care Surg. 2020;89(1):80–86. doi:10.1097/TA.0000000000002706
12. Berger RP, Adelson PD, Pierce MC, Dulani T, Cassidy LD, Kochanek PM. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J Neurosurg. 2005;103:61–68. doi:10.3171/ped.2005.103.1.0061
13. Hou L, Zhang L, Hong JS, Zhang D, Zhao J, Wang Q. Nicotinamide adenine dinucleotide phosphate oxidase and neurodegenerative diseases: mechanisms and therapy. Antioxid Redox Signal. 2020;33:374–393. doi:10.1089/ars.2019.8014
14. Ma MW, Wang J, Dhandapani KM, Wang R, Brann DW. NADPH oxidases in traumatic brain injury - Promising therapeutic targets? Redox Biol. 2018;16:285–293. doi:10.1016/j.redox.2018.03.005
15. Dang PM, Rolas L, El-Benna J. The dual role of reactive oxygen species-generating nicotinamide adenine dinucleotide phosphate oxidases in gastrointestinal inflammation and therapeutic perspectives. Antioxid Redox Signal. 2020;33:354–373. doi:10.1089/ars.2020.8018
16. Tang XN, Cairns B, Kim JY, Yenari MA. NADPH oxidase in stroke and cerebrovascular disease. Neurol Res. 2012;34(4):338–345. doi:10.1179/1743132812Y.0000000021
17. Radermacher KA, Wingler K, Langhauser F, et al. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid Redox Signal. 2013;18(12):1418–1427. doi:10.1089/ars.2012.4797
18. Li Z, Tian F, Shao Z, et al. Expression and clinical significance of non-phagocytic cell oxidase 2 and 4 after human traumatic brain injury. Neurol Sci. 2015;36(1):61–71. doi:10.1007/s10072-014-1909-z
19. Pan J, Lao L, Shen J, et al. Utility of serum NOX4 as a potential prognostic biomarker for aneurysmal subarachnoid hemorrhage. Clin Chim Acta. 2021;517:9–14. doi:10.1016/j.cca.2021.02.007
20. Khellaf A, Khan DZ, Helmy A. Recent advances in traumatic brain injury. J Neurol. 2019;266(11):2878–2889. doi:10.1007/s00415-019-09541-4
21. Mckee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66.
22. Polich G, Iaccarino MA, Zafonte R. Psychopharmacology of traumatic brain injury. Handb Clin Neurol. 2019;165:253–267.
23. Karve IP, Taylor JM, Crack PJ. The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol. 2016;173(4):692–702. doi:10.1111/bph.13125
24. Kaur P, Sharma S. Recent advances in pathophysiology of traumatic brain injury. Curr Neuropharmacol. 2018;16:1224–1238. doi:10.2174/1570159X15666170613083606
25. Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72:355–362. doi:10.1001/jamaneurol.2014.3558
26. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi:10.1152/physrev.00044.2005
27. Cooney SJ, Bermudez-Sabogal SL, Byrnes KR. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J Neuroinflammation. 2013;10:155. doi:10.1186/1742-2094-10-155
28. Ma MW, Wang J, Dhandapani KM, Brann DW. Deletion of NADPH oxidase 4 reduces severity of traumatic brain injury. Free Radic Biol Med. 2018;117:66–75. doi:10.1016/j.freeradbiomed.2018.01.031
29. Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013;34:1397–1411. doi:10.1016/j.neurobiolaging.2012.11.013
30. Zhang L, Li Z, Feng D, et al. Involvement of Nox2 and Nox4 NADPH oxidases in early brain injury after subarachnoid hemorrhage. Free Radic Res. 2017;51:316–328. doi:10.1080/10715762.2017.1311015
31. Xie J, Hong E, Ding B, et al. Inhibition of NOX4/ROS suppresses neuronal and blood-brain barrier injury by attenuating oxidative stress after intracerebral hemorrhage. Front Cell Neurosci. 2020;14:578060. doi:10.3389/fncel.2020.578060
32. Kleinschnitz C, Grund H, Wingler K, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8(9):e1000479. doi:10.1371/journal.pbio.1000479
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.