Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Serum Lipid Profiles of Patients Taking Efavirenz-Based Antiretroviral Regimen Compared to Ritonavir-Boosted Atazanavir with an Optimized Background at Zewditu Memorial Hospital, Addis Ababa, Ethiopia
Authors Muche Belete A, Seifu D, Menon M, Amogne W , Shewa A, Adela Tefera A
Received 7 December 2020
Accepted for publication 11 February 2021
Published 19 February 2021 Volume 2021:13 Pages 217—227
DOI https://doi.org/10.2147/HIV.S296170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Abebe Muche Belete,1 Daniel Seifu,2 Menakath Menon,3 Wondwossen Amogne,4 Aster Shewa,5 Alemu Adela Tefera1
1Department of Biochemistry, Medical Faculty, Debre Berhan University, Debre Berhan, Ethiopia; 2Department of Biochemistry, Division of Biomedical Sciences, University of Global Health Equity, Kigali, Rwanda; 3Department of Biochemistry, Medical Faculty, Addis Ababa University, Addis Ababa, Ethiopia; 4Department of Internal Medicine, Medical Faculty, Addis Ababa University, Addis Ababa, Ethiopia; 5Department of Internal Medicine, Zewditu Memorial Hospital, Addis Ababa, Ethiopia
Correspondence: Abebe Muche Belete
Department of Biochemistry, Medical Faculty, Debre Berhan University, P.O. Box 445, Debre Berhan, Ethiopia
Email [email protected]
Background: Dyslipidemia represents significant health care concerns in patients taking antiretroviral therapy due to their association with cardiovascular disease risk. There is limited data regarding the effects of boosted atazanavir (ATV/r) treatment in the lipid profiles of Ethiopian HIV patients. Thus, this study compares the mean values of lipid profile differences of HIV patients on ATV/r-based regimen compared to efavirenz (EFV)-based regimen, while the background is Tenofovir Disoproxil Fumarate/lamivudine.
Materials and Methods: A comparative hospital-based cross-sectional study was conducted among adult HIV-infected patients at Zewditu Memorial Hospital, Addis Ababa, Ethiopia, from July–September 2017. An equal number of EFV and ATV/r-treated patients (n=90 each) receiving for 1-year and over were included in the study. Serum total cholesterol (TC), triglyceride (TG), gigh-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) were measured. Data comparison used chi-square test, Student’s t-test and Mann–Whitney U-test. Multivariate logistic regression analysis and p-value< 0.05 were used to identify associated factors of serum lipid profiles.
Results: In the present study, the ATV/r-treated group results were significantly higher in the median values of TG [207 (56– 1094) vs 145 (42– 768) mg/dL; p=0.001] and the mean value of TG/HDL-c (6.6 vs 4.4; p=0.001) as compared to the EFV-treated group. The EFV-treated group showed significantly higher in the mean value of HDL-c (44.7 vs 38.7 mg/dL; p=0.001) as compared to the ATV/r-treated group. Body mass index was associate with LDL and HDL. CD4 was associated with TC. Current antiretroviral therapy was associated with TG. Duration of HIV since first diagnosis and duration of ART were associated with HDL.
Conclusion: ATV/r is associated with elevated in TG and TG/HDL-C, but low HDL as compared to EFV. Differences in LDL or HDL that were found were of unclear clinical significance. The long-term significance is unknown.
Keywords: dyslipidemia, antiretroviral therapy, efavirenz, ritonavir-boosted atazanavir
Introduction
HIV patients taking antiretroviral drugs have been associated with a number of metabolic and anthropometric abnormalities including dyslipidemia, lipodystrophy, and insulin resistance,1 all of which may contribute to an increased risk of cardiovascular disease (CVD).2 Efavirenz (EFV) and ritonavir-boosted atazanavir (ATV/r) are two commonly used antiretroviral drugs.3
Compared to other protease inhibitors, atazanavir (ATV) has no adverse impact on cholesterol, triglyceride, and other metabolic parameters, including insulin and glucose.4 In addition, in patients who developed hyperlipidemia due to prior Highly Active Anti-Retroviral Therapy (HAART) regimens, ATV has been shown to decrease lipid concentrations to near normal levels.5,6 Switching to ATV results in a significant improvement in HIV therapy induced hyperlipidemia and a valuable option to improve atherogenic lipid profiles while maintaining virologic control.7 Patients receiving un-boosted ATV seemed to have a better lipid profile than those on boosted ATV.8
EFV is a beneficial effect in the protective high-density lipoprotein-cholesterol (HDL-C) and related to EFV plasma concentrations.9 Additionally, it was associated with an improvement in the atherogenic index of LDL-C/HDL-C or TC/HDL-C ratios. A prospective study showed that after 36 months of follow-up the mean level of HDL was 44 mg/dL. This EFV concentration-dependent change in lipid profile may suggest an EFV-specific beneficial effect and explains the association between HDL-C increase and adequate suppression of HIV-1 infection.9
HIV-1 infected patients who received EFV compared with ATV/r both in combination with abacavir (ABC)+Lamivudine (3TC) or Tenofovir Disoproxil Fumarate (TDF)+emitrctabin had significantly greater increased in all cholesterol levels but not in TC/HDL-C ratios.10 ATV was associated with less mean values of lipid profiles as compared to EFV.11
From Los Angeles, CA, a randomized, 2-arm study comparing the antiviral efficacy and safety between ATV and EFV both in combination with open-label fixed-dose AZT+3TC showed that ATV-treated patients relative to EFV-treated patients did not demonstrate significant increases in TC, LDL-C, or TG over 48 weeks of therapy.12
A study done in the US showed that both EFV and ATV/r are similar beneficial declines in the TC/HDL-C ratio. Both ATV/r and EFV users had increased in serum HDL-C and decreased in TC/HDL-C ratio. In comparison to individuals initiating EFV, ATV/r users on average had lower HDL-C and non-HDL-C, but similar declines in TC/HDL-C ratio. In addition, the average levels of TC was almost similar in both groups.13
In a randomized trial of antiretroviral-naïve patients initiated to ATV/r and EFV both in combination with TDF+emtricitabine over 48 weeks showed that EFV users had greater increased in mean differences in TC, LDL-C, and HDL-C, as compared to ATV/r users. No significant differences were found in mean ratios of TC/HDL-C between the two groups.14
Comparative trials between EFV, LPV/r, and ATV/r indicated that the risk for hypertriglyceridemia is lower with EFV than with the use of LPV/r, but there is a greater likelihood of hypercholesterolemia compared to ATV/r. However, in most cases, no change in the TC/HDL-C ratio was seen between the EFV and ATV/r groups.15
A pressing need remains to better quantify non-AIDS related co-morbidities in people living with HIV in Sub-Saharan Africa. Most studies of cardiovascular co-morbidities with HIV represent populations from developed countries. The treatment disparities, combined with differences in demographics, lifestyle, and nutritional status between Ethiopian and Western populations, may make this population more susceptible to non-AIDS-related co-morbidities.
In resource-limited settings, EFV and ATV/r remain cornerstones of ART. The comparative safety of regimens based on ATV/r and EFV are, however, incomplete. Most studies come from the developed world. Very limited comparative data exist for ATV/r and EFV, both used in combination with TDF and 3TC on serum lipid profiles.
With rapid scale up of ART and the increasing usage of antiretroviral drugs to prevent HIV, the need to monitor the side-effects of these drugs has increased substantially. HIV-infected patients are known to have an increased risk of CVD compared with the general population, with a significantly elevated mortality rate from cardiovascular events.16 Dyslipidemia represents significant health care concerns in HIV infected patients due to its direct association with increased CVD risk.17 Assessing of serum lipid profiles in HIV patients on ART helps in early prediction of CVD.
Therefore, the present study will compare the mean values of serum lipid profiles between EFV and ATV/r-treated adult HIV patients and assess associated factors of serum lipid profiles in the study area and give recommendations so as to minimize CVD morbidities and mortalities.
Materials and Methods
Study Period and Area
The study was conducted at ART Clinic of Zewditu Memorial Hospital (ZMH) from July 1–September 30, 2017. ZMH is under Addis Ababa City Administration Health Bureau. It is the first ART services delivery site (since July 2003) and has grown to become the largest HIV care and treatment site in the country. As of August 2017, the number of adults and children who on ART was 6,818. From this, 6,666 were adults greater than or equal to 15 years, of which 6,132 were on first-line ART and the remaining 534 were on second-line ART. From the first-line, 3,111 patients were on a TDF+3TC+EFV-based regimen and, from the second-line, 144 patients were on a TDF+3TC+ATV/r-based regimen.
Study Design
A hospital-based cross-sectional study design of comparative nature was conducted.
Source Population
All adult HIV-1 infected people receiving HAART at the ART Clinic of ZMH.
Study Population
All adult HIV-1 infected people who were on follow-up for at least 1 year on TDF/3TC/EFV and TDF/3TC/ATV/r-based regimens at the ART Clinic of ZMH.
Inclusion Criteria and Exclusion Criteria
Age greater than or equal to 18-years-old HIV-positive subjects on EFV- and ATV/r-containing regimens for at least 1 year prior to the study were recruited from ZMH in Addis Ababa, Ethiopia to participate in this study. Subjects were excluded if they had active AIDS defining illnesses, thyroid disorders, diabetes mellitus, known renal problems, thyroid problems, pregnancy, use of lipid-lowering agent or were on anti-tuberculosis drugs (record review). Subjects fasted and refrained from tobacco products for at least 8 hours prior to the test.
Sample Size Determination
The size of the study population that was recruited into the research was calculated using the G* power version 3.1 software by selecting t-test of means. Sample size was calculated by considering alpha=0.05, power (1-Beta)=0.9 (90%) with EFV- to ATV-based regimens ratio of 1:1 and effect size (d)=0.5. The total sample became 172. The number of participants enrolled into the study was 180 (90 for EFV-based group, and 90 for ATV/r-based group).
Sampling Procedure and Techniques
A convenience sampling method was applied; all consecutive EFV and ATV/r-treated individuals fulfilling the inclusion criteria and attending ZMH ART clinic during the study period were included until the required sample size was achieved.
Study Variables
Independent Variables
Age, sex, smoking status, alcohol use, physical exercise, CD4 count, duration on HAART, duration of HIV-infection since first diagnosis, and anthropometric indicators.
Dependent Variable
Serum TC, TG, LDL-C, and HDL-C level.
Data Collection and Procedure
Participant age, sex, date of first HIV-sero positive test, last CD4+ cell count, and any viral load determinations, and date of initiation of current and all previous HAART regimens were obtained from the participant’s hospital card. Questionnaire-driven interviews were performed by a trained nurse at the ZMH HIV clinic. Self-reported personal and familial history of heart attack, kidney disease, diabetes, or lipid disorders and self-reported alcohol and cigarette use were recorded.
Anthropometric Measurements
Body weight, body height, waist and hip circumference were measured. Weight was measured using a Tanita scale; patients were fully dressed, without heavy clothing or shoes, and height was determined without shoes using a portable stadiometer. Weight to the nearest 100 g and height to the nearest 1 mm were measured. Body mass index (BMI) was calculated by dividing weight (kg) by height (m2).18 Waist circumference was measured over light clothing at the level halfway between the iliac crest and the costal margin in the mid-axillary line after exhaling, when the lungs are at their functional residual capacity, with the subject in standing position with the body weight evenly distributed across the feet. Hip circumference was measured over light clothing at the level of greater trochanters with the subject in standing position and both feet together. The cut-off point considered for waist circumference (WC) was >80 cm for females and >90 cm for males to define overweight, the cut-off taken for waist-to-hip ratio was >0.8 for females and >0.9 for males as per the criterion of the WHO.19
Blood Sample Collection and Analysis
For determination of serum lipid profiles such as TC, TG, HDL-C, and LDL-C, after overnight fasting, 5 mL venous blood samples were collected from both groups by phlebotomy laboratory technician under aseptic conditions. Measurement of serum samples such as serum TC, TG, LDL-C, and HDL-C were assessed using calibrated fully automated Mind ray BS-200E, clinical chemistry analyzer (China) according to the reagent manufacturer’s instruction in central laboratory of ZMH.
Data Processing and Analysis
Data was checked, cleaned, and entered to Epi-data software version 3.1, and then exported to SPSS version 22.0 software for analysis. The results of the descriptive statistics were expressed as frequency and percentage. Chi-square (χ2) test was used to compare categorical variables. Continuous variables were presented as mean±standard deviation and median (interquartile range). Student’s t-test was performed to detect differences between groups on continuous variables that did show normality. While Mann–Whitney U-test was performed on continuous variables that did not show normality.
Univariate logistic regression was performed to examine the association of independent variables with TC, TG, LDL-C, and HDL-C for all study participants using crude odds ratios (ORs) with 95% confidence intervals (CI). Those independent variables with a p-value<0.2 in univariate analysis were included in the multivariable logistic regression models. P-value<0.05 on multivariable logistic regression was considered as a statistical significant association.
Results
General Characteristics of Study Participants
One hundred and eighty (90 EFV and 90 ATV/r) treated adult HIV-1 infected patients participated in this study. The mean age for EFV and ATV/r treated patients was 41.2±8.8 and 42.2±8.8 years old, respectively. Therefore, EFV and ATV/r treated adult HIV-1 infected patients of average age were similar (p=0.4). The number of females was 55/90 (61.1%) in the EFV-treated group and 52/90 (57.8%) in the ATV/r-treated group. So, the sex of EFV-treated patients was similar with the ATV/r-treated patients (p=0.7) (Table 1).
 |
Table 1 Baseline Characteristics of Study Participants at ART Clinic of ZMH, Addis Ababa, Ethiopia, 2018 |
The clinical features obtained from patients’ medical records showed that the median last CD4+ cell count was lower in the ATV/r-treated as compared to the EFV-treated group. Months since first HIV positive diagnosis were higher in the ATV/r-treated group as compared to the EFV-treated group. The ATV/r-treated group was on HAART longer than the EFV-treated group. Patients in the EFV-treated group have been on their current regimen longer than the ATV/r-treated group (Table 1). Considering history of hypertension and family history of lipid disorder, all of the study participants responded “no”. Considering smoking status, all of the 180 participants responded “no” for current smoking status. Self-reported treatment or drug adherence rate showed ≥95% or good drug adherence in both groups. In the present study, no differences were observed in the average BMI of the two groups.
Serum Lipid Levels of Study Participants
Fifty-two (28.9%) had elevated TC, 82 (45.6%) had reduced HDL, 71 (39.4%) had elevated TGs, and 90 (50%) had elevated LDL. There were significant differences in dyslipidemia between patients treated with EFV and ATV/r. TG ≥200 mg/dL was detected in 24/90 (26.7%) of the EFV-treated group and 47/90 (52.2%) of the ATV/r-treated group. HDL-C<40 mg/dL was detected in 35/90 (38.9%) of the EFV-treated group and 47/90 (52.2%) of the ATV/r-treated group. The ratio of TC/HDL-C≥5 was detected in 24/90 (26.7%) of the EFV-treated group and 29/90 (32.2%) of the ATV/r-treated group (Table 2).
 |
Table 2 Lipid Levels Among Ethiopian, Adult HIV Patients Stratified by EFV/ATV/r and Sex, ZMH, Addis Ababa, Ethiopia, 2018 |
There were significant differences in the mean value of HDL, TGs, and TG/HDL between patients treated with EFV and ATV/r. The mean value of HDL-c was elevated in EFV-treated subjects as compared to ATV/r-treated subjects (p=0.001). The mean value of TGs were elevated in ATV/r-treated subjects as compared to EFV-treated subjects (p=0.001). TG/HDL-C ratio was higher in the ATV/r-treated subjects as compared to EFV-treated subjects (p=0.008) (Table 2).
Considering sex, in female EFV- and ATV/r-treated adult HIV-1 infected patients, the TGs and HDL-c were statistically significantly different between the two groups. In addition, the results of the present study showed that in the serum of male EFV and ATV/r-treated HIV-1 infected patients, the mean values of LDL-C were statistically significantly different in the two groups (Table 2).
Associated Factors of Elevated Lipids
Independent variables which were entered to Bivariate logistic regression were sex, age, regular exercise, CD4 count, duration with HIV since first diagnosis, duration of taking ART, duration on the current ART, current ART, and BMI. Among them, CD4 count and BMI were significantly associated with TC. Sex, duration of ART, duration of the current ART, and current ART were significantly associated with TG (Table 3). BMI is associated with LDL. Duration of ART and BMI were associated with HDL (Table 4).
 |
Table 3 Univariate and Multivariate Logistic Regression Analysis of TC and TG with the Independent Variables, Adult HIV Patients at ZMH, Addis Ababa, Ethiopia, 2018 |
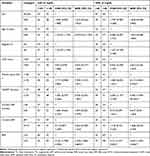 |
Table 4 Univariate and Multivariate Logistic Regression Analysis of LDL and HDL with the Independent Variables, Adult HIV Patients at ZMH, Addis Ababa, Ethiopia, 2018 |
In multivariable logistic analysis adjusted for various confounders, CD4 count was associated with elevated TC level. Those HIV patients who had low CD4 count was 2-times more likely to have elevated TC as compared with high CD4 count [AOR (95% CI)=2.227 (1.145–4.329)]. Sex and current ART were associated with TG. Female HIV patients were less likely to have elevated levels of TG as compared to males [AOR (95% CI)=0.269 (0.135–0.536)]. Additionally, HIV patients on current ART (TDF/3TC/EFV) were less likely to have elevated levels of TG as compared to HIV patients on TDF/3TC/ATV/r [AOR (95% CI)=0.387 (0.173–0.867)] (Table 3).
BMI was associated with LDL. Those patients who had BMI≥25 were 3-times more likely elevated LDL level as compared with patients who had BMI≤25 [AOR (95% CI)=3.128 (1.668–5.869)]. HIV since first diagnosis, duration of HAART, and BMI were associated with HDL. Patients on longer periods of time with the disease were less likely to have elevated HDL levels as compared to patients on short periods of time with the disease [AOR (95% CI)=0.213 (0.064–0.705)]. In addition, patients who were taking HAART for a long period of time were 6-times more likely to have reduced HDL level as compared to patients on HAART for a short period of time [AOR (95% CI)= 6.305 (1.939–20.502)]. Moreover, patients who had BMI≥25 are 2-times more likely to have elevated HDL levels as compared with BMI≤25 [AOR (95% CI)=2.632 (1.375–5.036)] (Table 4).
Discussion
Studies have shown that individual PIs have different effects on lipid metabolism. Both NNRTIs and NRTIs have low prevalence on inducing metabolic abnormalities compared to PIs. The present study compares EFV- and ATV/r-based regimens, both combined with TDF/3TC. Accordingly, we found a statistically significant difference between ATV/r- and EFV-treated groups in median TG values, mean values of HDL, and TG/HDL. For HDL, similar results were found in a randomized control trial that reported lower HDL-C in an ATV/r-treated group than in an EFV-treated group.12,13 Several confounding factors contribute to our observation. First, duration of current treatment with the EFV-treated group is nearly double that of the ATV/r-treated group. Second, CD4 count was higher in the EFV-treated group as compared to the ATV/r-treated group.
Available data also suggested that a long-term therapy with EFV and its concentration is directly proportional to HDL-C levels.9 In addition, genetic variation may also be attributed to the changes in HDL-C levels.20,21 The possible molecular mechanism by which EFV increase the HDL-C levels were through down-regulation of the activity of the plasma cholesterol transfer protein expression through antagonism of the lipid transcription factor.22
In the median value of TG there was also a significant difference between the EFV- and ATV/r-based regimens. HIV patients on ATV/r have elevated TG. The likely explanation of elevated levels of TG on the ATZ/r-treated group might be due to the addition of low dose ritonavir, which increases significantly serum or plasma TG levels.23 The study participants having TG≥200 mg/dL were higher in the ATV/r-treated group (52.2%) as compared to the EFV-treated group (26.7%). For the ATV/r-treated group, it was higher than the prevalence reported from the study conducted in Barcelona, Spain by Podzamczer et al24 that compared ATV/r to nevirapine and found 37.8% abnormal TG levels. This variation might be due to a difference in the duration of exposure to the treatment and some of the study participants in Podzamczer et al’s study were on lipid lowering drugs.
Even if there is no similar study regarding the TG/HDL-C ratio, it has greater predictive power than each of the single standard lipid parameters and superior to the other ratios in order to predict insulin resistance.25 Furthermore, a TG/HDL-C ratio of ≥3 has been shown to be closely correlated to insulin resistance.26 However, the capacity of TG/HDL-C to predict insulin resistance may vary by race.27,28
Even though there were no statistically significant differences, the mean values of TC and LDL-C were slightly higher in the EFV- than in the ATV/r-treated group. This is corroborated by the results of Ganesan et al13 and Squires et al.12 The ratio of TC/HDL-C and LDL-C/HDL-C was higher in the ATV/r- than the EFV-treated group. Other authors also found similar findings.14,15 TC/HDL-C and LDL-C/HDL-C ratios are indicators of CVD risk with greater predictive value than isolated parameters used independently.29
BMI is associated with LDL and HDL. CD4 is associated with TC. Current ART was associated with TG. Duration of HIV since first diagnosis and duration of ART were associated with HDL. Those patients who had low CD4 counts were 2-times more likely to have high TC as compared to patients who had a high CD4 count. In agreement with the results of this study, Belay et al30 and Kamoru et al31 reported a significantly positive correlation with CD4 among HAART patients. However, the current study was inconsistent with the study conducted in Malawi, in which there was no association between CD4 count and TC.32 This might be due to differences in socio-demographics.
Sex and current ART were significantly associated with TG. Females were less likely to have elevated TG levels as compared to males. Elevated levels of TG are a result of the combination of genetic and behavioral factors, and the TG levels are, in general, higher in males than in females.33 Our findings corroborate those of a previous study conducted in Brazil which showed that males are more likely to have hypertriglyceridemia than females.34 Moreover, HIV patients on a TDF/3TC/EFV-based ART regimen were less likely to have high values of TG as compared to a TDF/3TC/ATV/r based regimen. This might be due to the addition of ritonavir on ATV which increases TG levels.
Those patients who had BMI greater than 25 are more likely to have elevated levels of LDL as compared with their counterparts. This result was corroborated by a study done in Tanzania.35 HDL was associated with duration of HIV, duration of HAART, and BMI. Patients with longer times since first HIV diagnosis were more likely to have reduced HDL as compared to shorter times since first HIV diagnosis. Patients with a longer period of time on HAART are more likely to have a reduced level of HDL as compared with patients on a short period of time on HAART. Those patients who had a BMI greater than 25 were more likely to have a reduced HDL as compared with their counterparts. This was similar with the study conducted in Tanzania, which was conducted on HIV-naïve patients.35
Our study needs to be interpreted in the light of its limitations. The cross-sectional design precludes causal associations between dyslipidemia and patient characteristics. The study did not include a control group of HIV-uninfected persons which would have provided better insight into the role of HIV infection and antiretroviral drugs. There were baseline differences of the two groups on CD4 count, duration with HIV, duration on HAART, and duration on current treatment so it may be affected by the outcome variables. The sample size was also small.
Conclusion
Our research found elevated levels of mean TG and TG/HDL-C in ATV/r while HDL-C was higher in EFV than ATV/r-treated participants. Even though there were no statistically significant differences, the mean values of TC and LDL were slightly higher in the EFV- than in the ATV/r-treated group. The ratio of TC/HDL and LDL/HDL was higher in the ATV/r- than the EFV-treated group. Differences in LDL or HDL that they found are of unclear clinical significance. Their long-term significance is unknown.
BMI was associated with LDL and HDL. CD4 was associated with TC. Current ART was associated with TG. The duration of HIV since first diagnosis and duration of ART were associated with HDL.
Abbreviations
3TC, lamivudine; AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; ATV, atazanavir; ATV/r, ritonavir-boosted atazanavir; BMI, body mass index; CD4, cluster of differentiation four; CVD, cardiovascular disease; EFV, efavirenz; HAART, highly active antiretroviral therapy; HDL-C, high-density lipoprotein-cholesterol; HIV, human immune deficiency virus; LDL-C, low-density lipoprotein-cholesterol; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; SPSS, Statistical Package for Social Sciences; TDF, tenofovir disoproxil fumarate; TG, triglyceride; TC, total cholesterol; TC/HDL-C, total cholesterol/high-density lipoprotein-cholesterol; TG/HDL-C, triglyceride/high-density lipoprotein-cholesterol; WHO, World Health Organization; ZMH, Zewditu Memorial Hospital.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. An ethical clearance letter was obtained from the Departmental Research and Ethics Review Committee, Department of Biochemistry, College of Health Sciences, Addis Ababa University. The Ethical Review Committee of Department of Biochemistry, Addis Ababa University approved the informed verbal consent process for patients above the age of 18. The ethical review committee didn’t approve patients under the age of 18 because there were no patients under the age of 18. Additionally, parental informed consent was not obtained because there were no patients under the age of 18. A collaboration letter for data collection was also obtained from ZMH.
The objective of the study was briefly clarified and explained for each participant, before enrolling any of the eligible study participants. Samples and data were collected after informed consent had been obtained from the study participants. To assure confidentiality, a code number was used instead of the participants’ name or identification number.
Acknowledgment
We would like to acknowledge the support from Addis Ababa University, study participants and staff members of ZMH.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The funders had no role in the design of study, data collection and analysis, and interpretation of data and in writing the manuscript.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Tesfaye DY, Kinde S, Medhin G, et al. Burden of metabolic syndrome among HIV-infected patients in Southern Ethiopia. Diabetes Metab Syndr. 2014;8(2):102–107. doi:10.1016/j.dsx.2014.04.008
2. Altizani G, de Oliveira L, Dos Santos Tortajada J, Monteleone V, Bonafe S. Management of cardiovascular and metabolic alterations in HIV positive patients. Int J AIDS Res. 2016;3(7):105–113.
3. Fontas E, Van Leth F, Sabin C, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis. 2004;189(6):1056–1074. doi:10.1086/381783
4. Gatell J, Ceron DS, Lazzarin A, et al. Efficacy and safety of atazanavir-based highly active antiretroviral therapy in patients with virologic suppression switched from a stable, boosted or unboosted protease inhibitor treatment regimen: the SWAN Study (AI424-097) 48-week results. Clin Infect Dis. 2007;44(11):1484–1492. doi:10.1086/517497
5. Achenbach CJ, Darin KM, Murphy RL, Katlama C. Atazanavir/ritonavir-based combination antiretroviral therapy for treatment of HIV-1 infection in adults. Future Virol. 2011;6(2):157–177. doi:10.2217/fvl.10.89
6. Havlir DV, O’marro SD. Atazanavir: new option for treatment of HIV infection. Clin Infect Dis. 2004;38(11):1599–1604. doi:10.1086/420932
7. Ulrike M, Margrit L-R, Brigitte C-F, et al. Switching to atazanavir improves metabolic disorders in antiretroviral-experienced patients with severe hyperlipidemia. J AIDS. 2005;39(2):1–7.
8. Giuntini R, Martinelli C, Ricci E, et al. Efficacy and safety of boosted and unboosted atazanavir-containing antiretroviral regimens in real life: results from a multicentre cohort study. HIV Med. 2010;11:40–45. doi:10.1111/j.1468-1293.2009.00740.x
9. Pereira SA, Branco T, Côrte‐Real RM, et al. Long‐term and concentration‐dependent beneficial effect of efavirenz on HDL‐cholesterol in HIV‐infected patients. Br J Clin Pharmacol. 2006;61(5):601–604. doi:10.1111/j.1365-2125.2006.02619.x
10. Daar ES, Tierney C, Fischl MA, Sax PE, Mollan K, Collier AC. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1: a randomized trial. Ann Intern Med. 2011;154:445–456. doi:10.7326/0003-4819-154-7-201104050-00316
11. Jemsek JG, Arathoon E, Arlotti M, et al. Body fat and other metabolic effects of atazanavir and efavirenz, each administered in combination with zidovudine plus lamivudine, in antiretroviral-naive HIV-infected patients. Clin Infect Dis. 2006;42:273–280. doi:10.1086/498505
12. Squires K, Lazzarin A, Gatell JM, et al. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004;36(5):1011–1019. doi:10.1097/00126334-200408150-00003
13. Ganesan A, Benning L, Golub ET, et al. Serum lipid profiles among patients initiating ritonavir-boosted atazanavir versus efavirenz-based regimens. AIDS Res and Therapy. 2009;6(13):1–7. doi:10.1186/1742-6405-6-13
14. Gotti D, Cesana BM, Albini L, et al. Increase in standard cholesterol and large HDL particle subclasses in antiretroviral-naïve patients prescribed efavirenz compared to atazanavir/ritonavir. HIV Clin Trials. 2012;13(5):245–255. doi:10.1310/hct1305-245
15. Sension M, Deckx H. Lipid metabolism and lipodystrophy in HIV-1 infected patients: the role played by nonnucleoside reverse transcriptase inhibitors. AIDS Rev. 2015;17:21–36.
16. Chow D, Shikuma C, Ritchings C, Guo M, Rosenblatt L. Atazanavir and cardiovascular risk among human immunodeficiency virus-infected patients: a systematic review. Infect Dis Ther. 2016;5:473–489. doi:10.1007/s40121-016-0132-z
17. Husain NEO, Ahmed MH. Managing dyslipidemia in HIV/AIDS patients: challenges and solutions. HIV/AIDS – Res Palliative Care. 2015;7:1–10.
18. Organization WH. Obesity: Preventing and Managing the Global Epidemic. World Health Organization; 2000.
19. Organization WH. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008. 2011.
20. El Hadri K, Glorian M, Monsempes C, et al. In vitro suppression of the lipogenic pathway by the nonnucleoside reverse transcriptase inhibitor efavirenz in 3T3 and human preadipocytes or adipocytes. J Biol Chem. 2004;279(15):15130–15141. doi:10.1074/jbc.M312875200
21. Tarr PE, Taffé P, Bleiber G, et al. Modeling the influence of APOC3, APOE and TNF polymorphisms on the risk of antiretroviral therapy–associated lipid disorders. J Infect Dis. 2005;191(9):1419–1426. doi:10.1086/429295
22. Flint O, Bellamine A, Noor M, van der Hoorn J, Princen H, Parker R. Effects of efavirenz on lipid metabolism in APOE* 3* Leiden hCETP double-transgenic mice: evidence for antagonism of LXR pathway. Antivir Ther. 2007;12:L5.
23. Danner SA, Carr A, Leonard JM, et al. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. N Engl J Med. 1995;333(23):1528–1534. doi:10.1056/NEJM199512073332303
24. Podzamczer D, Andrade‐Villanueva J, Clotet B, et al. Lipid profiles for nevirapine vs atazanavir/ritonavir, both combined with tenofovir disoproxil fumarate and emtricitabine over 48 weeks, in treatment-naïve HIV-1-infected patients (the ARTEN Study). HIV Med. 2011;12(6):374–382.
25. Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13(1):146. doi:10.1186/s12933-014-0146-3
26. Iwani NAKZ, Jalaludin MY, Zin RMWM, et al. Triglyceride to HDL-C ratio is associated with insulin resistance in overweight and obese children. Sci Rep. 2017;7(1):1–7. doi:10.1038/srep40055
27. Knight MG, Goedecke JH, Ricks M, et al. The TG/HDL-C ratio does not predict insulin resistance in overweight women of African descent: a study of South African, African American and West African women. Ethn Dis. 2011;21(4):490.
28. Sumner AE, Zhou J, Doumatey A, et al. Low HDL-cholesterol with normal triglyceride levels is the most common lipid pattern in West Africans and African Americans with metabolic syndrome: implications for cardiovascular disease prevention. CVD Prev Control. 2010;5(3):75–80. doi:10.1016/j.cvdpc.2010.07.003
29. Millán J, Pintó X, Muñoz A, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757.
30. Belay E, Seifu D, Amogne W, Kibret K. Lipid profile derangements among human immunodeficiency virus infected adults receiving first line anti-retroviral therapy in Tikur Anbesa specialized hospital, Addis Ababa, Ethiopia: comparative cross-sectional study. J AIDS Clin Res. 2014;5:328.
31. Kamoru A, Japhet O, Adetunji A, et al. CD4+ cell count, lipid and lipoprotein levels in HIV patients on drug treatment. Int J AIDS Res. 2017;4:140–146.
32. Amberbir A, Singano V, Matengeni A, et al. Dyslipidemia among rural and urban HIV patients in south-east Malawi. PLoS One. 2018;13(5). doi:10.1371/journal.pone.0197728
33. Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Hypertriglyceridemia and its pharmacologic treatment among US adults. Arch Intern Med. 2009;169(6):572–578. doi:10.1001/archinternmed.2008.599
34. Mendicino CCP, Braga LP, CAMd P, Guimarães MDC. High incidence of hypertriglyceridemia in a Brazilian cohort of people living with HIV/AIDS undergoing antiretroviral treatment in Belo Horizonte, 2001–2010. Rev Soc Bras Med Trop. 2016;49(6):758–762. doi:10.1590/0037-8682-0078-2016
35. Armstrong C, Liu E, Okuma J, et al. Dyslipidemia in an HIV-positive antiretroviral treatment-naive population in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2011;57:141–145. doi:10.1097/QAI.0b013e318219a3d1
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
