Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Serum Human Epididymis Protein 4 is a Potential Biomarker for Early Chronic Kidney Disease in an Obese Population
Authors Tan S, Zeng Y, Kuang S, Li J
Received 12 January 2021
Accepted for publication 10 March 2021
Published 16 April 2021 Volume 2021:14 Pages 1601—1608
DOI https://doi.org/10.2147/DMSO.S300940
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
This paper has been retracted.
Shubo Tan, Yongmao Zeng, Shiliang Kuang, Jianjun Li
Department of Urology, Second Affiliated Hospital of the University of South China, Hengyang City, 421000, People’s Republic of China
Correspondence: Jianjun Li
Department of Urology, Second Affiliated Hospital of the University of South China, No. 35, Jiefang Avenue, Zhengxiang District, Hengyang City, 421000, Hunan Province, People’s Republic of China
Tel +861301734996
Email [email protected]
Background: At present, it is difficult to clinically diagnose early chronic kidney disease (CKD). As a novel biomarker of malignancies in the female reproductive tract, the human epididymis protein 4 (HE4) has been reported to be significantly expressed in CKD patients.
Aim: We sought to assess whether HE4 can be used as a potential biomarker of early-stage CKD.
Methods: The association between serum HE4 levels and CKD was analyzed in a retrospective study. A cohort of 506 patients with diabetic nephropathy who were hospitalized at Weihai Central Hospital, China, from January 2016 to November 2019 were included.
Results: Serum HE4 levels were increased with increasing stage of CKD and significantly elevated in patients with CKD3-5 than CKD1-2 (P< 0.001). In multivariate linear regression analyses, HE4 levels were strongly correlated with the estimated glomerular filtration rate (eGFR) in CKD patients (Model 2, P< 0.001). HE4 (area under the curve; AUC=0.934) had better diagnostic value than serum creatinine (SCr; AUC=0.770) and blood urea nitrogen (BUN; AUC=0.647) for patients with early-stage CKD (CKD1-2). Additionally, HE4 levels increased with increasing glomerular lesion (GL) and renal interstitial fibrosis (IF)/tubular atrophy (TA) scores in 51 CKD patients (P< 0.001).
Conclusion: Serum HE4 levels can be positively associated with the severity of CKD and are a very valuable clinical biomarker for predicting early-stage CKD.
Keywords: biomarker, chronic kidney disease, obese, human epididymis protein 4
Introduction
Chronic kidney disease (CKD) is a serious health problem worldwide. With increasing prevalence, CKD has enormous social and economic consequences in our society.1–3 However, most early-stage CKD patients have no obvious clinical symptoms, and the clinical indicators of renal function, such as serum creatinine (SCr) and blood urea nitrogen (BUN) levels, do not exceed the normal range because of the strong compensatory ability of the kidney. It is difficult to detect the reduction in renal function at an early stage by traditional urine and blood tests, which hampers the timely diagnosis and treatment for early-stage CKD patients.4
In 1991, the human epididymis protein 4 gene located on chromosome 20q12-13.1 was successfully cloned by Kirchhoff et al in the human epididymal epithelium, and the authors discovered that human epididymis protein 4 (HE4), encoded by the gene, is involved in the maturation of sperm.5,6 The immature HE4 protein contains two four-disulfide core domains (WFDC2) with antiproteinase activity, and mature HE4 is a 20–25 kDa glycoprotein found in the cytoplasm, cell membrane and circulation.7,8 HE4 is moderately or highly expressed in ovarian and endometrial cancer, breast cancer and pancreatic cancer.9,10 Meanwhile, studies have also shown that HE4 is expressed in normal tissues of the human body, including the kidney, digestive tract, and other organs.11,12
Thus far, the significance of HE4 in the context of CKD has not been extensively studied. Only several studies have shown that serum HE4 levels are elevated in CKD.13,14 It is uncertain whether HE4 could be a sufficiently sensitive marker to distinguish patients with early-stage CKD from healthy individuals. Accordingly, in this study, we studied whether serum HE4 can be used as a sensitive and specific indicator for predicting early-stage CKD. We additionally investigated the association of HE4 levels with pathological changes in CKD patients.
Materials and Methods
Study Population
Because most of the patients in the study have been discharged and cannot be contacted, for research needs, the Ethics Committee of Renmin Hospital of Weihai Central Hospital approved this retrospective study and the requirement for informed consent was waived according to the guidelines of the Declaration of Helsinki. In this study, the privacy of the included patients is strictly confidential and will not have any impact on the patients. The clinical characteristics of all subjects enrolled in this study were obtained by medical record review. The cohort consisted of 506 obese patients with CKD (body mass index [BMI] ≥28) who were hospitalized at Weihai Central Hospital, Weihai, China from January 2016 to November 2019: 487 patients without kidney disease (CKD 0) serving as a control group. Patients with neoplastic diseases, or other serious diseases were excluded, including 9 patients with severe liver or lung diseases, or ovarian cancer or other malignant diseases.
Inclusion Criteria, Definition of CKD
Inclusion criteria was based on the definitions for CKD according to the KDIGO guidelines:15 “CKD is defined as abnormalities of kidney structure or function, present for > 3 months”. Thus, the diagnosis of CKD was made when estimated glomerular filtration rate was reduced (eGFR≤60 mL/min/1.73 m2) and/or signs of kidney damage were present. Kidney damage was ascertained by kidney disease proven by kidney biopsy, pathological hematuria and proteinuria or abnormal imaging examination results (computed tomography, ultrasound, magnetic resonance imaging or nuclear imaging). According to the classification of the KDIGO guideline15 the included 506 CKD patients were divided into five subgroups according to their eGFR values: CKD1, eGFR>90 mL/min/1.73 m2; CKD2, eGFR=61–90 mL/min/1.73 m2; CKD3, eGFR=31–60 mL/min/1.73 m2; CKD4, eGFR=16–30 mL/min/1.73 m2; and CKD5, eGFR<15 mL/min/1.73 m2. Early CKD (CKD1-2) was defined as an eGFR >60 mL/min/1.73 m2. Advanced CKD (CKD3-5) was defined as eGFR≤60 mL/min/1.73 m2. We included 487 CKD0 patients without a medical history of CKD and/or a eGFR <60 mL/min. Emission computed tomography (eCT) has been considered the gold standard for clinical measurement of eGFR. In this study, the eGFR of all the CKD patients was measured by eCT.
Evaluation of HE4 in Clinical Practice
We collected a total of 102 CKD patients who had undergone eCT examination at our hospital from January 2020 to June 2020. We calculated the sensitivity and specificity of HE4 to verify the value of serum HE4 for diagnosis of early CKD. In addition, to further confirm the relationship between HE4 and CKD, we also collected a total of 51 CKD patients who had undergone renal biopsy at our hospital from January 2016 to November 2019, and evaluated whether serum HE4 concentrations were associated with pathological changes in CKD. All patients with malignant tumors were excluded.
Measurement of HE4 Levels
Serum samples were prepared immediately by centrifugation of peripheral venous blood and cryopreserved (−80°C) for the determination of HE4 levels. Serum levels of HE4 were measured by electrochemiluminescence immunoassays (Cobas e 601, F. Hoffmann-La Roche Ltd, Basel, Switzerland). The samples with HE4 concentrations over 1500 pmol/L were measured again (precision, coefficient of variation [CV]<5%; analytic measurement range, from 15 to 1500 pmol/L; detection limit, 5 pmol/L).
Laboratory Measurements and Definitions
Blood samples were collected from the patients after they had fasted overnight for at least 8 hours. SCr, BUN, beta 2 microglobulin (β2-MG), cystatin C (CysC), uric acid (UA), hemoglobin (Hb) and albumin (ALB) levels were measured using a Siemens ADVIA 2400 automatic biochemistry analyzer (Siemens AG).
Renal Biopsy and Histopathological Staining
In this study, renal biopsy was performed in 51 of 506 CKD patients. Pathologic materials were processed by conventional histological procedures. The pathologic samples were used to evaluate glomerular, renal tubular, and interstitial conditions by hematoxylin and eosin (H&E) and Periodic Schiff-Methenamine (PASM) staining in the pathology department of our hospital. The CKD scores were evaluated according to the 2007 Banff classification.16,17 The score for glomerular lesion (GL) was based on the percentage of diseased glomeruli as follows: GL0, GL=0% diseased glomeruli; GL1, ≤25%; GL2, 25–50%; and GL3: >50%. The score for renal interstitial fibrosis (IF)/tubular atrophy (TA) was based on the percentage of cortical parenchymal involvement as follows: IF/TA0, IF/TA≤5% cortical area; IF/TA1: 6–25%; IF/TA2; 26–50%; and IF/TA3: >50%.
Statistical Analyses
The data with normal distributions were expressed as the mean±standard deviation (SD). Comparisons between variables with normal distributions were performed by t test or analysis of variance (ANOVA). The data that were not normally distributed were expressed by median (interquartile range [IQR]) and were analyzed by the Mann–Whitney U-test and Kruskal–Wallis test. The distribution of categorical variables was studied using the Chi-square test. Multivariate linear regression modeling was performed with HE4 as the independent variable and eGFR as the dependent variable. To test if HE4 might distinguish between CKD0 and early CKD, the diagnostic performance of serum HE4, SCr, BUN, β2-MG and CysC levels for CKD was determined using receiver operating characteristic (ROC) curves, and sensitivity, specificity, area under the curve (AUC), and the 95% confidence intervals were calculated. IBM SPSS 24.0 was used for the statistical analyses. P-values less than 0.05 were considered statistically significant.
Result
Clinical Characteristics of the Study Population
Serum HE4 levels in the CKD patients were significantly higher than control subjects (CKD0, data not shown). The clinical characteristics of all CKD subjects are presented in Table 1. All patients were divided into two groups including patients with CKD1-2 and patients with CKD3-5. All variables were compared between the two groups. The disease history of the CKD patients was as follows: hypertension in 244 patients, diabetes in 73 patients, primary kidney disease in 146 patients, nephrolithiasis in 83 patients, and other diseases in 22 patients. Median levels of HE4 in patients with CKD1-2 were 267.8 pmol/L. Median levels of HE4 CKD3-5 were only 578.8 pmol/L. Significantly, The serum levels of HE4 were significantly higher in patients with CKD3-5 than that in patients with CKD1-2 (P<0.001).
 |
Table 1 Clinical Characteristics of CKD Patients |
Serum HE4 Levels Had a Strong Correlation with Conventional Biomarkers and eGFR Levels in CKD Patients
In order to clarify the relationship between serum HE4 levels and renal function in CKD patients, multivariate linear regression analysis was performed. Multivariate analysis revealed that HE4 levels remained significantly and strongly associated with eGFR after adjusting for age, gender, BMI, admission systolic blood pressure (SBP), admission diastolic blood pressure (DBP), current smoker, current drinker and CKD etiology and laboratory measurements (Table 2). In the model 3, these factors explained 87.5% of the variance in eGFR.
 |
Table 2 The Relationship Between the HE4 Level and eGFR in CKD Patients |
Serum HE4 Levels Had a Better Diagnostic Value Than SCr, β2-MG and CysC Levels for Early-Stage CKD Patients
To determine the performance of HE4 as a diagnostic biomarker for early CKD, we performed the area under the receiver operating characteristic curve (ROC-AUC) analysis (Table 3). Serum HE4 (AUC=0.982, 95% CI: 0.974–0.999, optimal cutoff 67.6 µmol/L, sensitivity 94.9%, and specificity 98.4%) had better diagnostic performance than SCr (AUC=0.912, 95% CI: 0.884–0.935, optimal cutoff 66.3 µmol/L, sensitivity 76.3%, and specificity 96.7%) and BUN (AUC=0.834, 95% CI: 0.812–0.856, optimal cutoff 6.9 µmol/L, sensitivity 61.1%, and specificity 92.6%) for patients with CKD1-5. The AUC for HE4 was 0.934 (95% CI: 0.925–0.967) for differentiating patients with CKD1-2 from CKD0, with an optimal cutoff value of 63.7 pmol/L (sensitivity 88.7% and specificity 98.1%). HE4 had a better diagnostic value than SCr (AUC=0.770, 95% CI: 0.724–0.840, optimal cutoff 65.4 µmol/L, sensitivity 53.4%, and specificity 93.0%) and BUN (AUC=0.647, 95% CI: 0.601–0.724, optimal cutoff 5.87 mmol/L, sensitivity 45.4%, and specificity 78.6%) for patients with CKD1-2.
 |
Table 3 Diagnostic Ability of Serum HE4 Values to Assess CKD |
To further investigate the diagnostic performance of serum HE4 in early CKD patients, we measured β2-MG and CysC levels in 89 of 506 CKD patients. Serum HE4 had significantly better diagnostic performance for CKD1-2 patients than β2-MG and CysC (data not shown), both of which have been confirmed to be useful indicators for early kidney diseases.18–21
Serum HE4 Levels Have High Sensitivity and Specificity in Clinical Practice for Predicting Patients with Early CKD
To verify the value of serum HE4 in the early diagnosis of CKD, we collected 102 CKD inpatients who had undergone eCT examination in our hospital. The mean age of these patients was 65.4 years. According to the optimal cutoff value of HE4 and SCr calculated above, we further calculated the sensitivity and specificity of HE4 and SCr (Table 4). HE4 (sensitivity 94.6% and specificity 86.7%) had higher sensitivity and similarly specificity than SCr (sensitivity 60.3% and specificity 85.4%) for patients with CKD1-2.
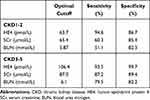 |
Table 4 Diagnostic Ability of Serum HE4 in Clinical Practice for Predicting CKD |
Serum HE4 Levels Were Strongly and Positively Correlated with GL and IF/TA Scores in CKD Patients
To investigate whether serum HE4 levels were associated with pathological changes in CKD, we categorized 51 of 506 CKD patients with renal insufficiency of different degrees from early to advanced stages who had undergone renal biopsy into four groups according to their GL and IF/TA scores. Indeed, serum HE4, SCr and BUN levels increased with increasing GL scores (P<0.001, Table 5). Serum HE4 levels were also strongly and positively correlated with IF/TA scores (P<0.001; Table 6), which further explained the close positive relationship between HE4 levels and renal function in CKD patients.
 |
Table 5 Major Laboratory Indicators According to Glomerular Lesion Scores in CKD Patients |
 |
Table 6 Major Laboratory Indicators According to the Renal Interstitial Fibrosis/Tubular Atrophy Scores in CKD Patients |
Discussion
The new and important findings from this study were (1) serum levels of HE4 were significantly higher in patients with CKD than in control subjects; (2) serum HE4 levels were strongly correlated with eGFR according to the multivariate linear regression analyses; (3) serum HE4 had high sensitivity and specificity for predicting CKD1-2; and (4) serum HE4 levels had a strong and positive correlation with GL and IF/TA scores in CKD patients.
GFR is the best indicator of renal function in CKD and is measured as the clearance rate of a filtration marker from serum by the kidneys.22 However, accurate measurements of GFR by inulin and nuclear medicine measurements require the appropriate infrastructure and are time consuming. SCr is not sensitive or specific for diagnosing early renal insufficiency, making it difficult for clinicians to timely prevent and treat early CKD. At present, clinical practice faces the challenge of detecting the early stages of CKD. No biomarker exists that reliably detects the early stages of CKD. β2-MG and CysC have been investigated as alternative markers of kidney function to estimate GFR with similar precision as SCr and with less bias due to muscle mass.23,24 However, age, gender, smoking, race and other factors influence serum CysC concentrations and reduce its reliability as a biomarker for diagnosing early CKD.23,25 Serum β2-MG levels can reflect GFR well, but it is also influenced by many factors in early CKD.24 Kidney injury molecule-1 (KIM-1), interleukin-18 (IL-18), neutrophil and gelatinase-associated lipocalin (NGAL) are considered biomarkers for acute kidney injury (AKI), but are limited in their use for early CKD.26–29 Recently, urinary proteomics analysis improved the diagnostic performance to detect early CKD.30,31 Unfortunately, this procedure is expensive and complex. A convenient and quick blood test is desirable, making urinary proteomics analysis difficult for routine clinical use.
Our study showed that compared with patients without CKD, serum HE4 levels were significantly elevated in advanced CKD patients. The results of our study are in accordance with the findings of previous studies.32,33 Therefore, we further explored the relationship between HE4 and early CKD. Importantly, we found that serum levels of HE4 were still significantly higher in patients with CKD1 and CKD2 than in CKD0 patients. Our study showed that serum HE4 had high specificity and sensitivity for predicting patients with CKD1-2, which suggested that HE4 may be useful for the timely diagnosis and treatment for early-stage CKD patients. Moreover, we also found that HE4 had higher diagnostic value for patients with CKD1-2 than β2-MG and CysC, both of which have been used for the early clinical diagnosis of early kidney diseases.18–21
In this study, according to the optimal cut-off value of 63.7 pmol/L for the diagnosis of CKD1-2, we calculated the specificity and sensitivity of HE4 in the tested population without malignant tumors to be high. Although studies have shown that HE4 is also associated with the age of the healthy population, smoking habit, inflammatory diseases, heart failure, etc.34–38 HE4 still showed a high specificity for the diagnosis of early CKD in the tested population. Furthermore, HE4 had a sensitivity of 95.7%, and this high sensitivity was very conducive for screening for early CKD. Therefore, the results further confirmed the important value of HE4 for predicting early CKD.
We showed that serum HE4 levels were significantly and positively associated with the severity of GL. The elevated serum HE4 levels in CKD patients may be due to upregulated HE4 expression and/or reduced eGFR. Because HE4, as a small molecule secreting protein, can be filtered freely in the glomerulus.7,39 Patients with CKD have a decline in renal function, which changes the removal of HE4 in the circulating blood, resulting in increased serum HE4, thereby explaining the finding in our study that serum HE4 was strongly correlated with eGFR. Moreover, research has shown that HE4 expression is upregulated in primary renal disease12 which may be associated with the fact that in early CKD, there is no significant decrease in renal function, but serum HE4 was significantly increased. Additionally, our results demonstrated that HE4 levels were clearly correlated with the severity of IF/TA. The increased serum HE4 levels may be related to renal fibrosis. HE4 often exhibits antiproteinase activity, indicating an important role of HE4 in fibrosis.40–42 Other proteins with WFDC have also been correlated with fibrosis formation and inflammatory processes.41 Importantly, these processes play an important role in the progression of CKD. The studies have suggested that HE4 is a mediator of renal fibrosis and when overexpressed, can prevent the degradation of type I collagen by inhibiting Prss35 and Prss23 serine protease activities during renal fibrosis.40,41 Recently, some researchers have reported that serum HE4 levels were associated with heart failure (HF) and speculated that elevated HE4 levels in patients with HF may be due to cardiac fibrosis.37,38 Since renal fibrosis has been involved in the development of CKD at an early stage, it may also give a possible explanation of why the serum levels of HE4 are significantly increased in the early stages of CKD.
Limitation
First, our study is a single-center study with a limited sample size and the results cannot be extrapolated. More studies are needed to identify the best HE4 cutoff or recognizing early-stage CKD in other centers or by multicenter studies. Second, this study excluded patients with malignant tumors, which may affect the accuracy of HE4 in detecting renal function in some tumor patients.
Conclusion
Our findings showed that serum HE4 levels were positively associated with the severity of CKD using two aspects of clinical indexes and renal pathology. HE4 can be considered a very valuable clinical biomarker for predicting early-stage CKD. In the future, multi-center and large sample studies are needed to further confirm the reliability of the research results.
Disclosure
All the authors declared no conflicts of interest and have nothing to disclose.
References
1. Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66(4):1310–1314. doi:10.1111/j.1523-1755.2004.00894.x
2. Riella MC. Malnutrition in dialysis: malnourishment or uremic inflammatory response? Kidney Int. 2000;57(3):1211–1232. doi:10.1038/sj.ki.4491447
3. Wang J, Wang F, Liu S, Zhou M, Zhang L, Zhao M. Reduced kidney function, albuminuria, and risks for all-cause and cardiovascular mortality in China: a Population-based Cohort Study. BMC Nephrol. 2017;18(1):188. doi:10.1186/s12882-017-0603-9
4. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–180. doi:10.1016/S0140-6736(11)60178-5
5. Kirchhoff C, Habben I, Ivell R, Krull N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod. 1991;45(2):350–357. doi:10.1095/biolreprod45.2.350
6. Kirchhoff C. Molecular Characterization of epididymal proteins. Rev Reprod. 1998;3(2):86–95. doi:10.1530/ror.0.0030086
7. Wang K, Gan L, Jeffery E, et al. Monitoring gene expression profile changes in ovarian carcinomas using cDNA microarray. Gene. 1999;229(1–2):101–108. doi:10.1016/S0378-1119(99)00035-9
8. Bingle L, Singleton V, Bingle CD. The putative ovarian tumour marker gene HE4 (WFDC2), is expressed in normal tissues and undergoes complex alternative splicing to yield multiple protein isoforms. Oncogene. 2002;21(17):2768–2773. doi:10.1038/sj.onc.1205363
9. Hellstrom I, Raycraft J, Hayden-Ledbetter M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63(13):3695–3700.
10. Li J, Chen H, Mariani A, et al. HE4 (WFDC2) promotes tumor growth in endometrial cancer cell lines. Int J Mol Sci. 2013;14(3):6026–6043. doi:10.3390/ijms14036026
11. Hertlein L, Stieber P, Kirschenhofer A, et al. Human epididymis protein 4 (HE4) in benign and malignant diseases. Clin Chem Lab Med. 2012;50(12):2181–2188. doi:10.1515/cclm-2012-0097
12. Galgano MT, Hampton GM, Frierson HF. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006;19(6):847–853. doi:10.1038/modpathol.3800612
13. Huang Y, Jiang H, Zhu L. Human epididymis protein 4 as an indicator of Acute Heart failure in patients with chronic kidney disease. Lab Med. 2020;51(2):169–175. doi:10.1093/labmed/lmz041
14. Yuan T, Li Y. Human epididymis Protein 4 as a potential biomarker of chronic kidney disease in female patients with normal ovarian function. Lab Med. 2017;48(3):238–243. doi:10.1093/labmed/lmx036
15. KDIGO 2012 clinical Practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3.
16. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
17. Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8(4):753–760. doi:10.1111/j.1600-6143.2008.02159.x
18. Deinum J, Derkx FH. Cystatin for estimation of glomerular filtration rate? Lancet. 2000;356(9242):1624–1625. doi:10.1016/S0140-6736(00)03152-4
19. Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36(1):29–34. doi:10.1053/ajkd.2000.8237
20. Liabeuf S, Lenglet A, Desjardins L, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82(12):1297–1303. doi:10.1038/ki.2012.301
21. Spanaus KS, Kollerits B, Ritz E, et al. Serum creatinine, cystatin C, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin Chem. 2010;56(5):740–749. doi:10.1373/clinchem.2009.138826
22. Rahman M, Smith MC. Chronic renal insufficiency: a diagnostic and therapeutic approach. Arch Intern Med. 1998;158(16):1743–1752. doi:10.1001/archinte.158.16.1743
23. Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65(4):1416–1421. doi:10.1111/j.1523-1755.2004.00517.x
24. Bianchi C, Donadio C, Tramonti G, et al. Reappraisal of serum beta2-microglobulin as marker of GFR. Ren Fail. 2001;23(3–4):419–429. doi:10.1081/JDI-100104725
25. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi:10.1038/ki.2008.638
26. Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290(2):F517–29. doi:10.1152/ajprenal.00291.2005
27. Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70(1):199–203. doi:10.1038/sj.ki.5001527
28. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi:10.1016/S0140-6736(05)74811-X
29. Wang Y, Wang J, Su T, et al. Community-acquired acute kidney injury: a Nationwide Survey in China. Am J Kidney Dis. 2017;69(5):647–657. doi:10.1053/j.ajkd.2016.10.034
30. Schanstra JP, Zurbig P, Alkhalaf A, et al. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol. 2015;26(8):1999–2010. doi:10.1681/ASN.2014050423
31. Siwy J, Schanstra JP, Argiles A, et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrol Dial Transplant. 2014;29(8):1563–1570. doi:10.1093/ndt/gfu039
32. Wan J, Wang Y, Cai G, et al. Elevated serum concentrations of HE4 as a novel biomarker of disease severity and renal fibrosis in kidney disease. Oncotarget. 2016;7:67748–67759. doi:10.18632/oncotarget.11682
33. Nagy BJ, Krasznai ZT, Balla H, et al. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann Clin Biochem. 2012;49(4):377–380. doi:10.1258/acb.2011.011258
34. Urban N, Thorpe J, Karlan BY, et al. Interpretation of single and serial measures of HE4 and CA125 in asymptomatic women at high risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2087–2094. doi:10.1158/1055-9965.EPI-12-0616
35. Chhikara N, Saraswat M, Tomar AK, et al. Human epididymis protein-4 (HE-4): a novel cross-class protease inhibitor. PLoS One. 2012;7(11):e47672. doi:10.1371/journal.pone.0047672
36. Doumas S, Kolokotronis A, Stefanopoulos P. Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immunol. 2005;73(3):1271–1274. doi:10.1128/IAI.73.3.1271-1274.2005
37. de Boer RA, Cao Q, Postmus D, et al. The WAP four-disulfide core domain protein HE4: a novel biomarker for heart failure. JACC Heart Fail. 2013;1(2):164–169. doi:10.1016/j.jchf.2012.11.005
38. Piek A, Meijers WC, Schroten NF, et al. HE4 serum levels are associated with heart failure severity in patients with chronic heart failure. J Card Fail. 2017;23(1):12–19. doi:10.1016/j.cardfail.2016.05.002
39. Bunnag S, Einecke G, Reeve J, et al. Molecular correlates of renal function in kidney transplant biopsies. J Am Soc Nephrol. 2009;20(5):1149–1160. doi:10.1681/ASN.2008080863
40. Lebleu VS, Teng Y, O’Connell JT, et al. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat Med. 2013;19(2):227–231. doi:10.1038/nm.2989
41. Bingle CD, Vyakarnam A. Novel innate immune functions of the whey acidic protein family. Trends Immunol. 2008;29:444–453. doi:10.1016/j.it.2008.07.001
42. Allison SJ. Fibrosis: HE4–a biomarker and target in renal fibrosis. Nat Rev Nephrol. 2013;9:124.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
