Back to Journals » Breast Cancer: Targets and Therapy » Volume 16
Serum HER2 Level Predicts Therapeutic Efficacy and Prognosis in Advanced Breast Cancer Patients
Authors Wang S, Chen Y, Li W , Hao C, Zhang L , Zhao W, Shi Y, Tong Z
Received 29 November 2023
Accepted for publication 26 March 2024
Published 3 April 2024 Volume 2024:16 Pages 163—179
DOI https://doi.org/10.2147/BCTT.S449510
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Shuling Wang,1,2,* Yuqin Chen,1– 3,* Weidong Li,2,4,* Chunfang Hao,1,2 Li Zhang,1,2 Weipeng Zhao,1,2 Yehui Shi,1,2 Zhongsheng Tong1,2
1Department of Breast Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, People’s Republic of China; 2Tianjin’s Clinical Research Center for Cancer, Key Laboratory of Breast Cancer Prevention and Therapy, Tianjin Medical University, Ministry of Education, Tianjin, People’s Republic of China; 3Department of Respiratory Center, The Second Affiliated Hospital of Xiamen Medical College, Xiamen, People’s Republic of China; 4Department of Breast Cancer Pathology and Research Laboratory, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yehui Shi; Zhongsheng Tong, Email [email protected]; [email protected]
Background: The purpose of this study was to investigate the therapeutic efficacy and prognosis of serum HER2 (sHER2) in patients with advanced breast cancer.
Methods: We analyzed the sHER2 levels of 200 patients with advanced breast cancer receiving first or second line treatment, the tissue HER2 (tHER2) level was also analyzed. Indicators of therapeutic efficacy and prognosis were objective response rate (ORR), disease control rate (DCR), and time to progression (TTP).
Results: The baseline sHER2 level was high in 132 patients and low in 68 patients. The high level of sHER2 is correlated with molecular subtype (p=0.016), visceral metastasis (p< 0.001), liver metastasis (p< 0.001), tissue HER-2 (tHER2) (p=0.001), and, among tHER2-low tumors (59 patients), the baseline sHER2 high level was associated with a higher proportion of brain metastasis. The ORR of patients with baseline sHER2 high level is higher than those with baseline sHER2 low level (p=0.026). The TTP of patients with baseline sHER2 low level is longer than the patients with baseline sHER2 high level (p=0.024). For patients with baseline sHER2 high level, a significant decrease in sHER2 after two cycles of treatment indicates higher ORR, DCR, and an extension of TTP. After multiple cycles of treatment, for patients with tHER-2 positive and baseline sHER2 high level, the DCR in the sHER2 decrease in the negative group was higher than that in the continuous positive group (p=0.037). Patients with a rapid decline type of sHER2 dynamic change curve had higher ORR and prolonged TTP compared with patients with other types of sHER2 dynamic change curve. There is no correlation between OS and sHER2 levels.
Conclusion: Our study showed that patients with advanced breast cancer had a high level of sHER2 at recurrence, regardless of whether they are tHER2 positive or negative. Dynamic detection of sHER2 can help predict therapeutic efficacy and prognosis, regardless of whether tHER-2 is positive or negative.
Keywords: serum HER2, advanced breast cancer, clinicopathological characteristics, therapeutic efficacy, prognosis
A Letter to the Editor has been published for this article.
Introduction
Breast cancer is one of the most common malignant tumors in women worldwide. In 2020, the World Health Organization’s International Agency for Research on Cancer (IARC) released the latest global cancer burden data,1 reporting 2.26 million new cases of breast cancer worldwide, making it the largest cancer in the world. Breast cancer is a heterogeneous disease which can be classified into four molecular subtypes: Luminal A, Luminal B, HER2-enriched (20–25%), and triple-negative.2,3 Advanced breast cancer is an incurable disease, but survival improvements have been reported with appropriate therapeutic strategies.4 CA 15–3, CA125, and CEA are commonly used in breast cancer patients as tumor markers. But these markers may lack sensitivity and specificity.5 Therefore, exploring new tumor markers is of great significance for monitoring breast cancer recurrence and metastasis.
HER2 is the key oncogene and tumor treatment target of HER2 positive breast cancer. The extracellular domain (ECD) of the HER2 protein on the surface of breast cancer cells can be cleaved by metalloproteases and enter the circulation, which can be detected as serum HER2 (sHER2).6,7 Nearly 50% of advanced breast cancer patients have elevated sHER2 levels (>15 ng/mL). Studies show that elevated sHER2 levels are usually associated with poor prognosis.8–10 Serum HER2 can be continuously measured, which can be used to monitor treatment response, predict recurrence and metastasis, and evaluate HER2 status in real time follow-up.11,12
However, the predictive and prognostic value of sHER2 levels in advanced breast cancer remains controversial, with even conflicting results from different studies.13–15 Retrospective studies16 have shown that the low expression of HER2 is highly unstable in the process of cancer development. The clinical utility of sHER2 in the therapeutic efficacy and prognosis in patients with advanced breast cancer is yet unclear.
Two hundred patients with advanced breast cancer treated with first-line or second-line chemotherapy were tested for sHER2 values at least three times. The correlation between the sHER2 level and clinicopathological characteristics, therapeutic efficacy, prognosis of the patients, and the change trend were analyzed. The purpose was to investigate the predictive value of dynamic detection of sHER2 in the clinical application of advanced cancer patients. The results may provide reference for clinical treatment decisions of patients with advanced breast cancer.
Materials and Methods
Patients
We retrospectively analyzed 200 patients with advanced breast cancer patients undergoing first or second line treatment in Tianjin Medical University Cancer Institute and Hospital between July 2016 and January 2020. Baseline levels of sHER2 were detected in all patients.
The tumor recurrence or metastasis site of the patients was confirmed by tumor biopsy, ultrasound, chest computed tomography (CT), magnetic resonance imaging (MRI), emission computed tomography (ECT), or positron emission tomography/computed tomography (PET/CT). Patients were treated as needed with chemotherapy, endocrine, and radiotherapy therapy according to NCCN and ESMO Clinical Practice guidelines, and HER2-positive patients were treated with anti-HER2 therapy. After every two treatment cycles, clinical examination and image-based assessment are used to evaluate the treatment efficacy. Evaluation of tumor lesions and lymph nodes was according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1).17 The progress of the patient’s condition is judged by two clinicians combined with imaging and clinical manifestations.
Clinicopathological information including primary tumor size, age at diagnosis, histological grading, lymph node status, ER, PR, HER2 and Ki67 expression, metastasis site (visceral, liver, lung, and brain metastasis), and therapeutic efficacy were retrospectively collected. Indicators of therapeutic efficacy and the prognosis of this study were the objective response rate (ORR), disease control rate (DCR), and time to progression (TTP).
HER2 Evaluation and Other Biomarkers Evaluation
The tHER2 status is assayed by immunohistochemistry (IHC) and HER2 gene amplification by fluorescence in situ hybridization (FISH). tHER2-low refers to patients with IHC 1+ or IHC 2+ who have a negative FISH test. ER, PR,18,19 and Ki-6720 were assayed by IHC. Immunohistochemistry was performed on formalin-fixed, paraffin-embedded samples.
Fasting venous blood was used to detect sHER2 level. Baseline sHER2 level was obtained from each patient before starting treatment. The sHER2 level was measured on an ADVIA Centaur XP automatic chemiluminescence immunoassay analyzer (Siemens, Germany). According to the recommendations of the Food and Drug Administration and manufacturers, the sHER2 cut-off value of 15 ng/mL was used as the cut-off point.21 sHER2 ≥15 ng/mL is defined as the threshold of high sHER2 level (positive) and sHER2 <15 ng/mL is considered as a low sHER2 level (negative).
According to previous studies,22,23 compared with the baseline sHER2, those with a level of sHER2 which had decreased by 20% were defined as the significant decrease group, those with a change of sHER2 of less than 20% were defined as the no significant change group, and a 20% or more increase in the level of sHER2 compared with baseline was defined as a significant increase group after two cycles treatment.
The patients in the baseline sHER2 high level group were divided into decrease to negative group and continuous positive group according to whether the value of sHER2 can be reduced to negative after treatment and last for at least two cycles. The patients in the baseline sHER2 low level group were divided into the continuous negative group and increase to the positive group according to whether the value of sHER2 remains negative after multiple cycles of treatment.
Objective Response Rate (ORR) and Disease Control Rate (DCR)
The objective response rate (ORR) is the proportion of patients whose tumors have shrunk to a certain extent and maintained for a certain time, including patients whose therapeutic efficacy has reached complete response (CR) (all lesions have disappeared and maintained for 4 weeks or more) and partial response (PR) (lesions have shrunk by≥30% and maintained for 4 weeks or more).
Disease control rate (DCR) refers to the percentage of cases with remission and stable lesions after treatment, including CR, PR, and stable disease (SD) (focal reduction less than 30% and increase less than 20%).
Follow-Up
Follow-up was conducted through examination, hospitalization records, and telephone communication. The follow-up time was up to the death of the patient or May 31, 2023. In this study, time to progress (TTP) refers to the time from the beginning of treatment to the occurrence of progress in the patient. Two clinicians judge whether the patient has progressed according to the imaging and clinical manifestations of the patient.
Statistical Analyses
The statistical analysis was carried out by SPSS 25.0 software. Pearson Chi square test was used for the comparison of quantitative data. Kaplan-Meier method was used for survival analysis. p<0.05 showed that the difference was statistically significant.
Results
The Relationship Between Baseline sHER2 Level and Clinicopathological Characteristics
There were 200 metastatic breast cancer patients in this study, including 102 tHER2 positive and 98 tHER2 negative patients (59 tHER2-low tumors were included). The baseline of sHER2 level was high in 132 (66.0%) and low in 68 (34.0%) patients. Regardless of the expression of tissue HER-2(tHER-2) is positive or negative by IHC and FISH, sHER2 high level can occur during relapse or metastasis in advanced breast cancer.
Baseline sHER2 levels were correlated with molecular subtypes of metastatic breast cancer (p=0.016, Table 1). Forty-five cases (34.09%) were tHER2 positive type in the sHER2 high level group, while 14 cases (20.59%) were tHER2 positive type in the sHER2 low level group. There were 30 (44.12%) patients with luminal B (tHER2 negative) type in the sHER2 low level group, and 35 (26.52%) patients with luminal B (tHER2 negative) type in the sHER2 high level group. Seventy-eight cases (59.10%) were tHER2 positive and 54 cases (40.90%) were tHER2 negative in the sHER2 high level group. Twenty-four cases (35.29%) were tHER2 positive and 44 cases (64.71%) were tHER2 negative in the sHER2 low level group.
 |
Table 1 Correlation Between Baseline sHER2 Levels with Clinicopathological Characteristics in Patients with Advanced Breast Cancer |
The incidence of visceral metastasis in sHER2 high level group (94 patients, 71.21%) was higher than that in the sHER2 low level group (30 patients, 44.12%) (p<0.001). The patients with liver metastasis were 53 cases (40.15%) in the sHER2 high level group and seven cases (10.29%) in the sHER2 low level group (p<0.001, Table 1).
Among the tHER2 low level group (59 cases), the baseline sHER2 high level patients had a higher proportion of brain metastasis (16.67% vs 26.83%, p<0.05).
The ORR of Patients with Baseline sHER2 High Level is Higher Than Those with Baseline sHER2 Low Level
In 132 patients with baseline sHER2 high level, ORR was 40.91% and DCR was 92.42% (including 54 cases of PR, 68 cases of SD, and 10 cases of PD). In 68 patients with baseline sHER2 low level, ORR was 25% and DCR was 92.65% (including 1 case of CR, 16 cases of PR, 46 cases of SD, and 5 cases of PD). The ORR of the baseline sHER2 high level group was higher than that of the baseline sHER2 low level group (40.91% vs 25.00%, p=0.026, Table 2). There was no significant difference in DCR between the two groups.
 |
Table 2 Correlation Between Baseline sHER2 Level and Therapeutic Efficacy |
Of the 132 patients in the baseline sHER2 high level group, 78 were tHER2 positive, the ORR was 51.28%, and the DCR was 93.59% (40 cases of CR/PR, 33 cases of SD, and 5 cases of PD). There were 54 patients who were tHER2 negative, of which the ORR was 25.93% and the DCR was 90.74% (14 cases of CR/PR, 35 cases of SD, and 5 cases of PD). The ORR of tHER2 positive was higher than that of tHER2 negative patients in the baseline high level group (51.28% vs 25.93%, p=0.004, Table 2). No significant correlation was observed in DCR between tHER2 positive and tHER2 negative patients in the baseline sHER2 high level group.
Of the 68 patients in the baseline sHER2 low level group, 24 cases were tHER2 positive. No significant difference in ORR and DCR was found between tHER2 positive and tHER2 negative patients in the baseline low level group (Table 2).
Of the 102 patients with tHER2 positive, 78 patients were in the baseline sHER2 high level group and 24 patients were in the baseline sHER2 low level group. Of the 98 patients with tHER2 negative, 54 patients were in the baseline sHER2 high level group and 44 patients in the baseline low level group (Table 2).
The TTP of Patients with Baseline sHER2 Low Level is Longer Than the Patients with Baseline sHER2 High Level
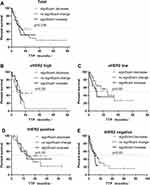
The median TTP in the low level group was longer than that in the high level group (17 m vs 12 m) (p=0.048, Figure 1). The median TTP of the tHER2 positive patients and the tHER2 negative patients in the sHER2 high level group was 13 m and 9 m, respectively, and there was no significant difference in TTP between the two groups (p=0.142). In tHER2 positive patients, the baseline sHER2 high level group had a longer TTP than the baseline sHER2 low level group (p=0.024, Figure 1).
The Decrease in sHER2 After Two Cycles of Treatment Indicates Higher ORR, DCR, and an Extension of TTP in Patients with Baseline sHER2 High Level
There were 70 patients in the significant decrease group, with an ORR of 58.57% and DCR of 97.14%. Among the 101 patients in the no significant change group, ORR was 23.76% and DCR was 90.10%. Among the 29 patients in the significant increase group, ORR was 20.69% and DCR was 89.66%. The ORR of the significant decrease group was higher than the no significant change group and significant increase group (p<0.001, Table 3). There were no statistically significant differences in DCR between the three groups (p=0.187, Table 3).
 |
Table 3 Correlation Between the Change of sHER2 Level and Therapeutic Efficacy in Baseline sHER2 High Level and Low Level Group After Two Treatment Cycles |
In the baseline sHER2 high level group (132 cases), there were 64 patients in the significant decrease group, and the cases of ORR and DCR were 60.94% and 98.44%, respectively. There were 58 patients in the no significant change group, ORR was 22.41%, and DCR was 86.21%. There were 10 patients in the significant increase group, with an ORR of 20.00% and DCR of 90.00%. The ORR and DCR of the significant decrease group were higher than the no significant change group and significant increase group (p<0.001, p=0.037, Table 3).
Among 102 tHER2-positive patients, 54 patients were in the significant decrease group, ORR was 61.11% and DCR was 98.15%; 37 patients were in the no significant change group, ORR was 35.14%, DCR was 89.19%; and 11 patients were in the significant increase group, ORR was 27.27%, DCR was 100%. The ORR of the significant decrease group was significantly higher than that of the no significant change group and the significant increase group (p=0.018, Table 3), and there was no statistically significant in DCR between the three groups (p=0.110, Table 3).
Among the 98 tHER2-negative patients, 16 patients were in the significant decrease group, ORR was 50% and DCR was 93.75%; 64 patients were in the no significant change group, ORR was 17.19%, DCR was 90.63%; and 18 patients were in the significant increase group, ORR was 16.67%, DCR was 83.33%. The ORR of the significant decrease group was higher than that of the no significant change group and the significant increase group (p=0.015, Table 3), and there was no significant difference in DCR between the three groups (p=0.565, Table 3).
In the baseline sHER2 high level group, there were 64 patients in the significantly decrease group, with a median TTP of 13 months. There were 58 patients in the no significant change group, with a median TTP of 7 months. There were 10 patients in the significant increase group, and the median TTP was 12 months. There was no statistically significant difference in TTP between the three groups (p>0.05, Figure 2). After pairwise comparison, TTP of the significant decrease group was longer than in the no significant changed group (13 m vs 7 m, p=0.003).
The DCR and ORR of sHER2 Decrease to the Negative Group Was Higher Than That of the Continuous Positive Group After Multi Cycles of Treatment in Patients with Baseline sHER2 High Level
Among the 132 patients with baseline sHER2 high level, there was a sHER2 decrease to negative in 43 patients after treatment. The ORR and DCR in the decrease to negative group were 60.47% and 100%. The level of sHER2 failed to fall into a negative range and continued as positive for at least two cycles in 89 patients. In the continuous positive group, the ORR was 31.46% and the DCR was 88.76%. The ORR and DCR in the sHER2 decreased to negative group were higher than those in the continuous positive group (60.47% vs 31.46%, p=0.001 and 100% vs 88.76%, p=0.022) (Table 4).
 |
Table 4 Correlation Between the Change of sHER2 Level and Therapeutic Efficacy in Baseline sHER2 High Level and Low Level Group After Multi Cycles of Treatment |
Among 68 patients with baseline sHER2 low level, 31 patients remained negative after treatment. The ORR of the continuous negative group was 35.48% and the DCR was 96.77%. In 37 patients, the level of sHER2 increased from negative to positive, ORR was 16.22% and DCR was 97.06%. There was no significant difference in ORR and DCR between the two groups.
In the 68 patients with baseline sHER2 low level, 44 were tHER2 negative and 24 were tHER2 positive. Among the 44 tHER2 negative patients, the ORR of the sHER2 continuous negative group was better than that of the sHER2 increase to positive group (35.00% vs 4.17%, p=0.008, Table 4), and there was no significant difference in DCR between the two groups.
The TTP in the Decrease to Negative Group Was Better Than That in the Continuous Positive Group After Multi Cycles of Treatment
The median TTP of 132 patients with baseline sHER2 high level was 13 months. There were 89 patients in the continuous positive group, of which 57 progressed during the follow-up period. The median TTP was 12 months. The TTP in the decrease to negative group was better than that in the continuous positive group (13 m vs 12 m, p=0.01, Figure 3A). There were 78 cases of tHER2 positive patients. The TTP of the sHER2 decreased to negative group was longer than that of the sHER2 continuous positive group after treatment in patients with baseline sHER2 high level and tHER2 positive (p=0.006, Figure 3B). There is no difference in patients with baseline sHER2 high level and tHER2 negative group (p=0.684, Figure 3C). The patients TTP in the continuous negative group was better than that in the increased to positive group in the baseline sHER2 low level patients (35 m vs 11 m, p=0.03, Figure 3D).
Patients with Rapid Decline Type of the sHER2 Dynamic Change Curve Group Had Higher ORR and Prolonged TTP
According to the characteristics of the dynamic change curve of sHER2 level during the treatment, 200 patients were divided into six types (Figure 4): 1) rapid decline type: decreased ≥50%; 2) stable type: exceeds ≥20% less than twice; 3) fluctuating type: near the baseline value of sHER2, but exceeds ≥20% more than twice; 4) slow ascending type: sHER2 level increased slowly; 5) flow descending type: the level of sHER2 decreased slowly; and 6) spoon type: the level of sHER2 decreased significantly and then increased significantly. Nineteen cases could not be classified into the above six types.
The different types of sHER2 dynamic change curves of patients were correlated with molecular subtype, surgical approach, and liver metastasis (p<0.001, <0.001, 0.020, and 0.022, respectively) (Table 5).
 |
Table 5 Clinicopathological Characteristics of Patients and Therapeutic Efficacy in Different Types of sHER2 Dynamic Change Curve |
ORR was 76.00% and DCR was 96% in the rapid decline type. ORR was 28.77% and DCR was 94.52% in the stable type. ORR was 18.18% and DCR was 95.45% in the fluctuating type. ORR was 23.53% and DCR was 100% in the slow ascending type. ORR was 46.88% and DCR was 93.75% in the slow descending type. ORR was 41.67% and DCR was 100% in the spoon type. ORR was 15.79% and DCR was 63.16% in the unable to classify group. The ORR of the rapid decline type was higher than that of other groups (p<0.001, <0.001, 0.001, 0.026, 0.041, and <0.001, respectively). There was no significant difference in DCR between any two groups (p>0.05).
The median TTP was 13 months in the rapid decline group, 17 months in the stable group, 13 months in the fluctuating group, 13 months in the slow ascending type group, and 7 months in the spoon group. Pairwise comparison of different curve types found that TTP of patients in the spoon group was shorter than that of the rapid decline type, stable type, and slow descending type (p=0.028, 0.038, and 0.007, respectively). Analysis of all curve types showed that there was no significant difference in patients TTP (Figure 5). The OS was 12 months to 307 months and OS after metastasis was 4 months to 149 months. There was no significant difference in OS and OS after metastasis between the sHER2 low level group and sHER2 high level group in this study.
Discussion
HER2 is a member of the human epidermal growth factor receptor (EGFR) family, and its overexpression is a poor prognostic factor for breast cancer. The predictive and prognostic value of serum HER2 (sHER2) levels in advanced breast cancer remains controversial. Previous studies on sHER2 level in breast cancer patients were mostly concentrated on tHER2 positive breast cancer patients. However, tHER2 negative breast cancer patients were rarely analyzed. The clinical utility and efficacy of sHER2 in the therapeutic efficacy and prognosis in patients with advanced breast cancer is yet unclear. Retrospective studies showed that the low expression of HER2 is highly unstable in the process of breast cancer development.16 As a tumor marker for dynamic detection, sHER2 is worthy of further exploration and discussion regarding the predictive value of its therapeutic efficacy and prognosis in advanced breast cancer.
Our study showed that patients with advanced breast cancer had a high level of sHER2 at recurrence, regardless of whether they were tHER2-positive or negative. Darlix et al also showed that 44 (33.6%) out of 131 tHER2 negative patients had abnormal sHER2 expression.24 The expression of sHER2 was correlated with tumor burden and breast tumor heterogeneity,24,25 which may be an explanation. Panis et al found that 16% of tHER2 negative tumors were positive for HER2 intracellular tyrosine kinase domain (ICD), and HER2 gene amplification was also found in some tHER2 negative tumors.25 A recent report analyzed 2,310 patients from four clinical trials, indicating the clinicopathological characteristics and clinical results of breast cancer with HER2 low expression.26 And sHER2 may present positive in HER2-low expression breast cancer which was included in tHER2 negative patients. In some patients, tHER2 status may changes during tumor recurrence and metastasis,27 and some studies consider that sHER2 can reflect the real-time state of HER2 expression in patients.28
Our study showed molecular subtype, visceral metastasis, and liver metastasis were correlated with baseline sHER2 level after recurrence or metastasis in advanced breast cancer, which is consistent with previous studies.29 Studies have found that the high level of sHER2 is associated with liver metastasis.30,31 Previous studies have shown that some breast cancer patients who were identified as tHER2 negative by IHC and/or FISH had therapeutic efficacy on anti-HER2 treatment.32 In patients with tHER2-low levels, the baseline sHER2 high level group had a higher proportion of brain metastasis. This was probably because none of these patients had received trastuzumab or other anti-HER2 targeted therapy. According to HER2 testing guidelines, these patients are currently considered to have HER2-negative breast cancer, therefore HER2-targeted treatments are not recommended.16 So, detecting sHER2 in this population is even more important. The Darlix et al study showed that, whether tHER2 is positive or not, the sHER2 level was significantly correlated with brain metastases in patients with advanced breast cancer.33 sHER2 level in this study was not correlated with the expression of ER and PR. Previous studies suggested that sHER2 levels in advanced breast cancer patients were negatively correlated with hormone receptor levels.34,35 In some early studies, sHER2 levels were measured in frozen serum samples at any time after breast cancer metastasis. The baseline sHER2 level in this study was mostly detected in patients with advanced breast cancer before receiving first- or second-line rescue therapy. This may be the reason for the difference from previous studies.
According to the previous research,23 taking 15 ng/mL as the cut-off value, this study found that the ORR of patients with baseline sHER2 high level was higher than that of patients with baseline sHER2 low level. However, analyzed in tHER2 positive and tHER2 negative patients, respectively, there was no significant difference in ORR between the baseline high level group and low level group. This may be because tHER2 positive patients all received anti-HER2 treatment effectively. The TTP of patients with baseline sHER2 low level was longer than those with baseline sHER2 high level. This advantage remained in tHER2 positive patients, but not in tHER2 negative patients. Lipton et al concluded that baseline sHER2 was associated with ORR, but not related to PFS in 138 breast cancer patients treated with lapatinib.36 It is reported that there is a significant difference in OS between the sHER2 low level group and the sHER2 high level group in advanced breast cancer patients,29,37 regardless of the tHER2 status of the primary breast tumor. sHER2 high level is an independent prognostic factor for advanced breast cancer.24 There was no difference in OS in this study. The inconsistency may be due to differences in the study population and follow-up time.
The ORR of the group with a sHER2 significant decrease was better than those with a significant increase and no significant change. In the baseline sHER2 high level, the ORR and DCR of the sHER2 significant decrease group were higher than the significant increase and no significant change groups. However, in the baseline sHER2 low level group, the ORR of the sHER2 significant decrease group was not statistically different from other groups. This may indicate that patients with a sHER2 high level at baseline are more sensitive in reflecting their disease status and treatment response at the time of recurrence/metastasis. Dynamic detection of sHER2 in patients with a sHER2 high level is more important.
In the patients with baseline sHER2 high level, the TTP of the sHER2 significant decrease group is longer than that of the no significant change group after treatment. The result is also consistent with previous studies,38,39 which suggested that elevated sHER2 and a lack of decline after treatment is associated with poorer survival. And sHER2 may be a useful surveillance biomarker to predict metastasis. In a study of 307 MBC patients treated with trastuzumab by Ali et al, the ORR of patients with a decrease of ≥20% of sHER2 is 57%, while the patients with a decrease of less than 20% of sHER2 is 28%. And the patients with a decrease of >20% of sHER2 had longer TTP times.22 Lipton et al obtained a similar conclusion in the study of 138 MBC patients treated with lapatinib.36 In patients with baseline sHER2 high level at the time of recurrence or metastasis, a significant decrease of sHER2 after treatment was correlated with a higher ORR and longer TTP.
It was found that, in baseline sHER2 high level groups, the ORR of patients with a decrease to negative and maintain at least two cycles during the treatment was higher than those who failed to reduce to negative. For patients with baseline sHER2 high level at the time of recurrence or metastasis, the sHER2 level decreased to the negative after treatment, indicating that the current treatment has obvious therapeutic efficacy. For the tHER2 negative patients, the ORR of the negative group was better than that of the positive group. And sHER2 rises from negative to positive in tHER2 negative patients during treatment, which may indicate tumor load increase or tHER2 status change. For this part of patients, pathological examination of metastasis site should be carried out to identify tHER2 status, and anti-HER2 therapy should be taken if necessary.
In patients with tHER2 positive and baseline sHER2 high level, the TTP of sHER2 level decrease to negative during the treatment was longer than those with a sHER2 high after treatment. This study found that, for patients with tHER2 negative and baseline sHER2 low level, the continuous sHER2 low level during treatment indicated that patients can obtain a longer remission period. If patients with tHER2 negative and baseline sHER2 high have increased to the positive range during treatment, it indicates a shorter TTP and lower ORR in this study. Clinically, dynamic monitoring of sHER2 level can be used to monitor whether such patients have disease progression. Some previous studies have also obtained similar results.40 The median PFS of patients with low level of sHER2 is significantly longer. The PFS of patients with low level of sHER2 is significantly longer than those with sHER2 high level or with an elevated level of sHER2 after treatment.40
It was found that the ORR of patients with rapid decline curve type is significantly better than that of other curve types. Fornier et al also found that patients whose sHER2 level decreased by 55% or more during treatment had a better therapeutic effect.41 It is worth paying attention to the spoon curve type, which is a kind of curve type where the level of sHER2 decreased rapidly and then increased rapidly at a certain point. The TTP of the spoon curve type is short, and the disease progression mostly occurred near the rapidly rising point. The results of patients with rapid decline and spoon curve type of sHER2 dynamic change suggest that the short-term sharp decline of sHER2 level suggests that patients have a obvious therapeutic effect, but whether they can benefit from survival still needs to be consider whether they can maintain a low level after the decline of sHER2 level. Ali has also found a similar result,26 suggesting that there is a correlation between the change of sHER2 level and the disease progression in patients with advanced breast cancer.
Conclusion
In conclusion, our results showed the predictive value of sHER2 in the treatment of advanced breast cancer patients undergoing first or second line treatment. Dynamic detection of sHER2 can help predict the therapeutic efficacy and prognosis, whether tHER-2 is positive or negative. ORR and TTP in patients of tHER-2 positive and negative all have significant differences in different types of sHER2 dynamic change curve groups. Therefore, in addition to the baseline, we recommend to measure sHER2 level every two treatment cycles. As advanced breast cancer is an incurable disease and a high sHER2 level is associated with a worse prognosis, sHER2 can be used as an important indicator to evaluate the therapeutic efficacy and progression of advanced breast cancer.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by The Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (Ek2017067). Informed consent of study participants was obtained prior to study commencement. We declared that patient data was confidential.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by National Natural Science Foundation of China (81602340, 81472183); Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A, TJYXZDXK-012A); Tianjin Medical University Cancer Hospital “14th Five-Year” Peak Discipline Support Program Project; Shenzhen Chipscreen Biosciences project; Science and Technology Project of Tianjin Health Commission (Grant NO, TJWJ2021MS009).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi:10.1038/35021093
3. Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–1167. doi:10.1200/JCO.2008.18.1370
4. Gao JJ, Cheng J, Bloomquist E, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020;21(2):250–260. doi:10.1016/S1470-2045(19)30804-6
5. Pedersen AC, Sorensen PD, Jacobsen EH, Madsen JS, Brandslund I. Sensitivity of CA 15-3, CEA and serum HER2 in the early detection of recurrence of breast cancer. Clin Chem Lab Med. 2013;51(7):1511–1519. doi:10.1515/cclm-2012-0488
6. Tse C, Gauchez AS, Jacot W, Lamy PJ. HER2 shedding and serum HER2 extracellular domain: biology and clinical utility in breast cancer. Cancer Treat Rev. 2012;38(2):133–142. doi:10.1016/j.ctrv.2011.03.008
7. Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999;59(6):1196–1201.
8. Pichon MF, Hacene K, Guepratte S, Neumann R. Serum HER-2 extracellular domain (ECD) before the first metastasis in 128 breast cancer patients. Clin Lab. 2004;50(3–4):163–170.
9. Ryu DW, Lee CH. Impact of serum HER2 levels on survival and its correlation with clinicopathological parameters in women with breast cancer. J Breast Cancer. 2012;15(1):71–78. doi:10.4048/jbc.2012.15.1.71
10. Kong Y, Dai S, Xie X, et al. High serum HER2 extracellular domain levels: correlation with a worse disease-free survival and overall survival in primary operable breast cancer patients. J Cancer Res Clin Oncol. 2012;138(2):275–284. doi:10.1007/s00432-011-1095-9
11. Carney WP, Bernhardt D, Jasani B. Circulating HER2 extracellular domain: a specific and quantitative biomarker of prognostic value in all breast cancer patients? Biomark Cancer. 2013;5:31–39. doi:10.4137/BIC.S12389
12. Lee MH, Jung SY, Kang SH, et al. The significance of serum HER2 levels at diagnosis on intrinsic subtype-specific outcome of operable breast cancer patients. PLoS One. 2016;11(10):e0163370. doi:10.1371/journal.pone.0163370
13. Lipton A, Ali SM, Leitzel K, et al. Elevated Serum HER-2/ neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol. 2002;20(6):1467–1472. doi:10.1200/JCO.2002.20.6.1467
14. Lennon S, Barton C, Banken L, et al. Utility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in metastatic breast cancer. J Clin Oncol. 2009;27(10):1685–1693. doi:10.1200/JCO.2008.16.8351
15. Zhang P, Xiao J, Ruan Y, Zhang Z, Zhang X. Monitoring value of serum HER2 as a predictive biomarker in patients with metastatic breast cancer. Cancer Manag Res. 2020;12:4667–4675. doi:10.2147/CMAR.S254897
16. Modi S, Park H, Murthy RK, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase ib study. J Clin Oncol. 2020;38(17):1887–1896. doi:10.1200/JCO.19.02318
17. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
18. Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–1366. doi:10.1200/JCO.19.02309
19. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of clinical oncology/college of American pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi:10.1200/JCO.2013.50.9984
20. Nielsen TO, Leung SCY, Rimm DL, et al. Assessment of Ki67 in breast cancer: updated recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst. 2021;113(7):808–819. doi:10.1093/jnci/djaa201
21. Cook GB, Neaman IE, Goldblatt JL, et al. Clinical utility of serum HER-2/neu testing on the Bayer Immuno 1 automated system in breast cancer. Anticancer Res. 2001;21(2B):1465–1470.
22. Ali SM, Carney WP, Esteva FJ, et al. Serum HER-2/ neu and relative resistance to trastuzumab-based therapy in patients with metastatic breast cancer. Cancer. 2008;113(6):1294–1301. doi:10.1002/cncr.23689
23. Zhang Z, Li C, Fan H, et al. Prognostic value of baseline serum HER2 extracellular domain level with a cut-off value of 15 ng/mL in patients with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2018;172:513–521.
24. Darlix A, Lamy PJ, Lopez-Crapez E, et al. Serum HER2 extra-cellular domain, S100ss and CA 15-3 levels are independent prognostic factors in metastatic breast cancer patients. BMC Cancer. 2016;16(1):428. doi:10.1186/s12885-016-2448-1
25. Panis C, Pizzatti L, Correa S, et al. The positive is inside the negative: HER2-negative tumors can express the HER2 intracellular domain and present a HER2-positive phenotype. Cancer Lett. 2015;357(1):186–195. doi:10.1016/j.canlet.2014.11.029
26. Denkert C, Seither F, Schneeweiss A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–1161. doi:10.1016/S1470-2045(21)00301-6
27. Santinelli A, Pisa E, Stramazzotti D, Fabris G. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int J Cancer. 2008;122(5):999–1004. doi:10.1002/ijc.23051
28. Aurilio G, Sandri MT, Pruneri G, et al. Serum HER2 extracellular domain levels and HER2 circulating tumor cell status in patients with metastatic breast cancer. Future Oncol. 2016;12(17):2001–2008. doi:10.2217/fon-2016-0081
29. Darlix A, Hirtz C, Thezenas S, et al. The prognostic value of the Tau protein serum level in metastatic breast cancer patients and its correlation with brain metastases. BMC Cancer. 2019;19(1):110. doi:10.1186/s12885-019-5287-z
30. Schondorf T, Hoopmann M, Warm M, et al. Serologic concentrations of HER-2/neu in breast cancer patients with visceral metastases receiving trastuzumab therapy predict the clinical course. Clin Chem. 2002;48(8):1360–1362. doi:10.1093/clinchem/48.8.1360
31. Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9(12):4423–4434.
32. Lam L, McAndrew N, Yee M, Fu T, Tchou JC, Zhang H. Challenges in the clinical utility of the serum test for HER2 ECD. Biochim Biophys Acta. 2012;1826(1):199–208. doi:10.1016/j.bbcan.2012.03.012
33. Darlix A, Lamy PJ, Lopez-Crapez E, et al. Serum NSE, MMP-9 and HER2 extracellular domain are associated with brain metastases in metastatic breast cancer patients: predictive biomarkers for brain metastases? Int J Cancer. 2016;139(10):2299–2311. doi:10.1002/ijc.30290
34. Di Gioia D, Dresse M, Mayr D, et al. Serum HER2 supports HER2-testing in tissue at the time of primary diagnosis of breast cancer. Clin Chim Acta. 2014;430:86–91. doi:10.1016/j.cca.2013.12.036
35. Garoufali A, Kyriakou F, Kountourakis P, et al. Extracellular domain of HER2: a useful marker for the initial workup and follow-up of HER2-positive breast cancer. J BUON. 2008;13(3):409–413.
36. Lipton A, Leitzel K, Ali SM, et al. Human epidermal growth factor receptor 2 (HER2) extracellular domain levels are associated with progression-free survival in patients with HER2-positive metastatic breast cancer receiving lapatinib monotherapy. Cancer. 2011;117(21):5013–5020. doi:10.1002/cncr.26101
37. Moreno-Aspitia A, Hillman DW, Dyar SH, et al. Soluble human epidermal growth factor receptor 2 (HER2) levels in patients with HER2-positive breast cancer receiving chemotherapy with or without trastuzumab: results from North Central Cancer Treatment Group adjuvant trial N9831. Cancer. 2013;119(15):2675–2682. doi:10.1002/cncr.28130
38. Reix N, Malina C, Chenard MP, et al. A prospective study to assess the clinical utility of serum HER2 extracellular domain in breast cancer with HER2 overexpression. Breast Cancer Res Treat. 2016;160(2):249–259. doi:10.1007/s10549-016-4000-z
39. Finn RS, Gagnon R, Di Leo A, Press MF, Arbushites M, Koehler M. Prognostic and predictive value of HER2 extracellular domain in metastatic breast cancer treated with lapatinib and paclitaxel in a randomized Phase III study. J Clin Oncol. 2009;27(33):5552–5558. doi:10.1200/JCO.2008.21.1763
40. Shao X, Wang X, Xu X, et al. Outcome prediction values of soluble human epidermal growth factor receptor-2 extracellular domain in metastatic breast cancer. Int J Clin Exp Pathol. 2014;7:1108–1113.
41. Fornier MN, Seidman AD, Schwartz MK, et al. Serum HER2 extracellular domain in metastatic breast cancer patients treated with weekly trastuzumab and paclitaxel: association with HER2 status by immunohistochemistry and fluorescence in situ hybridization and with response rate. Ann Oncol. 2005;16:234–239.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.