Back to Journals » Journal of Inflammation Research » Volume 16
Serum Exosomal Long Noncoding RNA Growth Arrest-Specific 5 Predicts 3-Month Mortality in Acute-on-Chronic Hepatitis B Liver Failure
Authors Sun CX , Han LY, Wang K, Gao S
Received 29 May 2023
Accepted for publication 9 October 2023
Published 17 October 2023 Volume 2023:16 Pages 4603—4616
DOI https://doi.org/10.2147/JIR.S423321
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Monika Sharma
Cheng-Xi Sun,1 Li-Yan Han,2,3 Kai Wang,2,3 Shuai Gao2,3
1Department of Clinical Laboratory, Qilu Hospital of Shandong University, Jinan, Shandong, 250012, People’s Republic of China; 2Department of Hepatology, Qilu Hospital of Shandong University, Jinan, Shandong, 250012, People’s Republic of China; 3Institute of Hepatology, Shandong University, Jinan, Shandong, 250012, People’s Republic of China
Correspondence: Shuai Gao, Department of Hepatology, Qilu Hospital of Shandong University, No. 107, Wenhuaxi Road, Jinan, Shandong, 250012, People’s Republic of China, Tel +86-531-82169593, Fax +86-531-86927544, Email [email protected]; [email protected]
Background: Acute-on-chronic hepatitis B liver failure (ACHBLF) is a clinical syndrome with an extremely high mortality. In this study, we aim to evaluate the potential role of serum exosomal long noncoding RNA (lncRNA) growth arrest-specific 5 (GAS5) in ACHBLF and its predictive value for 3-month mortality.
Methods: From December 2017 to June 2022, we enrolled 110 patients with ACHBLF and 42 healthy controls (HCs). Exosomes were isolated from the serum of the participants. Serum exosomal lncRNA GAS5 was detected using quantitative real-time polymerase chain reaction (qRT-PCR). The functional role of lncRNA GAS5 on hepatocyte phenotypes was investigated through loss-of-function and gain-of-function assays. Exosomal labeling and cell uptake assay were used to determine the exosomes-mediated transmission of lncRNA GAS5 in hepatocytes in vitro.
Results: The serum exosomal lncRNA GAS5 was identified to be an independent predictor for 3-month mortality of ACHBLF. It yielded an area under the receiver operating characteristic curve (AUC) of 0.88, which was significantly higher than MELD score (AUC 0.73; P < 0.01). Further study found that lncRNA GAS5 could inhibit hepatocytes proliferation and increase hepatocytes apoptosis. Exosomes-mediated lncRNA GAS5 transfer promoted hepatocytes injury. The knocked down of lncRNA GAS5 weakened H2O2-induced hepatocytes injury.
Conclusion: We revealed that serum exosomal lncRNA GAS5 might promote hepatocytes injury and showed high predictive value for 3-month mortality in ACHBLF.
Keywords: acute-on-chronic hepatitis B liver failure, exosomes, long noncoding RNA, growth arrest-specific 5, prognosis
Introduction
Acute-on-chronic liver failure (ACLF) is a complex clinical syndrome characterized by an acute episode of liver dysfunction in patients with pre-existing chronic liver diseases.1,2 The common causes of ACLF include viral infection, bacterial infection, alcoholic hepatitis and surgery injury.1,3–5 In Asia, hepatitis B virus (HBV) infection remains the main cause of ACLF, which is named acute-on-chronic hepatitis B liver failure (ACHBLF). The short-term mortality of ACHBLF is as high as 63%–72.3%.2 Until now, liver transplantation remains the only determined cure for ACHBLF. However, it is hard to be applied widely due to the limitation of liver donors and high cost in most Asia countries. Therefore, prognostic biomarkers for ACHBLF are urgently needed.
Exosomes are nanosized vesicles with a diameter of 40–200 nm, which can serve as mediators of intercellular communication through transferring proteins, nucleic acids and lipids among cells.6 Recent studies revealed that exosomes participated in the development and progression of several kinds of liver diseases.7–9 Long noncoding RNAs (lncRNAs) refer to a class of non-protein coding transcripts with a length of more than 200 nucleotides.10 They can regulate gene expression at the transcriptional and post-transcriptional levels through interacting with DNA, RNA or proteins.11 Recently, emerging evidences suggested that lncRNAs encapsulated in exosomes were involved in regulating physiological and pathological processes, such as immune response, inflammation, tumor growth and infection.12,13 In our previous study, we found that the serum exosomal lncRNA NEAT1 was aberrantly overexpressed in ACHBLF and might serve as a biomarker for short-term prognosis of ACHBLF.14
LncRNA growth arrest-specific transcript 5 (GAS5) has been proven to inhibit cells growth and sensitize cells to apoptosis.15,16 Low level of lncRNA GAS5 was found to be associated with early recurrence and poor prognosis of non-muscle-invasive bladder cancer.17 The up-regulated lncRNA GAS5 inhibited glioma cell growth, migration and invasion.18 Upregulation of lncRNA GAS5 suppressed cell proliferation, promoted cell apoptosis and reversed the resistance of cells to EGFR-TKIs via downregulation of the IGF-1R expression.19 In addition, lncRNA GAS5 played an important role in maintaining the self-renewal of human embryonic stem cell.20 Since ACHBLF was characterized by its increased hepatocytes apoptosis, we speculated that lncRNA GAS5 might also play a potential role in the pathogenesis of ACHBLF.
Therefore, we designed this study to evaluate the potential role of serum exosomal lncRNA GAS5 in ACHBLF and determine the predictive value of serum exosomal lncRNA GAS5 for 3-month mortality of ACHBLF.
Materials and Methods
Patients and Controls
In this study, a total of 110 patients with ACHBLF and 42 healthy controls (HCs) were enrolled from December 2017 to June 2022 at the Department of Hepatology, Qilu Hospital of Shandong University. ACHBLF was diagnosed according to the consensus recommendations of APASL: (1) the presence of serum hepatitis B surface antigen (HBsAg) for >6 month; (2) progressive jaundice (serum bilirubin≥5mg/dL); (3) coagulopathy (INR ≥ 1.5 or prothrombin activity <40%).2 Exclusion criteria included: (1) co-infected with human immunodeficiency virus (HIV), hepatitis A, C, D or E virus, Epstein-Barr virus, cytomegalovirus; (2) accompanied by other liver diseases, such as alcoholic hepatitis, autoimmune liver diseases; (3) decompensated liver cirrhosis; (4) liver cancer; (5) pregnancy. The routine therapies for patients with ACHBLF were in accordance with the APASL consensus recommendations, including anti-HBV treatment, absolute bed rest, energy supplements and vitamins, intravenous infusion of albumin, maintenance of water, electrolyte and acid–base equilibrium, prevention and treatment of complications.
Informed consent was obtained from each participants included. The study protocol was approved by the local Research and Ethics Committee at Qilu Hospital of Shandong University, in accordance with the guidelines of the 1975 Declaration of Helsinki (6th revision, 2008).
Serum Exosomes Isolation
Five milliliter of peripheral venous blood was drawn from each participant on the first day of diagnosis. All venous blood samples were firstly centrifuged at 3000 rpm for 10 min to separate serum, and then centrifuged at 11,000 rpm for 10 min to further remove cell debris. The serum was stored at −80°C for exosomes isolation.
The exosomes were isolated following the manufacturer’s instructions. Briefly, 250 µL of serum was mixed with 63 µL of Exo-Quick™ solution (EXOQ5A-1; SBI System Biosciences, United States). The mixture was incubated for 30 min, and then centrifuged twice at 4°C (1500 g for 30 min and 1500 g for 5 min). The supernatants were discarded, and the exosomes were resuspended in 50 µL of PBS.14
Transmission Electron Microscopy (TEM)
The morphological characteristics of exosomes were characterized by TEM. Briefly, isolated exosomes were resuspended in 50 μL PBS buffer and adsorbed onto the copper grid. Then, the copper grid of adsorbed exosomes was suspended above 2.5% glutaraldehyde droplet for fixation, and then suspended above 2% uranyl acetate for staining. Wash the copper grid 10 times with double distilled water, each time for 2 min. Images were collected with TEM (FEI, United States) at 185 kV.
Western Blot Assay
Cultured MIHA cells and isolated exosomes were lysed in RIPA buffer (Solarbio, China) supplemented with protease inhibitor cocktail (Solarbio, China). Protein concentrations were determined using BCA Protein Assay Kit (ThermoFisher Scientific, United States). Proteins were separated by a 10% SDS-polyacrylamide gel and electrophoretically transferred onto PVDF membranes (Millipore, United States). Subsequently, the membranes were blocked in 5% skimmed milk for 1h and incubated with primary antibodies at 4°C overnight. Following primary antibodies were used: anti-CD81 (ab79559, Abcam), anti-Hsp70 (ab45133, Abcam). After the membranes were incubated with HRP-conjugated secondary antibodies (ZSGB-BIO, China), immunoreactive signals were detected using enhanced chemiluminescence reagent (ThermoFisher Scientific, United States). GAPDH was used as internal reference control.
RNA Extraction and Reverse Transcription
Total RNA was extracted from exosomes by miRNeasyMicro Kit (Qiagen, Germany). Concentration and integrity of total RNA was evaluated using NanoDrop spectrophotometer (Thermo Fisher Scientific, United States). Purified RNA was reversely transcribed into cDNA using the TaKaRa Reverse Transcription Kit (TaKaRa, China).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
qRT-PCR was performed in a 25 µL system containing 12.5 µL of SYBR® Premix ExTaq™ (Takara, China), 0.5 µL of ROX Reference Dye α, 2 µL of diluted cDNA, 0.75 µL of gene specific forward and reverse primers (10 µmol/L), and 8.5 µL of RNase-free ddH2O. GAPDH was used as an endogenous control. The primers sequences for PCR were as follows: GAS5 (forward: 5’-CTGTGAGGTATGGTGCTGGG-3’, reverse: 5’-GGCTTGCTCTTTAGGACTTCA-3’), GAPDH (forward: 5’-AAGGTGAAGGTCGGAGTCAA-3’, reverse: 5’-GGAAGATGGTGATGGGATTT-3’). The reaction included an initial denaturation step at 95°C for 30 seconds, 42 cycles of amplifications at a melting temperature of 95°C for 5 seconds, and an annealing temperature of 60°C for 30 seconds. The product was confirmed using melting curve analysis. The serum exosomal lncRNA GAS5 level was calculated by 2−∆∆Ct method.
Cell Culture
Human hepatocyte MIHA was purchased from BeNa Culture Collection (Beijing, China). The cells were cultured in RPMI 1640 medium (Gibco, United States) supplemented with 10% fetal bovine serum (FBS) (Gibco, United States), 100 U/mL penicillin and 100 μg/mL streptomycin (Life Technologies, United States) in a humidified 37°C and 5% CO2 incubator.
Cell Transfection
The cDNA encoding lncRNA GAS5 was PCR-amplified by Thermo Scientific Phusion Flash High-Fidelity PCR Master Mix (ThermoFisher Scientific, United States) and subcloned into the XhoI and EcoRI sites of pIRES2-EGFP overexpression vector (Invitrogen, United States). The siRNAs specifically targeting GAS5 and control siRNA were synthesized by RiboBio (Guangzhou, China). Cells were transfected with the plasmids or siRNAs using Lipofectamine 3000 (Invitrogen, United States) according to the manufacturer’s instructions.
Cell Counting Kit-8 (CCK-8) Assay
CCK-8 (Dojindo, Japan) assay was conducted to evaluate cell viability. A total of 5000 MIHA cells were seeded into 96-well culture plates with corresponding treatment. Then, 10 μL CCK-8 reagent was added to each well. After incubation for 3h at 37°C, the absorbance was examined at 450 nm using the Infinite M200 spectrophotometer (Tecan, Switzerland).
Flow Cytometry
Cell apoptosis was detected using BD FACSCanto II flow cytometer (BD Biosciences, Belgium) with an Annexin V-FITC/7-amino-actinomycin (7-AAD) double-staining kit (BD Biosciences, Belgium). In brief, 5 µL of PE Annexin V and 5 µL 7-AAD were added into 100 µL of single-cell suspension (1 × 105 cells) followed by a 15 min incubation at RT (25°C) in the dark. Flow cytometry was performed within 1 hour. The cells are divided into four groups: dead cells (Q1), early apoptotic cells (Q2), viable cells (Q3), and late apoptotic cells (Q4).
Exosomes Isolation from Cell Culture Media
Exosomes in conditioned media of MIHA cells were isolated by differential ultracentrifugation. MIHA cells were incubated in RPMI 1640 medium containing 10% exosome-depleted FBS (SBI, United States) for 48 h. Then, conditioned media was collected and centrifuged at 2000g for 20 min at 4°C to remove cells followed by 10,000 g for 30 min at 4°C to remove cell debris. The resulting supernatant was filtered through a 0.22 mm filter (Millipore, United States). Exosomes were pelleted by ultracentrifuged at 100,000 g for 70 min at 4°C. After resuspension in phosphate-buffered saline (PBS), exosomes were pelleted again by ultracentrifuged at 100,000 g for 70 min at 4°C.
Exosomal Labeling and Cell Uptake Assay
To demonstrate that exosomes can be transmitted among hepatocytes, we labeled exosomes using PKH67 Fluorescent Cell Linker kits (Sigma-Aldrich, United States). A total of 10 μg exosomes isolated from culture medium supernatant were resuspended with PBS and labeled with PKH67 solution according to the manufacturer’s protocol. The exosomes labeled with PKH67 were co-cultured with MIHA cells for 24 h at 37°C. Cell nuclei were stained with DAPI (Solarbio, China).
Statistical Analysis
Quantitative variables were expressed as median (centile 25; centile 75). Categorical variables were expressed as number (percentage). The data were analyzed using GraphPad Prism 5.0 (GraphPad Software, LaJolla, CA, United States) and SPSS 16.0 (SPSS Inc., Chicago, IL, United States). The quantitative variables were compared by Student’s t-test and Mann–Whitney U-test. The categorical variables were compared by chi-square test. Spearman correlation analysis was used to evaluate correlation between serum exosomal lncRNA GAS5 level and clinicopathological parameters. The independent prognostic factors were identified using univariate and multivariate cox proportional hazards regression analysis. The receiver operating characteristic (ROC) curve was used to determine diagnostic performance. The area under the ROC curve (AUC) was used to evaluate the diagnostic value of lncRNA GAS5 and MELD score. From the ROC curve coordinates, the optimal cut-off point that associated with the maximum of the Youden index was chosen. Furthermore, sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), negative likelihood ratio (LR-) and positive likelihood ratio (LR+) was used to determine the diagnostic accuracy. Survival curve was used to evaluate the survival condition. All statistical analyses were two sided and P < 0.05 was considered statistically significant.
Results
General Characteristics
In this study, 153 patients with ACHBLF were screened from December 2017 to June 2022 at Department of Hepatology, Qilu Hospital of Shandong University. Eight patients were excluded for co-infection with HAV, HCV or HEV. Fifteen patients were excluded for alcoholic hepatitis and 7 patients were excluded for autoimmune hepatitis. Nine patients were excluded for history of hepatocellular carcinoma. Four patients were excluded for incomplete clinical data. Finally, 110 ACHBLF patients were enrolled (Figure 1). Meanwhile, 42 healthy controls were also enrolled at Qilu Hospital of Shandong University.
 |
Figure 1 The inclusive process of the participants. |
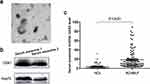
TEM was used to confirm the morphology of serum exosomes. The serum exosomes were spherical vesicles with double layer membrane structure and diameters about 40 to 200 nm (Figure 2a). Western blotting analysis was used to confirm the expression of specific protein markers in exosomes. CD81 and Hsp70 have been detected in the exosomes (Figure 2b).
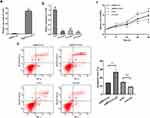
The general characteristics of enrolled participants are presented in Table 1. The serum exosomal lncRNA GAS5 levels in patients with ACHBLF were significantly higher than those in HCs (Z = −6.84, P < 0.01, Figure 2c). Then, we analyzed the association between serum exosomal lncRNA GAS5 expression and clinicopathological characteristics of ACHBLF patients. ACHBLF patients were categorized into high-expression group and low-expression group according to the median value of serum exosomal lncRNA GAS5. As shown in Table 2, high serum exosomal lncRNA GAS5 expression was significantly associated with TBIL (t = −5.75, P < 0.01), DBIL (t = −4.88, P < 0.01), PT-INR (Z = −2.38, P = 0.02) and MELD score (t = −4.04, P < 0.01). However, no significant association was found between serum exosomal lncRNA GAS5 expression and age (t = 0.90, P = 0.37), gender (χ2 = 0.23, P = 0.63), ALT (Z = −0.12, P = 0.90), AST (Z = −0.39, P = 0.70), ALB (t = −0.23, P = 0.82), Cr (Z = −1.08, P = 0.28), WBC (Z = −1.65, P = 0.10), HGB (t = 0.12, P = 0.91) and PLT (t = 0.42, P = 0.68). Through further statistical correlation analysis, we found that the expression level of serum exosomal lncRNA GAS5 was positively correlated with TBIL (Spearman’s r = 0.53, P < 0.01, Figure 3a), DBIL (Spearman’s r = 0.44, P < 0.01, Figure 3b), PT-INR (Spearman’s r = 0.28, P < 0.01, Figure 3c) and MELD score (Spearman’s r = 0.43, P < 0.01, Figure 3d).
 |
Table 1 Baseline Characteristics of the Enrolled Participants |
 |
Table 2 Relationship Between lncRNA GAS5 Expression and Clinicopathological Characteristics in ACHBLF |
Serum Exosomal lncRNA GAS5 Level Was an Independent Risk Factor for 3-Month Mortality of ACHBLF
After 3-month follow up, 60 patients with ACHBLF died and the mortality was 54.55%. The mean survival time was 54.04 (SE 3.50, 95% CI 47.18–60.89) days. Survivors (median 6.41, interquartile range 4.44–9.66) showed significantly lower serum exosomal lncRNA GAS5 levels than non-survivors (median 17.39, interquartile range 10.94–39.26; P < 0.01) (Figure 3e). Univariate Cox proportional hazards regression analysis showed that the 3-month mortality of ACHBLF was significantly associated with TBIL (P < 0.01), DBIL (P < 0.01), Cr (P = 0.04), PT-INR (P = 0.04), serum exosomal lncRNA GAS5 expression (P < 0.01) and MELD score (P < 0.01). Further multivariate Cox proportional hazards regression analysis demonstrated that serum exosomal lncRNA GAS5 expression (P < 0.01) was an independent predictor for 3-month mortality of ACHBLF (Table 3).
 |
Table 3 Univariate and Multivariate Analysis of Factors Associated with 3-Month Mortality of Patients with ACLF |
Diagnostic Value of Serum Exosomal lncRNA GAS5 Level and MELD Score for the 3-Month Mortality of ACHBLF Patients
In this study, serum exosomal lncRNA GAS5 yielded an AUC value of 0.88 (SE 0.03, 95% CI, 0.81–0.94; Figure 3f), which was markedly higher than MELD score (AUC 0.73; SE 0.05, 95% CI 0.64–0.81; P < 0.01), indicating that serum exosomal lncRNA GAS5 level was a better predictor than MELD score in predicting 3-month mortality of ACHBLF.
An optimal cut-off value of 10.12 was selected for serum exosomal lncRNA GAS5 level to discriminate survivors and non-survivors, with a sensitivity of 81.67%, specificity of 84.00%, PPV of 86.00%, NPV of 79.20%, LR+ of 5.10, and LR- of 0.22. The mean survival time of patients with serum exosomal lncRNA GAS5 levels >10.12 was 32.83 (SE 4.03, 95% CI 24.93–40.73) days. Meanwhile, the mean survival time of those patients with serum exosomal lncRNA GAS5 levels ≤10.12 was 77.69 (SE 3.77, 95% CI 70.31–85.07) days. Compared with patients with serum exosomal lncRNA GAS5 levels ≤10.12, survival in those with serum exosomal lncRNA GAS5 levels >10.12 was significantly poorer (χ2 = 47.41, P < 0.01, Log rank test) (Figure 3g).
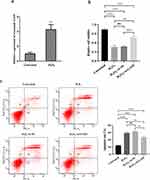
LncRNA GAS5 Inhibited Hepatocytes Proliferation and Increases Hepatocytes Apoptosis in vitro
The development of ACHBLF is largely due to an imbalance between hepatocytes apoptosis and proliferation. To determine the regulatory effect of lncRNA GAS5 on hepatocyte functions, MIHA cells were transfected with pIRES2-GAS5 or siRNAs. LncRNA GAS5 was overexpressed after transfected with pIRES2-GAS5 (Figure 4a) and knocked down by siRNAs (Figure 4b). Because of the highest knockdown efficiency of siRNA-3, it was applied to subsequent knockdown experiments (referred to as “si-GAS5” hereafter). Through carrying out CCK-8 assay, we found that cell viability was inhibited in MIHA cells with overexpressed lncRNA GAS5 and enhanced in MIHA cells after knockdown of lncRNA GAS5 (Figure 4c). To gain insight into the role of lncRNA GAS5 on cell apoptosis, flow cytometry was performed on Annexin V PE/7-AAD double staining hepatocytes. As shown in Figure 4d, the apoptosis proportion was increased by the overexpression of lncRNA GAS5 and reduced by the knockdown of lncRNA GAS5.
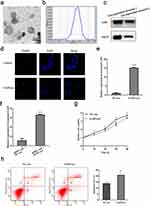
Exosomes-Mediated lncRNA GAS5 Transfer Promoted Hepatocytes Injury
We isolated exosomes from conditioned medium of MIHA cells and confirmed their identity. Exosomes exhibited typical cup-shaped morphology under TEM (Figure 5a). Size distribution and number of exosomes were quantified by nano-particle analysis (Figure 5b). The expression of exosomal markers CD81 and Hsp70 were confirmed by Western blot assay (Figure 5c). Subsequently, we verified exosomes could be transmitted among hepatocytes by PKH67 staining (Figure 5d). Then, we identified that exosomes could mediate the transfer of lncRNA GAS5 among hepatocytes and thus affect cell phenotypes. Exosomes were isolated from the conditioned media of lncRNA GAS5 overexpressed MIHA cells (GAS5-exo) and control-transfected cells (NC-exo). It was confirmed that lncRNA GAS5 level was higher in GAS5-exo than NC-exo (Figure 5e). As expected, MIHA cells incubated with GAS5-exo showed increased lncRNA GAS5 expression than those incubated with NC-exo (Figure 5f). CCK-8 assay demonstrated that MIHA cells incubated with GAS5-exo for 24 h showed impaired viability than those incubated with NC-exo (Figure 5g). Meanwhile, flow cytometry confirmed that MIHA cells incubated with lncRNA GAS5-exo for 24 h showed increased apoptosis rate than that incubated with NC-exo (Figure 5h).
Knocked Down of lncRNA GAS5 Weakened H2O2-Induced Hepatocytes Injury
H2O2 was used to induce hepatocytes injury. After treated with 0.1 mmol/L H2O2 for 3 h, the expression of lncRNA GAS5 was increased significantly in MIHA cells (Figure 6a), accompanied by impaired cell viability (Figure 6b) and elevated apoptosis rate (Figure 6c). Then, we knocked down lncRNA GAS5 in MIHA cells with si-GAS5. It weakened H2O2-induced hepatocytes injury by enhancing cell viability (Figure 6b) and decreasing apoptosis rate (Figure 6c).
Discussion
In this study, we evaluated the expression pattern of lncRNA GAS5 in serum exosomes of ACHBLF patients. We demonstrated that serum exosomal lncRNA GAS5 was elevated in ACHBLF and correlated with adverse clinical characteristics. Further study revealed that lncRNA GAS5 was a robust inhibitor of hepatocytes proliferation and promoted hepatocytes apoptosis. Exosomes-mediated intercellular communication might enhance the regulatory effect of lncRNA GAS5 on hepatocyte phenotypes. These findings implied a high application value of serum exosomal lncRNA GAS5 in clinical practice. The ACHBLF patients with serum exosomal lncRNA GAS5 levels >10.12 might have a worse prognosis and require more clinical treatment measures.
ACHBLF is a severe clinical syndrome with high mortality. Until now, the only effective treatment of ACHBLF is liver transplantation. However, liver transplantation cannot be widely the performed in Asia because of the limited organ source and high cost.21 Therefore, effective prognostic evaluation is helpful to organ allocation and treatment guidance. Previous studies have demonstrated that lncRNAs engaged in the pathogenesis of a variety of diseases.22,23 Specific lncRNA expression patterns coordinate different cellular states in physiological or pathological conditions.24,25 Therefore, this study aimed to identify a differentially expressed lncRNA in ACHBLF which could be used as a biomarker to predict ACHBLF prognosis and guide individualized treatment.
LncRNAs encapsulated into exosomes have been recognized as promising biomarker for occurrence and development of several diseases. The double-layer lipid structure of exosomes keeps its contents intact and bioactive, which is beneficial to improve the sensitivity of detection. Moreover, exosomes carry specific substances of their parental cells and exert obvious signal amplification effect, avoiding the interference of complex components in body fluids on the detection of biomarkers.26–28 In this study, we found that lncRNA GAS5 was up-regulated in serum exosomes of ACHBLF patients. Clinical implication of serum exosomal lncRNA GAS5 was further demonstrated. The expression of serum exosomal lncRNA GAS5 in ACHBLF patients was positively correlated with TBIL, DBIL, PT-INR and MELD score. It suggested that serum exosomal lncRNA GAS5 was an independent predictor for 3-month mortality of ACHBLF.
LncRNA GAS5 is a well-known lncRNA encoded by the GAS5 gene localized at 1q25.1. It is comprised of 12 exons (about 0.7 kb) and originally identified in growth-arrested mouse fibroblast cells.29 A short open reading frame (ORF) lying in exons of lncRNA GAS5 is able to encode for polypeptides; however, the sequence is poor conserved and not able to encode any functional protein.30 The expression level of lncRNA GAS5 is regulated by the interplay between the mammalian target of rapamycin (mTOR) and nonsense-mediated decay (NMD) pathways.31 Through the direct interaction with the DNA binding domain of the glucocorticoid receptor (GR), LncRNA GAS5 regulates the activity of glucocorticoid.16,32 Another major molecular mechanism of lncRNA GAS5 is to serve as “sponges” of miRNAs.18,33 According to these different mechanisms, lncRNA GAS5 plays a role in inhibiting cell proliferation and promoting apoptosis. In this study, we proved that overexpression of lncRNA GAS5 had the same regulatory effect on hepatocyte phenotypes including inhibition of cell proliferation and stimulation of apoptosis. Moreover, knockdown of lncRNA GAS5 in hepatocytes reduced H2O2-induced cell injury by enhancing cell viability and inhibiting apoptosis. Exosomes-mediated lncRNA GAS5 transfer among hepatocytes played an important role in the regulation of cell phenotypes. Therefore, elevated expression of lncRNA GAS5, at least partially, contributed to the exaggeration of liver injury in ACHBLF.
In this study, we found that serum exosomal lncRNA GAS5 was over-expressed in patients with ACHBLF. A study performed by Feng et al found that HBV could induce the expression of GAS5, while GAS5 did not modulate the replication of HBV.34 Another study showed that lncRNA GAS5 was downregulated in the sera from CHB patients compared with the sera from healthy controls.35 The possible reasons for the inconsistency among these studies might be multifaceted. In our study, we firstly isolated exosomes from serum and then detected lncRNA GAS5 expression within them. However, previous studies usually directly detected lncRNA GAS5 expression from serum or cells. Meanwhile, the lncRNA GAS5 was proved to inhibit cell proliferation and promote apoptosis. Hepatocytes apoptosis was much more obvious in patients with ACHBLF than CHB.
There were also several limitations in this study. Firstly, the clinical study was a single center study. A multicenter, large sample validation was still needed prior to its clinical application. Secondly, we revealed the potential role of exosomal lncRNA GAS5 in the development and progression of liver injury. However, the inner mechanism and signaling pathways associated with the function of lncRNA GAS5 was still unclear, which will be investigated in our further study.
In conclusion, we revealed that overexpressed serum exosomal lncRNA GAS5 was a promising prognostic biomarker for 3-month mortality in ACHBLF. LncRNA GAS5 could inhibit hepatocytes proliferation and increase hepatocytes apoptosis. Exosomes-mediated lncRNA GAS5 transfer promoted hepatocytes injury and the knocked down of lncRNA GAS5 weakened H2O2-induced hepatocytes injury in vitro. Therefore, lncRNA GAS5 had the potential to be a molecular therapeutic target for ACHBLF.
Data Sharing Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee at Qilu Hospital of Shandong University.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
The authors affirm that human research participants provided informed consent for publication of the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFC2301801), National Natural Science Foundation of China (82272313), Natural Science Foundation of Shandong Province (ZR2022MH006, ZR2020QH287, ZR2016HQ42), Jinan Science and Technology Development Plan (201704094).
Disclosure
The authors declare no competing interests in this work.
References
1. Bernal W, Jalan R, Quaglia A, et al. Acute-on-chronic liver failure. Lancet. 2015;386(10003):1576–1587. doi:10.1016/S0140-6736(15)00309-8
2. Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13(4):353–390. doi:10.1007/s12072-019-09946-3
3. Gustot T, Jalan R. Acute-on-chronic liver failure in patients with alcohol-related liver disease. J Hepatol. 2019;70(2):319–327. doi:10.1016/j.jhep.2018.12.008
4. Wu T, Li J, Shao L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67(12):2181–2191. doi:10.1136/gutjnl-2017-314641
5. Fernández J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67(10):1870–1880. doi:10.1136/gutjnl-2017-314240
6. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–289. doi:10.1146/annurev-cellbio-101512-122326
7. Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14(8):455–466. doi:10.1038/nrgastro.2017.71
8. Murphy AG, Selaru FM. Extracellular vesicles as novel therapeutics in hepatic failure. Hepatology. 2018;67(3):1158–1160. doi:10.1002/hep.29576
9. Jin Y, Wang J, Li H, et al. Extracellular vesicles secreted by Human Adipose-derived Stem Cells (hASCs) improve survival rate of rats with acute liver failure by releasing lncRNA H19. EBioMedicine. 2018;34:231–242. doi:10.1016/j.ebiom.2018.07.015
10. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407. doi:10.1016/j.cell.2018.01.011
11. Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov. 2013;12(6):433–446. doi:10.1038/nrd4018
12. Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77(1):13–27. doi:10.1146/annurev-physiol-021014-071641
13. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88(1):487–514. doi:10.1146/annurev-biochem-013118-111902
14. Gao S, Fan YC, Han LY, et al. Serum exosomal long noncoding RNA nuclear-enriched abundant transcript 1 predicts 90-day mortality in acute-on-chronic hepatitis B liver failure. Expert Rev Clin Immunol. 2021;17(7):789–797. doi:10.1080/1744666X.2021.1933442
15. Bockmann JH, Dandri M, Lüth S, et al. Combined glucocorticoid and antiviral therapy of hepatitis B virus-related liver failure. World J Gastroenterol. 2015;21(7):2214–2219. doi:10.3748/wjg.v21.i7.2214
16. Kino T, Hurt DE, Ichijo T, et al. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3(107):ra8. doi:10.1126/scisignal.2000568
17. Avgeris M, Tsilimantou A, Levis PK, et al. Loss of GAS5 tumour suppressor lncRNA: an independent molecular cancer biomarker for short-term relapse and progression in bladder cancer patients. Br J Cancer. 2018;119(12):1477–1486. doi:10.1038/s41416-018-0320-6
18. Zhao X, Wang P, Liu J, et al. Gas5 exerts tumor-suppressive functions in human glioma cells by targeting miR-222. Molecular Therapy. 2015;23(12):1899–1911. doi:10.1038/mt.2015.170
19. Dong S, Qu X, Li W, et al. The long non-coding RNA, GAS5, enhances gefitinib-induced cell death in innate EGFR tyrosine kinase inhibitor-resistant lung adenocarcinoma cells with wide-type EGFR via downregulation of the IGF-1R expression. J Hematol Oncol. 2015;8(1):43. doi:10.1186/s13045-015-0140-6
20. Xu C, Zhang Y, Wang Q, et al. Long non-coding RNA GAS5 controls human embryonic stem cell self-renewal by maintaining NODAL signalling. Nat Commun. 2016;7(1):13287. doi:10.1038/ncomms13287
21. Arroyo V. Acute-on-chronic liver failure in cirrhosis requires expedited decisions for liver transplantation. Gastroenterology. 2019;156(5):1248–1249. doi:10.1053/j.gastro.2019.03.004
22. Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. doi:10.1038/nrg.2015.10
23. Khorkova O, Hsiao J, Wahlestedt C. Basic biology and therapeutic implications of lncRNA. Adv Drug Deliv Rev. 2015;87:15–24. doi:10.1016/j.addr.2015.05.012
24. Flynn RA, Chang HY. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell. 2014;14(6):752–761. doi:10.1016/j.stem.2014.05.014
25. Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi:10.1016/j.cell.2013.02.012
26. Sun Z, Yang S, Zhou Q, et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer. 2018;17(1):82. doi:10.1186/s12943-018-0831-z
27. Hong CS, Funk S, Muller L, et al. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016;5(1):29289. doi:10.3402/jev.v5.29289
28. Yu X, He L, Pentok M, et al. An aptamer-based new method for competitive fluorescence detection of exosomes. Nanoscale. 2019;11(33):15589–15595. doi:10.1039/C9NR04050A
29. Fujiwara K, Yasui S, Yonemitsu Y, et al. Efficacy of combination therapy of antiviral and immunosuppressive drugs for the treatment of severe acute exacerbation of chronic hepatitis B. J Gastroenterol. 2008;43(9):711–719. doi:10.1007/s00535-008-2222-5
30. Zhao J, Zhang JY, Yu HW, et al. Improved survival ratios correlate with myeloid dendritic cell restoration in acute-on-chronic liver failure patients receiving methylprednisolone therapy. Cell Mol Immunol. 2012;9(5):417–422. doi:10.1038/cmi.2011.51
31. Pickard MR, Williams GT. Molecular and cellular mechanisms of action of tumour suppressor GAS5 LncRNA. Genes. 2015;6(3):484–499. doi:10.3390/genes6030484
32. Hudson WH, Pickard MR, de Vera IM, et al. Conserved sequence-specific lincRNA-steroid receptor interactions drive transcriptional repression and direct cell fate. Nat Commun. 2014;5(1):5395. doi:10.1038/ncomms6395
33. Zhang Z, Zhu Z, Watabe K, et al. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013;20(11):1558–1568. doi:10.1038/cdd.2013.110
34. Feng S, Ji G, Ma J, et al. Long noncoding RNA GAS5 does not regulate HBV replication. J Med Virol. 2019;91(11):1949–1959. doi:10.1002/jmv.25547
35. Guo Y, Li C, Zhang R, et al. Epigenetically-regulated serum GAS5 as a potential biomarker for patients with chronic hepatitis B virus infection. Cancer Biomarkers. 2021;32(2):137–146. doi:10.3233/CBM-203169
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.