Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 9
Serum-derived bovine immunoglobulin for children with diarrhea-predominant irritable bowel syndrome
Authors Arrouk R, Herdes RE, Karpinski AC, Hyman PE
Received 15 December 2017
Accepted for publication 26 September 2018
Published 24 October 2018 Volume 2018:9 Pages 129—133
DOI https://doi.org/10.2147/PHMT.S159925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Roosy Aulakh
Rami Arrouk,1 Rachel E Herdes,1 Aryn C Karpinski,2 Paul E Hyman1
1Department of Pediatrics, Louisiana State University, New Orleans, LA 70118, USA; 2Evaluation and Measurement Program, School of Foundations, Leadership, and Administration, College of Education, Health, and Human Services, Kent State University, Kent, OH 44240, USA
Background: Oral serum-derived bovine immunoglobulin (SBI)/protein isolate is a medical food intended to manage chronic diarrhea. It has been shown to improve pain and diarrhea in adults with diarrhea-predominant irritable bowel syndrome (d-IBS).
Aim: To determine if SBI can improve symptoms in children with d-IBS.
Methods: We performed a randomized, double-blind, placebo-controlled, pilot study (NCT02609529) to evaluate the effectiveness of SBI in children 8–18 years with d-IBS. We recorded stool number, abdominal pain, and stool form in all patients for 1 week and then assigned the patients at a ratio of 2:1 to treatment with SBI 5 g BID or placebo for 3 weeks. The patients and their parents completed the Pediatric Quality of Life Inventory™ for Gastrointestinal Symptoms (PedsQOL) and the Pediatric Functional Disability Index (FDI). In addition, complete blood counts and serum chemistries were recorded at the start and end of treatment to evaluate safety.
Results: Fifteen patients (nine SBI, six placebo) completed the study. Both SBI and placebo groups reported nonstatistical reductions in stool frequency per week. The SBI group showed a significant reduction in stool frequency at weeks 1 and 2 but not at the end of treatment. The SBI group also demonstrated statistical improvements in abdominal pain and stool form by 3 weeks. The placebo group did not achieve similar improvements. The overall FDI and PedsQOL scores, as well as PedsQOL subscale scores for pain, discomfort when eating, diarrhea, worry about stomach aches, and communication, improved significantly in the SBI group, but not in the placebo group. No serious adverse events occurred. Serum chemistries and hemograms were normal at baseline and at the end of study in all patients.
Conclusion: In this single-center, exploratory pilot study, we demonstrated that 10 g SBI per day was safe in children with d-IBS and improved symptoms. Larger studies, with longer treatment duration, seem warranted based on these initial positive results.
Keywords: pediatric, irritable bowel syndrome, diarrhea, abdominal pain
Introduction
Irritable bowel syndrome (IBS) is one of the most common diagnoses in new patients in pediatric gastroenterology clinics.1 In addition to abdominal pain, pediatric IBS patients often have diarrhea, constipation, or both. About one-third of pediatric IBS subjects have diarrhea-predominant IBS (d-IBS).2 There are no US Food and Drug Administration (FDA)-approved treatments for children with d-IBS. There is evidence that d-IBS is caused by a mild inflammation in the intestinal mucosa.3 Oral serum-derived bovine immunoglobulin (SBI)/protein isolate is a medical food intended to manage chronic diarrhea. Its mechanism of action involves binding of immunoglobulin (IgG) to microbial components to prevent their penetration into the lamina propria, which may help to ameliorate inflammation in the small intestine associated with some cases of d-IBS, especially the inflammation arising after acute gastroenteritis.4–6 SBI has been shown to improve pain and diarrhea in adults with d-IBS.7 In this study, we aim to determine if SBI can also improve symptoms in children with d-IBS.
Methods
The study protocol was approved by the institutional review board of Louisiana State University – Health Science Center and the Children’s Hospital of New Orleans (ClinicalTrials.gov Identifier, NCT02609529). All patients gave their assent, and their legal guardians provided written informed consent before the beginning of the study.
We designed a randomized, double-blind, placebo-controlled, weighted pilot study to evaluate the effect of SBI on symptoms in children 8–18 years, newly diagnosed with d-IBS according to ROME III criteria.8 The ROME III criteria consists of recurrent abdominal pain or discomfort for at least 2 days per week in the last 3 months associated with two or more of the following: improvement with defecation, onset associated with a change in frequency of stool, and onset associated with a change in form (appearance) of stool.8
To be included in the study, patients needed to be male and/or nonpregnant females between 8 and 18 years at the time of consent. Exclusion criteria included concurrent pharmacologic treatment for d-IBS, non-English speaker, known allergy or hypersensitivity to beef or any component of SBI, and pregnancy.
We assessed baseline symptoms for 1 week (stool number, abdominal pain, and stool type using the Bristol stool scale) during a screening phase and assigned patients to 5 g BID SBI (Entera Health, Inc., Ankeny, IA, USA) or matched placebo (hydrolyzed gelatin protein isolate) at a ratio of 2:1 for 3 weeks. Simple randomization was performed by a pharmacist who kept the trial blinded until all patients had completed the study.
SBI/protein isolate contains a minimum of 50% IgG along with other serum proteins. Neither SBI nor the placebo contained any milk-derived proteins or sugars. The isolate is a light-colored powder composed of finely ground flakes, easily dissolved in water or other drink. A minimum of 4 ounces of fluid was used to dissolve the protein formulations. The patients and their parents completed the Pediatric Quality of Life Inventory™ for Gastrointestinal Symptoms (PedsQOL)9 and the Pediatric Functional Disability Index (FDI)10,12 during screening and at the end of treatment. We also performed laboratory assessments (complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein) during screening and at the end of treatment. We conducted a urine pregnancy test for female patients during the screening visit.
The patients recorded gastrointestinal symptoms using a daily diary. The primary end point was change in number of stools or bowel movements per week from the screening phase compared to the end of treatment. The secondary clinical outcome variables included changes in abdominal pain, and stool consistency, at the end of treatment compared to the screening phase. The secondary psychosocial outcomes included PedsQOL and FDI scores at the end of treatment compared to the screening phase.
All analyses were conducted using SPSS version 24.0. Demographic and descriptive statistics were calculated as means, SDs, frequencies, and percentages reported. Skewness and kurtosis statistics were used to examine normality. Inferential statistical analyses using dependent t-tests were conducted comparing two time points at baseline and at the end of treatment (week 3). Changes in the number of bowel movements, abdominal pain ratings, stool consistency, and the FDI and PedsQOL psychosocial outcomes were examined in both groups. From these planned analyses, an a priori power analysis was conducted using G*Power 3 (Power =0.80, a=0.05). The minimum total sample size required to detect a medium effect was 27.10,11
Results
We randomized 20 patients in this study, of which 15 completed the study (overall, age 13.5±3.6 years, six male, 12 white, one black, two Hispanic; SBI, n=9, age 14.3±3.7 years, four male; placebo, n=6, age 12±3.3 years, two male). In the SBI group, one patient dropped out after 2 days due to emesis potentially related to treatment, one patient dropped out due to palatability, and two patients were lost to follow-up and could not be evaluated. One placebo patient dropped out after the first visit. In total, nine patients received SBI and six received placebo completing 3 weeks of the study. There were no serious adverse events. Serum chemistries and hemograms were normal at baseline and at the end of study in all patients.
There was a statistical reduction in stool frequency per week compared to the screening phase in the SBI group from 18.0±5.3 per week during the screening week to 12.2±5.9 per week after 1 week (P=0.02) and 12.7±5.6 per week after week 2 (P=0.03). There was no significant reduction in stool frequency per week comparing the screening phase to 13.6±5.4 per week at the end of treatment (P=0.07). The placebo group showed significantly less stool frequency per week compared to the screening phase from 19.7±8.3 per week during screening to 15.0±16.7 per week after 1 week (P=0.34), 12.5±15.0 per week after 2 weeks (P=0.06), and 15.5±13.6 per week at the end of treatment (P=0.12). The differences in stool number between the two groups at the end of treatment were also not significant (P=0.70; Figure 1). The treatment group reported a reduction in abdominal pain (P=0.02) and improved stool form (P=0.05) after 3 weeks (Table 1). The placebo group did not show a significant reduction in abdominal pain (P=0.08) or improvement in stool form (P=0.28).
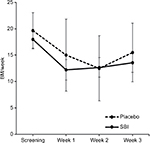  | Figure 1 The change in number of stools per week throughout the duration of the study. Abbreviation: BM, bowel movement. |
  | Table 1 Changes in gastrointestinal symptom scores in both groups between the screening week and end of treatment Abbreviation: SBI: serum-derived bovine immunoglobulin. |
The FDI scores of patients improved significantly in the SBI group (P=0.01), but not in the placebo group (P=0.23; Table 2). The PedsQOL scores also improved significantly with SBI (P=0.01), but not with placebo (P=0.14). The parent-assessed PedsQOL scores were significant for patients on SBI (P=0.004) and trended toward improvement for patients on placebo (P=0.06; data not shown). For IBS-related subscales of the PedsQOL questionnaire, there was statistical improvement of the scores from baseline to end of treatment in the SBI group for pain and discomfort (P=0.008), discomfort when eating or postprandial discomfort (P=0.01), constipation (P=0.03), diarrhea (P=0.009), worry about stomach aches (P=0.03), and communication (P=0.03). There was no significant improvement in these scores in the placebo group (P>0.05 for all).
Discussion
In this exploratory pilot study, we demonstrated that the 10 g SBI per day was safe in children ranging from 8 to 18 years of age. The study did not meet the primary end point of significant reduction in stool frequency at end of treatment with SBI but showed a trend toward significance (P=0.07). There was a significant reduction in stool frequency at weeks 1 and 2 in SBI group, but not in placebo group. In addition, SBI group demonstrated a significant reduction in abdominal pain (P=0.02) and improved stool form (P=0.05) compared to placebo group by the end of treatment. Finally, the FDI scores and scores of symptomology subscales of the PedsQOL questionnaire were significant compared to placebo by end of treatment.
In a recent study, only modest differences in stool form and frequency were found between children with IBS and healthy children, and childhood IBS subtypes could not be differentiated by stool frequency.13 However, we enrolled only patients who had stool frequencies averaging 18.7±6.4 per week, a higher number than normal children, and greater than those reported for d-IBS, and hence chose stools frequency as the primary end point.13 The nonsignificant reduction in stool frequency at the end of treatment in both groups could be related in part to the placebo response observed in FGID treatment trials, that has been attributed in part to the attention given to enrolled patients, including detailed explanation, reassurance, close monitoring, and ready access to the researchers, which may produce a therapeutic effect, a bias leading to an error in estimating the treatment effect.14 In addition, there was slight difference in age and number of male and female participants in each group with the SBI group being slightly older (14.3±3.7 vs 12±3.3 years) and having a higher proportion of male patients (44% vs 33%) which could have biased the results.
A statistical weakness in our study was that the study was underpowered; the minimum total sample size needed to detect at least medium effects for the paired samples t-tests conducted was 27. The reason for low recruitment number (20 vs 27) may be due to that the study population included children with d-IBS who had on average >18 stools per week. Weidler et al13 reported that the mean daily stool number in children with d-IBS was 1.01±0.44 compared to healthy children with 0.7±0.30 per day. This represents approximately 7±3 bowel movements per week, far below that of the population recruited for this study. However, the main assumption for the test (eg, normality) was met in our study, even though the sample sizes for both groups were small.
Another factor that may have contributed to our results is the 3 weeks treatment duration that we chose for a condition with symptoms that are known to be of intermittent and fluctuating severity.15 Three weeks is a shorter duration than the one recommended in the European Medicines Agency (EMA) guidelines,16 which is a duration of 4 weeks or longer for trials to establish short-term efficacy, and a minimum of 6 months for trials intended to establish long-term efficacy. For shorter study periods, it may be valuable to measure blood levels of amino acids or even fecal IgG levels to assure compliance in delivery of the bovine IgG. Shaw et al17 found in healthy adults, for example, that 10 g of SBI per day statistically increased blood levels of essential amino acids and specifically tryptophan between 2 and 3 weeks. In addition, they found the presence of bovine IgG in the feces. Unfortunately, other markers of tryptophan metabolism or inflammation in d-IBS have not yet been validated utilizing SBI in the management of this condition.18 Larger studies with longer treatment duration seem warranted.
Acknowledgments
The authors would like to acknowledge Bruce Burnett, PhD, and Raymond Panas, PhD (Medical Affairs, RedHill Biopharma, Inc., Cary, NC, USA), for their editorial support and review efforts in the preparation and finalization of this manuscript. The abstract of this manuscript was presented as a poster at the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Annual Meeting on 14 November 2017 in Las Vegas, NV, USA.
Author contributions
Each of the authors contributed to the planning and development of the manuscript. Dr Rami Arrouk, Dr Rachel E Herdes, and Dr Paul E Hyman recruited subjects for the study and served as the attending physicians for the subjects care. Dr Aryn C Karpinski provided statistical support for the analysis of the data. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Rouster AS, Karpinski AC, Silver D, Monagas J, Hyman PE. Functional gastrointestinal disorders dominate pediatric gastroenterology outpatient practice. J Pediatr Gastroenterol Nutr. 2016;62(6):847–851. | ||
Giannetti E, de’angelis G, Turco R, et al. Subtypes of irritable bowel syndrome in children: prevalence at diagnosis and at follow-up. J Pediatr. 2014;164(5):1099–1103. | ||
Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303(7):G775–G785. | ||
Asmuth DM, Ma ZM, Albanese A, et al. Oral serum-derived bovine immunoglobulin improves duodenal immune reconstitution and absorption function in patients with HIV enteropathy. AIDS. 2013;27(14):2207–2217. | ||
Jiang R, Chang X, Stoll B, et al. Dietary plasma protein reduces small intestinal growth and lamina propria cell density in early weaned pigs. J Nutr. 2000;130(1):21–26. | ||
Bosi P, Casini L, Finamore A, et al. Spray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J Anim Sci. 2004;82(6):1764–1772. | ||
Wilson D, Evans M, Weaver E, Shaw AL, Klein GL. Evaluation of serum-derived bovine immunoglobulin protein isolate in subjects with diarrhea-predominant irritable bowel syndrome. Clin Med Insights Gastroenterol. 2013;6:49–60. | ||
Di Lorenzo C. Childhood functional gastrointestinal disorders: child/ adolescent. In: Drossman DA, editor. Rome III: The Functional Gastrointestinal Disorders. McLean, VA: Degon Associates; 2006;741. | ||
Varni JW, Kay MT, Limbers CA, Franciosi JP, Pohl JF. PedsQL gastrointestinal symptoms module item development: qualitative methods. J Pediatr Gastroenterol Nutr. 2012;54(5):664–671. | ||
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. | ||
Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. | ||
Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16(1):39–58. | ||
Weidler EM, Self MM, Czyzewski DI, Shulman RJ, Chumpitazi BP. Stooling Characteristics in Children With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2017;15(1):140–141. | ||
Irvine EJ, Tack J, Crowell MD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1469–1480. | ||
Palsson OS, Baggish J, Whitehead WE. Episodic nature of symptoms in irritable bowel syndrome. Am J Gastroenterol. 2014;109(9):1450–1460. | ||
European Medicines Agency. Guideline on the Evaluation of Medicinal Products for the Treatment of Irritable Bowel Syndrome. London, UK: EMA; 2013. | ||
Shaw AL, Mathews DW, Hinkle JE, et al. Absorption and safety of serum-derived bovine immunoglobulin/protein isolate in healthy adults. Clin Exp Gastroenterol. 2016;9:365–375. | ||
Valentin N, Camilleri M, Carlson P, et al. Potential mechanisms of effects of serum-derived bovine immunoglobulin/protein isolate therapy in patients with diarrhea-predominant irritable bowel syndrome. Physiol Rep. 2017;5(5):e13170. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

