Back to Journals » OncoTargets and Therapy » Volume 13
Serum CXCL13 Level is Associated with Tumor Progression and Unfavorable Prognosis in Penile Cancer
Authors Mo M, Tong S, Li T, Zu X, Hu X
Received 28 May 2020
Accepted for publication 11 August 2020
Published 31 August 2020 Volume 2020:13 Pages 8757—8769
DOI https://doi.org/10.2147/OTT.S263980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tohru Yamada
Miao Mo,1 Shiyu Tong,1 Tao Li,2 Xiongbing Zu,1 Xiheng Hu1
1Department of Urology, Xiangya Hospital, Central South University, Changsha, Hunan 410008, People’s Republic of China; 2Xiangya School of Medicine, Central South University, Changsha, Hunan 410008, People’s Republic of China
Correspondence: Xiheng Hu
Department of Urology, Xiangya Hospital, Central South University, Changsha 410008, People’s Republic of China
Email [email protected]
Background: Chemokine (C-X-C motif) ligands (CXCLs) are important regulators of tumor progression in many cancers and could serve as potential cancer biomarkers. However, the expression patterns as well as functions of CXCLs remain unclear in penile cancer (PC). The aim of this study was to evaluate the usefulness of serum CXCL13 as a potential cancer biomarker for PC.
Patients and Methods: This retrospective study enrolled 76 patients diagnosed with PC between 2016 and 2018. Serum CXCL13 level was detected by enzyme-linked immunosorbent assay. Univariable and multivariable Cox regression analyses were conducted to identify the prognostic factors that influence disease-free survival. Human penile cancer cell lines Penl1, Penl2, 149RCa and LM156 were used as in vitro models. The expression of CXCL13 protein in PC cell lines was analyzed by Western blotting.
Results: Our initial analysis on GSE57955 dataset identified CXCL13 as a top CXCL gene enriched in PC. Higher preoperative serum CXCL13 level was detected in PC cohorts than in healthy male controls (P< 0.001). The area under the curve was 0.911 with the sensitivity of 84.2% and specificity of 87.0% to distinguish PC. Preoperative serum CXCL13 level was associated with pathological grade (P=0.048), T stage (P=0.009), nodal status (P< 0.001) and pelvic lymph node metastasis (P=0.005) in PC. Serum CXCL13 level could serve as an independent prognostic factor for disease-free survival with a HR of 3.818 (95%CI: 1.126– 12.946). Furthermore, autocrine expression of CXCL13 was detected in PC tissues and cell lines. Knockdown of CXCL13 expression suppressed malignant phenotypes (cell proliferation, clonogenesis, apoptosis escape, migration and invasion), attenuated STAT3 and ERK1/2 signaling and reduced MMP2/9 secretion in PC cell lines.
Conclusion: Serum CXCL13 could serve as a novel diagnostic and prognostic biomarker for PC. CXCL13 signaling might activate oncogenic signaling pathways to promote malignant progression of PC.
Keywords: penile cancer, CXCL13, cancer biomarker, tumor progression
Introduction
Penile cancer (PC) is a rare cancer in developed countries (0.5 to 1.6 per 100,000 men), despite its much higher incidence in some regions of South America, Asia, and Africa.1 Clinical outcomes of penile cancer are reported to be associated with histological subtype, pathological grade and TNM stage, with the presence of nodal metastasis being the most important prognostic factor for patient survival.2 Despite recent progress in multimodal therapies, the clinical outcome of PC still remains unsatisfactory, as the survival of PC patients has not improved during the last two decades.2 Serum cancer biomarkers have been a promising noninvasive tool for cancer diagnosis and treatment.3 However, there is still a lack of serum biomarkers for penile cancer. Although serum cancer biomarkers including CEA, AFP, CA125, CA19-9, and CA15-3 have been very useful to assist cancer diagnosis and disease monitoring in many cancers,4–6 the usefulness of such markers in PC remains to be demonstrated. Despite squamous cell carcinoma antigen (SCCa) level correlated with PC tumor burden, it could not predict the clinical outcome.7
Chemokines belong to a group of proinflammatory cytokines that recruit various immune cells to diverse tissues in response to pathogen-related infection.8 C-X-C motif chemokine ligands (CXCLs) is originally identified as important chemokines secreted by immune cells (monocytes, T lymphocytes, etc).8,9 Until now, 16 CXCLs have been identified (CXCL1, CXCL2, CXCL3, PF4, CXCL5, CXCL6, PPBP, CXCL8, CXCL9, CXCL10, CXCL11, CXCL12, CXCL13, CXCL14, CXCL16 and CXCL17). The connection between CXCLs and cancer has been unveiled in recent studies. The dysregulated expression of CXCLs as well as their relationship with clinicopathological characteristics and clinical outcomes is documented in many cancers.10–16 Some CXCLs including CXCL1, CXCL5, CXCL8, CXCL10, and CXCL13 are considered potential oncogenes and potential cancer biomarkers.10 CXCL1 is overexpressed in breast, bladder, gastric, and lung cancer;11 CXCL5 is overexpressed in breast, colorectal, pancreatic, and hepatocellular cancer.12 CXCL8 were upregulated markedly in gastric, pancreatic, bladder, and colorectal cancer.13 Nevertheless, increased CXCL10 expression is associated with malignant progression of melanoma, colorectal, and bladder cancer.14 Recently, CXCL9 and CXCL13 are reported to be associated with renal, breast and oral cancer.15,16 However, the expression patterns as well as functions of CXCLs remain unclear in penile cancer. Herein, we aimed to examine the expression of CXCLs in PC, and assess the usefulness of serum CXCL13 as a potential cancer biomarker for PC.
Patients and Methods
Patient Characteristics
This study was conducted in accordance with the Declaration of Helsinki. The patients (n=76) included in this retrospective study were performed surgery and diagnosed with PC (2016–2018) at Xiangya Hospital, Central South University. Patients with prior chemotherapy or brachytherapy history were excluded. Blood samples were collected one day prior to surgery (preoperative) or at day 28 after surgery (postoperative). Serum samples were separated and stored at −80ºC for later analysis. Serum samples of healthy male donors (n=46) were obtained from Health Examination Center (Xiangya hospital, Central South University). These healthy healthy male donors (median age: 53, range: 39–86) could match the age of the PC cohort (median age: 52, range: 40–75). This study was approved by the Research Ethics Committee of Xiangya Hospital Central South University (Rev. No. 201,805,847). Written informed consent was obtained from the patients and participants. The clinical parameters of PC patients included age, stage, nodal status/distant metastasis, histological subtype, pathological grade, and body mass index (BMI) as well as phimosis, etc. TNM staging was assigned with reference to the American Joint Committee on Cancer, 8th edition.17 All patients were prescribed a follow-up regimen based on the National Comprehensive Cancer Network guidelines, with a physical examination every six months. Cancer and vital status were recorded by clinical follow-up at our department.
Reagents and Cell Lines
Primary antibodies against CXCL13 and β-actin were obtained from Abcam (Cambridge, MA, USA); Primary antibodies against p-STAT3 (Tyr705), STAT3, p-ERK1/2 (Thr202/Tyr204), ERK1/2, p-AKT (Ser473) and AKT were obtained from Cell Signaling Technology (Beverly, MA, USA). Human penile cancer cell lines Penl1, Penl2, 149RCa and LM156 were kindly provided by Prof. Hui Han (Department of Urology, Sun Yat-sen University Cancer Center).18 These cell lines was routinely grown in DMEM supplemented with 10% FBS as described previously.18 The use of the cell lines was approved by the Research Ethics Committee of Xiangya Hospital Central South University.
Real-time PCR
Fresh PC and matched adjacent penile tissues (AT) (n=23) were obtained from Department of Urology, Xiangya Hospital Central South University with informed consent. The mRNA level of CXCL13 in PC and AT was analyzed using real-time PCR as described previously.19 Briefly, total RNA was extracted from tissues using TRIzol reagent and further purified using the Qiagen RNeasy kit (Germantown, MD, USA). Total RNA (1 µg) was used to generate cDNA, which was then used for the quantitative PCR using Invitrogen SYBRGreen PCR assays (Carlsbad, CA, USA). Relative gene expression was determined based on the threshold cycles (Ct values) of the CXCL13 and of the internal reference gene β-Actin. PCR Primers for CXCL13 and β-Actin are listed as follows:
CXCL13:
Forward: 5ʹ GCTTGAGGTGTAGATGTGTCC 3ʹ
Reverse: 5ʹ CCCACGGGGCAAGATTTGAA 3ʹ
β-Actin:
Forward: 5ʹ CATGTACGTTGCTATCCAGGC 3ʹ
Reverse: 5ʹ CTCCTTAATGTCACGCACGAT 3ʹ
The relative gene expression (RE) was calculated as the fold change relative to internal reference, which was based on the following equation:
RE = 2−ΔΔCt,
ΔΔCt = (meanCtPC − meanCtreference) − (meanCtAT − meanCtreference).
Enzyme-linked Immunosorbent Assay (ELISA)
The serum CXCL13 level of the aforementioned PC patients (n=76) and healthy male subjects (n=46) was measured using Abcam CXCL13 ELISA kit (Cambridge, MA, USA). The secretion of CXCL13, MMP2 and MMP9 into the supernatants of cell cultures was also determined by ELISA.
Immunohistochemistry (IHC)
Archival paraffin-embedded normal penile tissues (n=36) and PC tissues (n=40) were collected for IHC staining. These patients were performed surgery and diagnosed with PC (2017–2018) in Xiangya hospital, Central South University. IHC for CXCL13 were conducted as described previously.19,20 Antigen-antibody reactions (CXCL13 antibody dilution, 1:100) were visualized by exposure to 3.3-diaminobenzidine and hydrogen peroxide chromogen substrate (DAKO, Denmark). The expression of CXCL13 in PC cases was classified as high expression (≥30% of PC cells showed immunopositivity) or low expression (<30% of PC cells showed immunopositivity).
Western Blotting
Whole cell lysates were prepared with radio immunoprecipitation assay (RIPA) lysis buffer. The experimental procedure of Western blotting was conducted as described previously.21–23 Protein blots were visualized using the Abcam enhanced chemiluminescence system (Cambridge, MA, USA).
GEO Dataset
GEO dataset GSE57955 could be downloaded from NCBI GEO website (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE57955). Gene expression data were analyzed as described previously.24 Genes with a mean log2 signal ratio (penile cancer/normal glans pool) of ≥1.0 and ≤−1.0 within a 99%CI were considered differentially expressed.
Statistical Analysis
Statistical analyses were carried out using SPSS 16.0 software. The level of serum CXCL13 of two groups was compared by Mann–Whitney tests. The pre- and postoperative serum CXCL13 level was compared by Wilcoxon matched pairs test. The optimal cut-off value of serum CXCL13 was determined based on receiver-operating characteristic (ROC) analysis. Kaplan–Meier curves of disease-free survival were plotted and survival in the groups was compared by log rank test. The prognostic factors that influence disease-free survival were identified by univariable and multivariable Cox regression analysis. P<0.05 was considered statistically significant.
Results
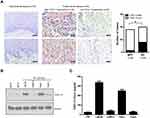
CXCL13 Was Highly Expressed in PC Tissues
The mRNA expression of CXCLs in PC (n=39) was analyzed in public GEO dataset GSE57955. As shown in Figure 1A, CXCL1, CXCL5, CXCL8, CXCL9, CXCL10, CXCL11, CXCL13 and CXCL16 were highly expressed in PC with reference to normal glans pool (NG) (Mean Log2(PC/NG) ≥1); while CXCL12 was low in PC (Mean Log2(PC/NG) ≤1). CXCL13 was the top CXCL highly enriched in PC (Mean Log2(PC/NG)=3.77). More than 70% of PC cases (28/39) exhibited high level of CXCL13 expression (Log2 (PC/NG) ≥1), Figure 1B). We also examined the mRNA level of CXCL13 in 23 pairs of fresh PC tissues and adjacent nontumor tissues (AT) using real-time PCR. The result showed that the mRNA level of CXCL13 was considerably higher in PC tissues than in matched adjacent nontumor tissues (P<0.001, Figure 1C). We did not observe the association between human papilloma virus (HPV) infection and CXCL13 expression in GSE57955 dataset, as CXCL13 expression in HPV– cases did not significantly differ from those HPV+ cases (Figure 1D, P=0.210).
Preoperative Serum CXCL13 Level was Significantly Elevated in PC Patients
A total of 76 men diagnosed with PC were enrolled in this study. Detailed summary of patient and tumor characteristics including treatment plan, TNM stage, histological subtype and pathological grade was shown in Table 1. Serum CXCL13 level was measured in healthy male control and PC patients. Preoperative serum CXCL13 level was significantly higher in PC cohort (98.5 ± 71.9 pg/mL) than that in healthy male control (20.6 ± 14.9 pg/mL) (P < 0.001. Figure 2A). The area under the curve (AUC) was 0.911 with the sensitivity of 84.2%, and specificity of 87.0% to distinguish penile cancer (Cutoff =36.3 pg/mL, Figure 2B). Moreover, Serum CXCL13 level was significantly decreased after surgery (P < 0.001, Figure 2C).
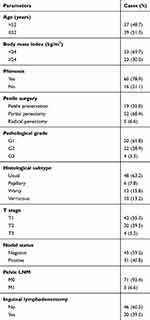 |
Table 1 Clinicopathologic Characteristics of PC Patient Cohort |
Preoperative Serum CXCL13 Level was Associated with Tumor Progression and Unfavorable Clinical Outcome
The association between preoperative serum CXCL13 level and clinicopathological parameters (age, body mass index, pathological grade, phimosis, histological subtype, TNM stage) was analyzed. As shown in Figure 3, preoperative serum CXCL13 level was significantly associated with clinicopathological parameters including pathological grade (P=0.048), T stage (P=0.009), nodal status (P<0.001), and pelvic lymph node metastasis (LNM) (P=0.005). Preoperative serum CXCL13 level was not significantly associated with histological subtype (P=0.265), body mass index (P=0.137), phimosis (P=0.499), and age (P=0.751). ROC analysis showed that serum CXCL13 level had a sensitivity of 83.3% and a specificity of 79.3% with reference to cancer recurrence (cutoff value: 107 pg/mL, AUC=0.857, Figure 4A). Survival analysis showed that PC patients with high serum CXCL13 level (≥107 pg/mL) exhibited unfavorable disease-free survival (P<0.001) (Figure 4B).
 |
Figure 4 (A) ROC curve for preoperative serum CXCL13 level with reference to cancer recurrence. (B) PC patients with high preoperative serum CXCL13 level exhibited shorter disease-free survival. |
Univariable Cox regression analysis showed that nodal status (P<0.001), Pelvic LNM (P<0.001), and higher preoperative serum CXCL13 level (P<0.001) were associated with shorter disease-free survival in our PC cohort (Table 2). Meanwhile, multivariable Cox regression analysis indicated that nodal status (P=0.021), pelvic LNM (P=0.005), and higher preoperative serum CXCL13 level (P=0.031) could serve as independent prognostic factors for unfavorable disease-free survival (Table 2).
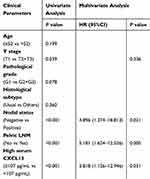 |
Table 2 Cox Univariate and Multivariate Proportional Hazard Model for Factors Affecting Disease-free Survival in PC Cases |
Expression of CXCL13 in PC Tissues, Cell Lines and Culture Supernatant
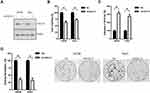
The expression of CXCL13 protein in normal penile tissues (n=36) and PC tissues (n=40) was analyzed by IHC. CXCL13 exhibited cytoplasmic staining in PC cells (Figure 5A). Overall, 40% of PC cases (16/40) exhibited high CXCL13 expression (immunopositivity ≥30%). The expression of CXCL13 protein in PC (16/40) was much higher than that in normal penile tissues (3/36) (P=0.0015; Figure 5A). The expression of CXCL13 in PC tissues was significantly associated with T stage (P=0.025) and nodal status (P=0.034) (Supplementary Table S1). The expression of CXCL13 protein was also analyzed in normal penile tissues (NPT1, NPT2) and a panel of PC cell lines (Penl1, Penl2, 149Rca and LM156). CXCL13 expression was low in normal penile tissues. In contrast, high expression of CXCL13 was seen in PC cell line Penl1and LM156 (Figure 5B). Consistently, ELISA analysis showed that high CXCL13 level was detected in culture supernatant from PC cell lines with high endogenous CXCL13 expression (Figure 5C).
Knockdown of CXCL13 Suppressed Cell Proliferation, Clonogenesis, Migration and Invasion in Penile Cancer Cell Lines
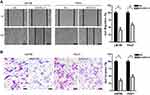
We further investigated the oncogenic function of CXCL13 in PC cell line LM156 and Penl2. Endogenous CXCL13 expression in LM156 and Penl2 was considerably reduced by shRNAs targeting CXCL13 (Figure 6A). CCK-8 assay showed that knockdown of CXCL13 markedly suppressed cell proliferation compared to the Scr control in LM156 and Penl2 cells (P<0.05; Figure 6B). However, caspase-3 activity was increased following CXCL13 knockdown in Penl1 and LM156 cell lines (P<0.05; Figure 6C). Colony formation in the shCXCL13 group was greatly attenuated compared to the Scr control group in LM156 and Penl2 cells (P<0.05, Figure 6D). Moreover, wound healing assay showed that cell migration was remarkably reduced in the shCXCL13 group compared to the Scr control (P<0.05; Figure 7A). Further, transwell invasion assay showed that depletion of CXCL13 expression significantly inhibited the invasiveness of PC cells compared to the Scr control (P<0.05; Figure 7B).
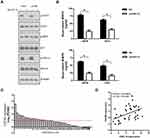
CXCL13 Regulated Downstream STAT3 and ERK1/2 Signaling Pathway and MMP2/9 Secretion
The cancer-related signaling pathways such as PI3K/AKT, ERK1/2 and STAT3 were analyzed using Western blotting. As shown in Figure 8A, knockdown of CXCL13 greatly attenuated p-STAT3 and p-ERK1/2 levels in Penl1 and LM156 cells. Meanwhile, ELISA assay revealed that depletion of CXCL13 reduced secretion of two invasion/metastasis-related molecules MMP2 and MMP9, compared with the Scr control (P<0.05) (Figure 8B). We further evaluated the expression of CXCL13 receptors CXCR5 in GSE57955 dataset. As shown in Figure 8C, CXCR5 expression was high in 51.3% (23/39) PC cases (Log2 (PC/NG) ≥1). High correlation of CXCL13:CXCR5 expression was observed in GSE57955 dataset (Pearson correlation r=0.435, P=0.006, Figure 8D).
Discussion
The crosstalk between tumor cells and their microenvironment is possibly mediated by a network of chemokines. Enormous evidence suggests that chemokine CXCLs and their corresponding receptors CXCRs can greatly affect cell proliferation, migration, invasion, and metastasis in many cancers.25 Exploring the expression and oncogenic function of CXCLs may help to identify potential cancer biomarkers and therapeutic targets for cancer treatment. However, the expression patterns as well as functions of these CXCLs in PC still remain largely unknown. To our knowledge, our study may be the first to analyze the expression patterns of CXCLs in PC. Our analysis on the GSE57955 dataset showed that several CXCLs (CXCL1, CXCL5, CXCL8, CXCL9, CXCL11, and CXCL13) were remarkably upregulated in penile cancer, suggesting these CXCLs might play an important role in penile cancer. Further study would be necessary to investigate the possible oncogenic function of these CXCLs in PC.
CXCL13, also known as B-Cell chemoattractant 1 (BCA1), could regulate the homing of B lymphocytes to spleen and lymph nodes.26 Recently, the involvement of CXCL13 in cancer development has been reported. Dysregulated CXCL13 expression is seen in gastric cancer, colorectal, breast, prostate, oral, lung, and hepatocellular cancer.16 Based on the GSE57955 dataset analysis and real-time PCR validation, we showed that CXCL13 was highly expressed in PC tissues compared to normal penile tissues. Although HPV is an important contributing factor for PC,27 our findings did not reveal the association between CXCL13 expression and HPV status, suggesting aberrant CXCL13 expression in PC might not be caused by HPV infection.
The connection between serum CXCL13 and cancer was also investigated in some cancers. Serum CXCL13 level is highly elevated in gastric cancer, hepatocellular carcinoma, clear cell renal carcinoma, non-small-cell lung cancer, breast cancer, and lymphoma. In gastric cancer, serum CXCL13 concentrations were higher in patients with lymph node metastasis and high-grade disease.28 In breast cancer, CXCL13 expression was associated with higher grade, lymph node metastasis and ER negative status.29 In clear cell renal carcinoma, CXCL13 could induce proliferation and migration in cancer cells, and was identified as a prognostic marker for cancer progression.30 CXCL13 was also shown to have better clinical values of diagnosis and prognosis than IL-31 in hepatocellular carcinoma.31 Our present findings showed that PC patients exhibited markedly higher preoperative serum CXCL13 level than healthy male controls; preoperative serum CXCL13 exhibited good sensitivity of 84.2%, and specificity of 87.0% to distinguish PC. Traditional clinical manifestation (painless lump or ulcer) could only be seen in 30~40% PC cases.32 Therefore, serum CXCL13 might be superior to the traditional clinical manifestation in diagnostic accuracy. We also observed that serum CXCL13 level was significantly reduced in postoperative cases when compared with matched preoperative PC cases, suggesting serum CXCL13 level might be caused by the tumor burden in PC patients. Moreover, preoperative serum CXCL13 level was significantly associated with clinicopathological characteristics including pathological grade, higher T stage, nodal status, and pelvic LNM in PC. In addition, our survival analysis showed that high preoperative serum CXCL13 level was associated with a shorter disease-free survival in our PC cohort. Consistently, Cox regression analysis revealed that high preoperative serum CXCL13 level could serve as an independent prognostic factor for unfavorable disease-free survival. Therefore, serum CXCL13 might serve as a diagnostic and prognostic cancer biomarker for PC. However, due to the limitation of our retrospective study (single center, small cohort, relatively short follow-up period, diversity of treatment, etc), a large-scale prospective study would be necessary to further validate the potential value of serum CXCL13 as a diagnostic and prognostic biomarker for PC.
Aberrant CXCL13 expression has been shown to activate multiple oncogenic signaling pathways to promote malignant phenotypes of cancer (uncontrolled cell proliferation, local invasion, distant metastasis, etc). CXCL13 could stimulate MMPs secretion and enhance the migratory and tumorigenic properties of prostate cancer.33 Xu et al showed that CXCL13 could induce ERK1/2 activation and promote breast cancer growth and metastasis in breast cancer.34 Zheng et al revealed that CXCL13/CXCR5 axis promotes tumor progression through PI3K/AKT/mTOR pathway in clear cell renal cell carcinoma.30 We detected high CXCL13 expression in PC tissues, cell lines and their culture supernatants, suggesting CXCL13 could act in an autocrine manner in PC cells. Further, knockdown of CXCL13 suppressed malignant phenotypes (cell proliferation, clonogenesis, migration/invasion, apoptosis escape), attenuated oncogenic signaling (ERK1/2, STAT3) and reduced metastasis-related MMP2/9 secretion in penile cancer cell lines. CXCL13 expression was significantly correlated with its receptor CXCR5 in PC. These results suggest that CXCL13 might promote PC progression through activating oncogenic signaling pathways (STAT3, ERK1/2) and inducing metastasis-related MMP2/9. However, contrary to the Zheng et al study,30 the attenuation of AKT signaling following knockdown of CXCL13 in the PC cell lines was not observed, suggesting that CXCL13 signaling might activate differential downstream signaling dependent on specific cancer types.
In conclusion, we have shown that serum CXCL13 is closely associated with tumor progression and might serve as diagnostic and prognostic biomarker for PC. Further, CXCL13 might activate downstream oncogenic signaling (STAT3, ERK1/2, and MMP2/9) to promote malignant phenotypes in PC. New discoveries in the CXCL13 signaling would also aid clinical decision-making for PC patients, bringing us closer to the promise of translational precision medicine.
Data Sharing Statement
All data generated or analyzed during this study are included in this article.
Ethics Approval and Consent to Participate
This study was approved by the Research Ethics Committee of Xiangya Hospital Central South University (Rev. No. 201,805,847). Written informed consent was obtained from the patients.
Acknowledgments
This work was supported by National Nature Science Foundation of China (Grant No. 81902605); Natural Science Foundation of Hunan Province, China (Grant No. 2020JJ5899).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Douglawi A, Masterson TA. Updates on the epidemiology and risk factors for penile cancer. Transl Androl Urol. 2017;6(5):785–790. doi:10.21037/tau.2017.05.19
2. Barski D, Georgas E, Gerullis H, Ecke T. Metastatic penile carcinoma - an update on the current diagnosis and treatment options. Cent European J Urol. 2014;67(2):126–132. doi:10.5173/ceju.2014.02.art2
3. Capone F, Guerriero E, Sorice A, Colonna G, Ciliberto G, Costantini S. Serum Cytokinome Profile Evaluation: A Tool to Define New Diagnostic and Prognostic Markers of Cancer Using Multiplexed Bead-Based Immunoassays. Mediators Inflamm. 2016;2016:3064643. doi:10.1155/2016/3064643
4. Wang J, Chu Y, Li J, et al. The clinical value of carcinoembryonic antigen for tumor metastasis assessment in lung cancer. PeerJ. 2019;7(7):e7433. doi:10.7717/peerj.7433
5. Felder M, Kapur A, Gonzalez-Bosquet J, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13(1):129. doi:10.1186/1476-4598-13-129
6. Nam SE, Lim W, Jeong J, et al. The prognostic significance of preoperative tumor marker (CEA, CA15-3) elevation in breast cancer patients: data from the Korean Breast Cancer Society Registry. Breast Cancer Res Treat. 2019;177(3):669–678. doi:10.1007/s10549-019-05357-y
7. Zhu Y, Ye DW, Yao XD, et al. The value of squamous cell carcinoma antigen in the prognostic evaluation, treatment monitoring and followup of patients with penile cancer. J Urol. 2008;180(5):2019–2023. doi:10.1016/j.juro.2008.07.040
8. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi:10.1038/nrc1388
9. Chen X, Chen R, Jin R, Huang Z. The role of CXCL chemokine family in the development and progression of gastric cancer. Int J Clin Exp Pathol. 2020;13(3):484–492.
10. Do HTT, Lee CH, Cho J. Chemokines and their Receptors: multifaceted Roles in Cancer Progression and Potential Value as Cancer Prognostic Markers. Cancers. 2020;12(2):287. doi:10.3390/cancers12020287
11. Amiri KI, Richmond A. Fine tuning the transcriptional regulation of the CXCL1 chemokine. Prog Nucleic Acid Res Mol Biol. 2003;74:1–36.
12. Aldinucci D, Colombatti A. The inflammatory chemokine CXCL5 and cancer progression. Mediators Inflamm. 2014;2014:292376. doi:10.1155/2014/292376
13. Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics. 2017;7(6):1543–1588. doi:10.7150/thno.15625
14. Karin N, Razon H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine. 2018;109:24–28. doi:10.1016/j.cyto.2018.02.012
15. Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev. 2018;63:40–47. doi:10.1016/j.ctrv.2017.11.007
16. Kazanietz MG, Durando M, Cooke M. CXCL13 and Its Receptor CXCR5 in Cancer: inflammation, Immune Response, and Beyond. Front Endocrinol. 2019;10:471. doi:10.3389/fendo.2019.00471
17. Paner GP, Stadler WM, Hansel DE, Montironi R, Lin DW, Amin MB. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73(4):560–569. doi:10.1016/j.eururo.2017.12.018
18. Zhou QH, Deng CZ, Li ZS, et al. Molecular characterization and integrative genomic analysis of a panel of newly established penile cancer cell lines. Cell Death Dis. 2018;9(6):684. doi:10.1038/s41419-018-0736-1
19. Hu X, Chen M, Li Y, Wang Y, Wen S, Jun F. Overexpression of ID1 promotes tumor progression in penile squamous cell carcinoma. Oncol Rep. 2019;41(2):1091–1100. doi:10.3892/or.2018.6912
20. Jun F, Hong J, Liu Q, et al. Epithelial membrane protein 3 regulates TGF-β signaling activation in CD44-high glioblastoma. Oncotarget. 2017;8(9):14343–14358. doi:10.18632/oncotarget.11102
21. Hu X, Chen M, Liu W, Li Y, Fu J. Preoperative plasma IGFBP2 is associated with nodal metastasis in patients with penile squamous cell carcinoma. Urol Oncol. 2019;37(7):452–461. doi:10.1016/j.urolonc.2019.04.013
22. Hu X, Chen M, Li Y, Wang Y, Wen S, Aberrant JF. CEACAM19 expression is associated with metastatic phenotype in penile cancer. Cancer Manag Res. 2019;11:715–725. doi:10.2147/CMAR.S192385
23. Peng H, Li Z, Fu J, Zhou R. Growth and differentiation factor 15 regulates PD-L1 expression in glioblastoma. Cancer Manag Res. 2019;11:2653–2661. doi:10.2147/CMAR.S192095
24. Kuasne H, Cólus IM, Busso AF, et al. Genome-wide methylation and transcriptome analysis in penile carcinoma: uncovering new molecular markers. Clin Epigenetics. 2015;7(1):46. doi:10.1186/s13148-015-0082-4
25. Spaks A. Role of CXC group chemokines in lung cancer development and progression. J Thorac Dis. 2017;9(Suppl 3):S164–S171. doi:10.21037/jtd.2017.03.61
26. Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187(4):655–660. doi:10.1084/jem.187.4.655
27. Stratton KL, Culkin DJ, Contemporary A. Review of HPV and Penile Cancer. Oncology. 2016;30(3):245–249.
28. Meng X, Yu X, Dong Q, et al. Distribution of circulating follicular helper T cells and expression of interleukin-21 and chemokine C-X-C ligand 13 in gastric cancer. Oncol Lett. 2018;16(3):3917–3922. doi:10.3892/ol.2018.9112
29. Panse J, Friedrichs K, Marx A, et al. Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients. Br J Cancer. 2008;99(6):930–938. doi:10.1038/sj.bjc.6604621
30. Zheng Z, Cai Y, Chen H, et al. CXCL13/CXCR5 Axis Predicts Poor Prognosis and Promotes Progression Through PI3K/AKT/mTOR Pathway in Clear Cell Renal Cell Carcinoma. Front Oncol. 2019;8:682. doi:10.3389/fonc.2018.00682
31. Li B, Su H, Cao J, Zhang L. CXCL13 rather than IL-31 is a potential indicator in patients with hepatocellular carcinoma. Cytokine. 2017;89:91–97. doi:10.1016/j.cyto.2016.08.016
32. Ritchie AW, Foster PW, Fowler S. BAUS Section of Oncology. Penile cancer in the UK: clinical presentation and outcome in 1998/99. BJU Int. 2004;94(9):1248–1252. doi:10.1111/j.1464-410X.2004.05152.x
33. El-Haibi CP, Singh R, Gupta P, et al. Antibody Microarray Analysis of Signaling Networks Regulated by Cxcl13 and Cxcr5 in Prostate Cancer. J Proteomics Bioinform. 2012;5(8):177–184. doi:10.4172/jpb.1000232
34. Xu L, Liang Z, Li S, Ma J. Signaling via the CXCR5/ERK pathway is mediated by CXCL13 in mice with breast cancer. Oncol Lett. 2018;15(6):9293–9298. doi:10.3892/ol.2018.8510
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.