Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 14
Serum Collagen Triple Helix Repeat Containing-1 Levels are Related to Radiological Affection and Disease Activity in Rheumatoid Arthritis
Authors Nassef EM , Elabd HA, Elzomor HM, El Nagger BMMA, Ibrahim AS , Ibrahim AH , Kotb HG, Hassan DA, Abd ElAziz REM , Mohamed EES
Received 27 September 2022
Accepted for publication 16 November 2022
Published 9 December 2022 Volume 2022:14 Pages 291—299
DOI https://doi.org/10.2147/OARRR.S391494
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Eman Mostafa Nassef,1 Hemmat Ahmed Elabd,2 Hala Mohamed Elzomor,2 Basma Mohamed Mohamed Ali El Nagger,2 Amira Shahin Ibrahim,2 Amal Hussein Ibrahim,1 Hend Gamal Kotb,1 Donia Ahmed Hassan,3 Rasha Elsayed Mohamed Abd ElAziz,4 Eman El Sayed Mohamed3
1Internal Medicine Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 2Rheumatology and Rehabilitation Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 3Clinical Pathology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 4Biochemistry Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
Correspondence: Eman Mostafa Nassef, Internal medicine Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt, Tel +201098002232, Email [email protected]
Background: Rheumatoid arthritis (RA) is a common systemic inflammatory disease. Collagen triple helix repeat containing-1 (CTHRC1) is a unique gene product able to reduce collagen deposition. The present study aimed to assess CTHRC1 level in RA patients and to uncover its relation to clinical, laboratory and radiological findings.
Methods: The study included 60 adult RA patients. In addition, there were 60 control subjects who included patients with osteoarthritis (n = 20) and reactive arthritis (n = 20) and healthy controls (n = 20). Serum CTHRC1 levels were assessed by Enzyme-Linked Immunosorbent Assay (ELISA). Disease activity was calculated using the Disease Activity Score (DAS28-CRP). Radiological damage was evaluated using the Simple Erosion Narrowing Score (SENS).
Results: There was significantly higher serum CTHRC1 levels in RA patients when compared to OA, ReA and control groups [median (IQR): 4.66 (1.68– 11.7) versus 1.88 (1.14– 2.94), 1.55 (0.98– 3.15) and 1.14 (0.85– 1.3) mg/dL, respectively, p < 0.001]. There was significantly higher CTHRC1 levels in patients with higher disease activity [median (IQR): 2.23 (1.4– 4.73) versus 6.55 (4.66– 12.0) mg/dL, p = 0.004]. Patients with higher SENS had significantly higher CTHRC1 [median (IQR): 1.99 (1.4– 4.66) versus 9.75 (4.39– 12.63) mg/dL, p < 0.001] and DAS28 [median (IQR): 4.25 (2.9– 5.2) versus 5.4 (4.65– 5.8), p = 0.01].
Conclusion: Serum CTHRC1 levels are related to disease severity and radiological affection in RA patients.
Keywords: rheumatoid arthritis, osteoarthritis, CTHRC1
Introduction
Rheumatoid arthritis (RA) is a common systemic inflammatory disease with significant affection of patients’ quality of life and marked economic burden. Related risk factors encompass host (genetic, hormonal, and neuroendocrine) and environmental (smoking, infectious agents and diet) factors.1 RA primarily affects small and large joints and adjacent periarticular tissues in a symmetrical pattern and progressive course. The associated systemic inflammation and related immune reactions are expressed in a wide spectrum of extra-articular manifestations.2,3
Management of RA had witnessed revolutionary changes in the recent years with the introduction of biological therapies and integrated treatment approaches. However, in spite of the conspicuous achievements in RA diagnosis and treatment, the disease remains far from being preventable or curable.4
Basic laboratory work-up for diagnosis of RA includes C-reactive protein (CRP), rheumatoid factor (RF), anti-cyclic citrullinated protein (Anti-CCP). While these biomarkers are evidently useful as diagnostic and prognostic markers, practice has identified many shortcomings that undermined their clinical performance. Pursuit of new markers with better reliability is strongly advocated.5
Collagen is the primary building block of the extracellular matrix of tissues including cartilages and tendons.6 Collagen triple helix repeat containing-1 (CTHRC1) is a unique gene product able to reduce collagen deposition by inhibiting Smad2/3 activation.7 CTHRC1 proved to have diverse roles affecting bone mass, myelination, collagen synthesis, synoviocytes migration and angiogenesis.8 Overexpression of CTHRC1 was associated with poor survival in some cancers.9–12
In RA, CTHRC1 was found to be overexpressed in activated synoviocytes of murine models which in turn initiates cartilage destruction.13 Moreover, it was linked to reduced bone resorption and formation.14 Clinically, only 3 reports showed elevated plasma levels of CTHRC1 in RA patients with significant correlation with disease activity15–17 and one report identified a correlation between CTHRC1 levels and proinflammatory cytokines in RA patients.15 However, none of these studies assessed the relation between CTHRC1 levels and radiological damage in RA patients. The present study aimed to assess CTHRC1 level in RA patients and to uncover its relation to clinical, laboratory and radiological findings.
Subjects and Methods
The present study was conducted at Al-Azhar University Hospitals, Cairo, Egypt. The study protocol was approved by Ethical Committee of Al-Azhar Faculty of Medicine for Girls and all participants provided informed consent before the study in line with Declaration of Helsinki on clinical research involving human subjects.
The study included 60 adult RA patients. They were diagnosed according to the classification criteria recommended by the American College of American College of Rheumatology and European League Against Rheumatism (ACR/EULAR).18 In addition, there were 60 control subjects who included patients with osteoarthritis (n = 20) and reactive arthritis (n = 20) and healthy controls (n = 20). Osteoarthritis (OA) was diagnosed according to the ACR criteria19 while reactive arthritis (ReA) was diagnosed according to Braun criteria.20 Patients were excluded if they had associated cancers, severe systemic diseases, systemic infections, autoimmune diseases or osteoporosis. Pregnant or lactating women were also excluded.
Upon recruitment, all participants were subjected to careful history taking and through clinical examination. For laboratory assessment, a volume of 10 mL of venous blood was withdrawn from each subject under complete aseptic conditions. The blood sample was divided into three aliquots; 1st aliquot of 2 mL of blood was collected into 3.8% sodium tri-sodium citrate for measuring ESR by Westergren method. The 2nd aliquot of 2 mL of blood was collected into EDTA tube for measuring complete blood count (CBC) (Sysmex KX-21, Japan). The 3rd aliquot of 6 mL was collected into 2 serum gel separator tubes and centrifuged for measuring RF and CRP by qualitative latex agglutination tests and Anti-CCP and CTHRC1 by Enzyme-Linked Immunosorbent Assay (ELISA). Serum for ELISA analysis was kept at −20 and measured by AS 1851 DAS, Italy (reader), and 16041412 BioTek, Winooski, Vermont, USA (washer). Serum CTHRC1 levels were assessed by ELISA using commercially available kits (Cat.No E6798Hu, Bioassay Technology Laboratory, Shanghai, China).
Disease activity was calculated using the Disease Activity Score (DAS28-CRP). According to DAS28-CRP results, patients were classified into 4 categories: remission (<2.6), low (2.6 - ≤3.2), moderate (>3.2 - ≤5.1) and high (>5.1) activity.21 Functional performance was assessed using the modified Health Assessment Questionnaire (HAQ).22 Radiological damage was evaluated using the Simple Erosion Narrowing Score (SENS).23,24 Typically, the original SENS is calculated from hands and feet images. However, in the present study, we used only radiographic hand images because this is the standard of care at our center.
Data obtained from the present study were presented as mean and standard deviation (SD), median and interquartile range (IQR) or number and percent. Numerical variables were compared using t-test, Mann–Whitney U-test or chi-square test as appropriate. Correlation analysis was achieved using Spearman correlation coefficient. All statistical tests were performed using SPSS version 25 with p value less than 0.05 considered statistically significant.
Results
The present study included patients with RA (n = 60), OA (n = 20), ReA (n = 20) and healthy controls (n = 20). Comparison between the studied groups showed significantly higher serum CTHRC1 levels in RA patients when compared to OA, ReA and control groups [median (IQR): 4.66 (1.68–11.7) versus 1.88 (1.14–2.94), 1.55 (0.98–3.15) and 1.14 (0.85–1.3) mg/dL, respectively, p < 0.001] (Figure 1). Comparison between RA patients with remission-to-moderate and patients with high disease activity revealed significantly higher CTHRC1 levels in patients with higher disease activity [median (IQR): 2.23 (1.4–4.73) versus 6.55 (4.66–12.0) mg/dL, p = 0.004]. In addition, it was noted that patients with high disease activity had significantly higher erosion [median (IQR): 2.0 (1.0–3.0) versus 4.0 (2.5–9.5), p < 0.001], narrowing [median (IQR): 3.0 (2.0–5.0) versus 7.0 (4.0–11.0), p < 0.001] and total SENS [median (IQR): 6.0 (3.0–8.0) versus 11.0 (6.0–20.5), p < 0.001] scores (Table 1).
 |
Table 1 Relation Between Clinical and Laboratory Findings and Disease Activity |
 |
Figure 1 CTHRC1 in the studied groups. |
RA patients were classified into those with SENS < median (n = 26) and others with SENS ≥ median (n = 34). Patients with higher SENS had significantly higher CTHRC1 [median (IQR): 1.99 (1.4–4.66) versus 9.75 (4.39–12.63) mg/dL, p < 0.001] and DAS28 [median (IQR): 4.25 (2.9–5.2) versus 5.4 (4.65–5.8), p = 0.01] (Table 2).
 |
Table 2 Relation Between Clinical and Laboratory Findings and Radiological Damage |
Correlation analysis identified significant correlation between CTHRC1 levels and tender joints count (r = 0.33), mHAQ (r = 0.47), DAS28 (r = 0.42), erosion score (r = 0.57), narrowing score (r = 0.62) and total SENS (r = 0.62) (Table 3, Figures 2 and 3).
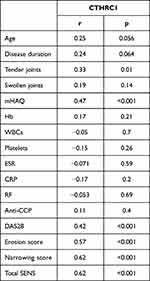 |
Table 3 Correlation Between CTHRC1 Levels and Clinical and Laboratory Data in RA Patients |
 |
Figure 2 Correlation between CTHRC1 and DAS28. |
 |
Figure 3 Correlation between CTHRC1 and total SENS. |
Discussion
In the present study, RA patients expressed significantly higher levels of serum CTHRC1 in comparison with the other studied groups in conformity with other reports. In their work, Myngbay et al15 recognized CTHRC1 as a sensitive and feasible marker for diagnosis of RA. Furthermore, Hu et al16 suggested that adding CTHRC1 to anti-CCP can improve the diagnosis of RA. Similar conclusions were reported by Selim et al.17 In our work, comparative and correlation analysis identified a strong relation between CTHRC1 levels and disease activity in RA patients in accordance with previous reports.15,17
While other studies investigating the value of CTHRC1 assessment in RA patients focused on its diagnostic reliability and relation to disease activity and inflammatory markers, our study further examined in the relation between CTHRC1 and radiological affection. Interestingly, we could find a significant association between elevated serum CTHRC1 levels and advanced radiological erosion and narrowing in RA patients which is a new finding to the best of our knowledge.
The mechanisms responsible for this relation in humans remain to be elucidated. However, CTHRC1 probably mediates its effects on RA cartilage through multiple pathways. Augmented CTHRC1 was found to be associated with exaggerated proinflammatory response as shown by the study of Myngbay et al,15 could detect a significant relation between elevated CTHRC1 levels and increased levels of the proinflammatory cytokine interleukin (IL)-1β, IL-6, IL-8 and interferon γ in RA patients. Interestingly, CTHRC1 was highly expressed in gingival tissues of periodontitis patients.25 While the relation between periodontitis and RA is well-established26 the suggestion that CTHRC1 constitutes a link between periodontitis and RA needs further investigation.
Moreover, it was found that Wnt5A-Fzd5-LRP5/6-CTHRC1 complex can promote the expression of hypoxia inducible factor-1α (HIF-1α) leading to high expression of vascular endothelial growth factor (VEGF) and enhancing angiogenesis.27 Angiogenesis is known to play a major role in RA progression. A plethora of angiogenic factors is primarily produced from RA synovial tissue and neovascularization helps to maintain synovitis.28 CTHRC1 is also involved in the Wnt signaling pathway significantly contributed to RA pathogenesis.29 Remarkably, experimental evidence suggested that sex disparities in RA prevalence and severity may be related to the sex-specific expression of some genes including CTHRC1.30
Findings of the present study may have therapeutic implications. Targeting CTHRC1 was suggested as a therapeutic approach in many malignant conditions.31–33 However, this approach was not investigated in RA until recently which is a clear research gap that is expected to be addressed in the near future.
In conclusion, the present study suggested that serum CTHRC1 levels are related to disease severity and radiological affection in RA patients. However, these conclusions are limited by the cross-sectional study design and the relatively small sample size.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by Ethical Committee of Al-Azhar Faculty of Medicine for Girls. Informed consent was obtained from all participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Romão VC, Fonseca JE. Etiology and risk factors for rheumatoid arthritis: a state-of-the-art review. Front Med. 2021;8:689698. PMID: 34901047; PMCID: PMC8661097. doi:10.3389/fmed.2021.689698
2. Cush JJ. Rheumatoid arthritis: early diagnosis and treatment. Rheum Dis Clin North Am. 2022;48(2):537–547. doi:10.1016/j.rdc.2022.02.010
3. Rivellese F, Pitzalis C. Cellular and molecular diversity in rheumatoid arthritis. Semin Immunol. 2022:101519. PMID: 35033462. doi:10.1016/j.smim.2021.101519
4. Mucke J, Krusche M, Burmester GR. A broad look into the future of rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2022;14:1759720X221076211. PMID: 35154419; PMCID: PMC8832593. doi:10.1177/1759720X221076211
5. Abdelhafiz D, Baker T, Glascow DA, Abdelhafiz A. Biomarkers for the diagnosis and treatment of rheumatoid arthritis - a systematic review. Postgrad Med. 2022:1–10. PMID: 35275765. doi:10.1080/00325481.2022.2052626
6. Kirkness MW, Lehmann K, Forde NR. Mechanics and structural stability of the collagen triple helix. Curr Opin Chem Biol. 2019;53:98–105. PMID: 31606538. doi:10.1016/j.cbpa.2019.08.001
7. LeClair R, Lindner V. The role of collagen triple helix repeat containing 1 in injured arteries, collagen expression, and transforming growth factor beta signaling. Trends Cardiovasc Med. 2007. 17(6):202–205. Erratum in: Trends Cardiovasc Med. 2010 Oct;20(7):247. PMID: 17662915. doi:10.1016/j.tcm.2007.05.004
8. Wu Q, Yang Q, Sun H. Role of collagen triple helix repeat containing-1 in tumor and inflammatory diseases. J Cancer Res Ther. 2017;13(4):621–624. PMID: 28901303. doi:10.4103/jcrt.JCRT_410_17
9. Guo B, Yan H, Li L, Yin K, Ji F, Zhang S. Collagen triple helix repeat containing 1 (CTHRC1) activates Integrin β3/FAK signaling and promotes metastasis in ovarian cancer. J Ovarian Res. 2017;10(1):69. PMID: 29021002; PMCID: PMC5637322. doi:10.1186/s13048-017-0358-8
10. Fu SW, Chen HY, Lin XL, Yang L, Ge ZZ. Collagen triple helix repeat containing 1 promotes tumor angiogenesis in gastrointestinal stromal tumors. Oncol Lett. 2017;14(6):7499–7505. PMID: 29344195; PMCID: PMC5755008. doi:10.3892/ol.2017.7111
11. Liu Y, Abulimiti N, Wang C. Collagen triple helix repeat containing 1 expression in osteosarcoma: a new predictor of prognosis. Ann Clin Lab Sci. 2018;48(3):338–344. PMID: 29970438.
12. Liu Y, Hu T, Li X, et al. Application of collagen triple helix repeat containing-1 and mitotic spindle apparatus antibody in small cell lung cancer diagnosis. J Clin Lab Anal. 2022;36(5):e24412. PMID: 35385156; PMCID: PMC9102652. doi:10.1002/jcla.24412
13. Shekhani MT, Forde TS, Adilbayeva A, et al. Collagen triple helix repeat containing 1 is a new promigratory marker of arthritic pannus. Arthritis Res Ther. 2016;18:171. PMID: 27430622; PMCID: PMC4950773. doi:10.1186/s13075-016-1067-1
14. Jin YR, Stohn JP, Wang Q, et al. Inhibition of osteoclast differentiation and collagen antibody-induced arthritis by CTHRC1. Bone. 2017;97:153–167. PMID: 28115279; PMCID: PMC6746321. doi:10.1016/j.bone.2017.01.022
15. Myngbay A, Bexeitov Y, Adilbayeva A, et al. CTHRC1: a new candidate biomarker for improved rheumatoid arthritis diagnosis. Front Immunol. 2019;10:1353. PMID: 31249576; PMCID: PMC6582781. doi:10.3389/fimmu.2019.01353
16. Hu T, Liu Y, Tan L, et al. Value of serum collagen triple helix repeat containing-1(CTHRC1) and 14-3-3η protein compared to anti-CCP antibodies and anti-MCV antibodies in the diagnosis of rheumatoid arthritis. Br J Biomed Sci. 2021;78(2):67–71. PMID: 32813981. doi:10.1080/09674845.2020.1810400
17. Selim ZI, Gamal RM, Araby LA, Badawy ER, Gamal NM. Collagen triple helix repeat containing 1 (CTHRC1) protein: a promising biomarker for evaluation of rheumatoid arthritis patients. Egypt Rheumatol. 2022;44(1):11–14. doi:10.1016/j.ejr.2021.07.003
18. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. PMID: 20872595. doi:10.1002/art.27584
19. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. diagnostic and therapeutic criteria committee of the American rheumatism association. Arthritis Rheum. 1986;29:1039–1049. doi:10.1002/art.1780290816
20. Braun J, Kingsley G, van der Heijde D, Sieper J. On the difficulties of establishing a consensus on the definition of and diagnostic investigations for reactive arthritis. Results and discussion of a questionnaire prepared for the 4th International Workshop on Reactive Arthritis, Berlin, Germany, July 3–6, 1999. J Rheumatol. 2000;27(9):2185–2192. PMID: 10990232.
21. Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. PMID: 7818570. doi:10.1002/art.1780380107
22. Pincus T, Summey JA, Soraci SA, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346–1353. doi:10.1002/art.1780261107
23. Van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 1999;26:743–745.
24. Dias EM, Lukas C, Landewé R, Fatenejad S, van der Heijde D. Reliability and sensitivity to change of the simple erosion narrowing score compared with the sharp-van der heijde method for scoring radiographs in rheumatoid arthritis. Ann Rheum Dis. 2008;67(3):375–379. PMID: 17644537. doi:10.1136/ard.2007.072785
25. Guo Y, Jiang C, Yao S, et al. CTHRC1 knockdown promotes inflammatory responses partially by p38 MAPK activation in human periodontal ligament cells. Inflammation. 2021;44(5):1831–1842. PMID: 33846931. doi:10.1007/s10753-021-01461-8
26. Li R, Tian C, Postlethwaite A, et al. Rheumatoid arthritis and periodontal disease: what are the similarities and differences? Int J Rheum Dis. 2017;20(12):1887–1901. PMID: 29341486. doi:10.1111/1756-185X.13240
27. Tai Y, Zhu Y, Mei D, et al. IgD promotes pannus formation by activating Wnt5A-Fzd5-CTHRC1-NF-κB signaling pathway in FLS of CIA rats and the regulation of IgD-Fc-Ig fusion protein. Int Immunopharmacol. 2021;101(Pt A):108261. PMID: 34688134. doi:10.1016/j.intimp.2021.108261
28. Elshabrawy HA, Chen Z, Volin MV, Ravella S, Virupannavar S, Shahrara S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis. 2015;18(4):433–448. PMID: 26198292; PMCID: PMC4879881. doi:10.1007/s10456-015-9477-2
29. Miao CG, Yang YY, He X, et al. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal. 2013;25(10):2069–2078. PMID: 23602936. doi:10.1016/j.cellsig.2013.04.002
30. Kudryavtseva E, Forde TS, Pucker AD, Adarichev VA. Wnt signaling genes of murine chromosome 15 are involved in sex-affected pathways of inflammatory arthritis. Arthritis Rheum. 2012;64(4):1057–1068. PMID: 22005949; PMCID: PMC3288823. doi:10.1002/art.33414
31. Chen G, Wang D, Zhao X, et al. miR-155-5p modulates malignant behaviors of hepatocellular carcinoma by directly targeting CTHRC1 and indirectly regulating GSK-3β-involved Wnt/β-catenin signaling. Cancer Cell Int. 2017;17:118. PMID: 29234238; PMCID: PMC5721693. doi:10.1186/s12935-017-0469-8
32. He W, Zhang H, Wang Y, et al. CTHRC1 induces non-small cell lung cancer (NSCLC) invasion through upregulating MMP-7/MMP-9. BMC Cancer. 2018;18(1):400. PMID: 29631554; PMCID: PMC5891957. doi:10.1186/s12885-018-4317-6
33. Peng D, Wei C, Zhang X, et al. Pan-cancer analysis combined with experiments predicts CTHRC1 as a therapeutic target for human cancers. Cancer Cell Int. 2021;21(1):566. PMID: 34702252; PMCID: PMC8549344. doi:10.1186/s12935-021-02266-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
