Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Serum Aldo-Keto Reductase Family 1 Member B10 (AKR1B10) as a Potential Biomarker for Diagnosis of Hepatocellular Carcinoma
Authors Wang Z, Kong L, Zhang R, Yang X, Cao Z, Xu T , Zhang H, Dou Y
Received 18 October 2023
Accepted for publication 11 January 2024
Published 16 January 2024 Volume 2024:11 Pages 131—143
DOI https://doi.org/10.2147/JHC.S443006
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Ziran Wang,1,* Lingjun Kong,1,* Rui Zhang,1 Xiaobo Yang,2 Zhe Cao,3 Tengda Xu,4 Han Zhang,1 Yaling Dou1
1Department of Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 2Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 3Hunan Light of Life Biotechnology Co., Ltd., Ningxiang, Hunan, People’s Republic of China; 4Department of Health Management, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yaling Dou, Department of Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, 100730, People’s Republic of China, Email [email protected]
Objective: To evaluate the diagnostic performance of aldo-keto reductase family 1 member B10 (AKR1B10) in a Beijing cohort with hepatocellular carcinoma (HCC).
Methods: This study included 521 subjects who visited Peking Union Medical College Hospital from June 2017 to May 2023, including 109 cases of HCC, 165 cases of healthy controls, 106 cases of benign liver diseases, and 141 cases of other cancers. Serum AKR1B10 levels were measured and compared across various groups. Diagnostic performances of serum AKR1B10 and other tumor markers were assessed using receiver operator characteristic (ROC) curves. In addition, a subset of HCC patients who underwent surgical resection were recruited for clinical follow-up study.
Results: We found that serum AKR1B10 expression was higher in patients with HCC relative to other control groups. The association between serum AKR1B10 and clinical features of HCC was not observed. Serum AKR1B10 showed a high diagnostic performance for HCC, and when combined with AFP, the diagnostic effectiveness was significantly improved. Specifically, serum AKR1B10 showed superior diagnostic effectiveness for AFP-negative HCC. The clinical follow-up study indicated a gradual decrease in serum AKR1B10 after surgery.
Conclusion: Our study demonstrated that serum AKR1B10 is a promising biomarker for HCC, and when used in combination with AFP can significantly improve the detection rate of HCC.
Keywords: AKR1B10, AFP, hepatocellular carcinoma, diagnostic performance
Introduction
Hepatocellular carcinoma (HCC) is a common malignant gastrointestinal tumor. In United States, there would be approximately 41,210 new cases of HCC and approximately 29,380 deaths caused by HCC in 2023.1 The situation seems to be even worse in China. According to a national survey by the National Cancer Center (NCC) of China, there were an estimated 388,800 new cases of HCC in 2016, ranking the fourth highest number of new cases in all cancers, while the estimated number of deaths was 336,400, accounting for the second leading cause of death among all cancers.2 The pathogenesis of HCC is complex and diverse, involving numerous environmental factors in addition to genetic factors. Risk factors for HCC have been identified for several conditions including hepatitis B and C virus infection, alcohol ingestion, ingestion of the fungal metabolite aflatoxin B1, and non-alcoholic steatohepatitis.3 Some treatment options (curative treatment, palliative treatments, transplantation, and molecular targeted therapy) are now available based on individual condition. Recently, several studies have indicated that patients may benefit from immunotherapy and immune-based combinations, which has shed light on the treatment and management of HCC.4–7 Nevertheless, the occurrence of metastases and recurrences as well as the heterogeneity among patients make the prognosis of HCC still unsatisfactory.
Patients with HCC in early-stage have the option of surgical resection as the first-line therapy, and the 5-year survival rate can reach at 70%, while patients with HCC in more developed stages have shorter survival periods and only benefit from chemoembolization or sorafenib.3 Considering the malignancy of HCC, it is critical to accurately identify HCC patients early in their treatment and prognostic management. Serum alpha-fetoprotein (AFP) is a widely-used biomarker for HCC in clinical practice at present, but its diagnostic effectiveness for early-stage HCC is remarkably limited. A meta-analysis indicated that when using AFP to detect early-stage HCC, the sensitivity was 44.4%, the specificity was 84.8% and the area under the curve (AUC) was only 0.52.8 Besides, Lens culinaris agglutinin A-reactive fraction of α-fetoprotein (AFP-L3, an isoform of AFP) and des-gamma-carboxy prothrombin (DCP) have been reported as biomarkers for HCC, but their lower sensitivity prevents them from being recommended for screening or diagnosis for HCC.9 Notably, with the development of liquid biopsy techniques in recent years, new molecular markers such as circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) have made encouraging progress in the diagnosis and prognosis of HCC.10 However, drawbacks such as complex operations, expensive costs and difficulties in standardization and automation may limit their application, especially in clinical laboratories. Consequently, there is an imperative need to explore new reliable serological markers with desirable diagnostic and prognostic value for HCC.
Aldo-keto reductase family 1 member B10 (AKR1B10), also known as ARL-1, is a NADPH-dependent enzyme containing 316 amino acids that efficiently reduces aliphatic and aromatic aldehydes and is less active against hexoses.11 AKR1B10 can protect host cells from the toxicity of dietary and lipid-derived unsaturated carbonyls.12 Furthermore, AKR1B10 can exhibit retinaldehyde reductase activity in vivo and in vitro, leading to depletion of retinoic acid, thereby promoting cell proliferation.13 AKR1B10 has been reported to be expressed predominantly in the small intestine and the colon, with low expression in the liver but overexpression in HCC.11 Several studies have shown that AKR1B10 was highly expressed in HCC as well as being an indicator of poor prognosis.9,14–17 Specifically, some investigations have pointed out the diagnostic performance of AKR1B10 was superior to that of AFP for early-stage HCC.9,18,19 Collectively, these results highlighted AKR1B10 as a promising biomarker in the diagnosis and prognostic management for HCC. However, there were also a few studies with conflicting conclusions. Schmitz et al found that higher AKR1B10 was associated with less malignant tumor behavior in HCC.20 Another study demonstrated that patients with HCC who underwent curative hepatectomy have better prognosis when having higher expression AKR1B10.21 In this context, selecting diverse regions and populations to evaluate the clinical performance of serum AKR1B10 is highly meaningful to clarify its application value in the real-world. In this study, we evaluated the diagnostic performance of serum AKR1B10 based on HCC and control cohorts in Beijing.
Materials and Methods
Subjects
This study included 521 subjects who visited Peking Union Medical College Hospital from June 2017 to May 2023, including 109 cases of HCC, 165 cases of healthy controls (HC), 106 cases of benign liver diseases (BLD), and 141 cases of other cancers (OC). All controls (AC) group was defined as containing all cases from HC, BLD, and OC groups. The diagnosis of HCC is made according to the combination of patients’ clinical manifestation, imaging, pathological examination, and laboratory tests, based on the recommended guideline of China.22 The HC group was originated from subjects who came to the hospital for their annual physical examination and were confirmed to have no abnormalities in laboratory indicators and liver imaging. The BLD group included patients with benign liver lesions such as hepatitis, cirrhosis, hepatic cysts, and hepatic hemangiomas. The OC group recruited patients diagnosed with other tumors such as lung cancer, gastric carcinoma, colon cancer and breast cancer. Clinical data and laboratory data of all subjects were extracted through the hospital information system (HIS) and laboratory information system (LIS).
The following laboratory parameters were collected from the subjects: AFP, albumin (ALB), alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), direct bilirubin (Dbil), gamma-glutamyl transpeptidase (GGT), lactic dehydrogenase (LD), total bilirubin (Tbil), total cholesterol (TC), total triglycerides (TG), and total protein (TP). AFP, CA19-9, and CEA were measured using Roche Cobas e801 (Roche, Switzerland) while ALB, ALP, ALT, AST, Dbil, GGT, LD, Tbil, TC, TG, and TP were measured with Beckman AU 5800 (Beckman Coulter, USA). Samples were assayed after satisfactory quality control (QC) results were obtained. Our laboratory is accredited by China National Accreditation Service for Conformity Assessment (CNAS) and College of American Pathologists (CAP) and is under their external supervision.
Serum AKR1B10 Assay
Serum AKR1B10 was measured using the commercial AKR1B10 assay kit (Hunan Light of Life Biotechnology Co., Ltd). The methodology of the kit is double antibody sandwich time-resolved fluorescence immunoassay (TRFIA). The reaction plate of the kit has been pre-coated with rabbit anti-AKR1B10 polyclonal antibody. Firstly, add 100 μL of the serum samples or AKR1B10 standards (0 pg/mL, 375 pg/mL, 750 pg/mL, 1500 pg/mL, 3000 pg/mL, 6000 pg/mL) to the microtiter wells in the reaction plate and incubate for 1 h at room temperature with vibration. After undergoing 4 rounds of washing with washing solution, 100 μL of biotin-labelled murine anti-AKR1B10 monoclonal antibody was added to the microtiter wells and incubated for 40 min at room temperature with vibration. Subsequently, after 4 rounds of washing, 100 μL of europium-labelled streptavidin was added to microtiter wells and incubated for 30 min at room temperature with vibration. After completion of the above incubation, 4 rounds of washing were performed to remove free streptomycin. Subsequently 100 μL of enhancement solution was added to each microwell for 5 min of incubation and then the fluorescence values were read using the matching instrument. The fluorescence values of serum samples were converted to quantitative values of AKR1B10 by the standard curves fitted with standards and their fluorescence values. For samples with AKR1B10 content greater than 6000 pg/mL, the assays were performed again after appropriate dilution using saline. The recommended reference interval (RI) for AKR1B10 in the instruction of kit is (0–215) pg/mL.
Clinical Follow-Up Study
After screening, 44 patients with HCC who underwent surgical resection at our institution were enrolled for clinical follow-up study. Blood was collected and serum was separated for AKR1B10 measurement at three time points: pre-surgery, 1–2 days after surgery, and 3–4 days after surgery, respectively. Serum AKR1B10 levels were measured as described above.
Statistical Analysis
The clinical data were collected and processed using Excel 2019 (Microsoft, USA), and statistical analysis was performed using R Project software (version 4.2.0), RStudio software (Open-Source Edition), and Prism GraphPad software (version 9.0). Comparisons of quantitative data were made using the t-test or Wilcoxon test whereas comparisons of qualitative data were conducted using the chi-square test. The Spearman correlation analysis was used to calculate the correlation between serum AKR1B10 and other laboratory indicators. It is considered statistically significant when P<0.05.
Results
Baseline Characteristics of Subjects
The baseline characteristics data of the subjects included in this study were displayed in Table 1. The proportion of males in the HCC group was significantly higher when compared to the other three groups (all P<0.001). Subjects in the HCC group were older compared to the HC and BLD groups (both P<0.001), but were not significantly different from the OC group (P=0.053). Additionally, when comparing to the HC group, the HCC group had higher levels of AFP, ALB, ALT, AST, CA19-9, CEA, Dbil, GGT, LD, Tbil, and TG as well as lower levels of ALB and TP. When using the BLD group as the reference, the HCC group had higher levels of AFP, ALB, ALT, AST, CA19-9, CEA, Dbil, GGT, LD, and TG as well as lower levels of ALB and TP. The HCC group had higher levels of AFP, ALP, ALT, AST, Dbil, GGT, LD, and Tbil while having lower levels of TC and TG, relative to the OC group.
 |
Table 1 The Baseline Characteristics of the Subjects Included in This Study |
Repeatability Validation of Serum AKR1B10 Assay
To confirm the analytical stability of the serum AKR1B10 kit, we selected three samples (high, medium, and low levels of AKR1B10, respectively) for repeatability validation. The above three samples were assayed for 12 repetitions using the kit and the coefficient of variation (CV) values were then calculated. The analytical results were presented in Table S1. The CV values for samples with high, medium, and low concentrations of AKR1B10 were 6.24%, 3.18% and 0, respectively. The above CV values were below the performance indicator (10%) which claimed in the instruction of kit, indicating that the kit can stably detect the level of AKR1B10 in serum samples.
Elevated Serum AKR1B10 in Patients with HCC
Serum AKR1B10 was measured for all subjects in this study using the kit. As shown in Figure 1 and Table 1, the levels of serum AKR1B10 were significantly higher in the HCC group (median: 361.05 pg/mL) than in the HC (median: 87.73 pg/mL, P<0.001), BLD (median: 0 pg/mL, P<0.001) and OC (median: 0 pg/mL, P<0.001) groups. After performing multiple regression analyses adjusted for sex and age, serum AKR1B10 remained elevated in the HCC group relative to the HC (P<0.001), BLD (P=0.014), and OC (P<0.001) groups.
Association Between Serum AKR1B10 with Clinical Features in Patients with HCC
To investigate the correlation between serum AKR1B10 and clinical features in patients with HCC, we first analyzed the correlation between serum AKR1B10 and laboratory indicators in HCC group. As shown in Figure 2, the results indicated that serum AKR1B10 did not correlate with selected laboratory parameters regarding liver function, tumor markers and lipid metabolism. Furthermore, we analyzed the correlation between serum AKR1B10 and clinicopathological parameters of HCC. As depicted in Table 2, serum AKR1B10 did not correlate with age, sex, HBV, tumor differentiation and tumor size.
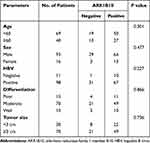 |
Table 2 Association Between AKR1B10 Expression and the Clinicopathological Parameters of HCC Patients |
Diagnostic Performance of Serum AKR1B10 for HCC
To sufficiently evaluate the diagnostic performance of AKR1B10 in HCC, we used receiver operator characteristic (ROC) curves to compare the diagnostic values of serum AKR1B10 with that of AFP, CEA, and CA19-9. When the HC group was used as the reference, AFP (AUC=0.920) and AKR1B10 (AUC=0.820) have higher diagnostic effectiveness while CEA and CA19-9 have relatively lower diagnostic effectiveness (Figure 3A). Similarly, AFP (AUC=0.877) and AKR1B10 (AUC=0.834) have relatively higher diagnostic values for HCC when compared with the BLD group (Figure 3B). Interestingly, when compared to the OC group, serum AKR1B10 exhibited the highest diagnostic effectiveness (AUC=0.882, Figure 3C). Furthermore, when we used the AC as the reference group, the ROC curve demonstrated the higher diagnostic values of AFP (AUC=0.896) and ARK1B10 (AUC=0.845) in comparison to CEA and CA19-9 (Figure 3D). The detailed diagnostic parameters were shown in Table 3. In view of these results, we further evaluated the effectiveness of a combination of AFP and ARK1B10 in the diagnosis of HCC. The results showed that when combining AFP with ARK1B10, satisfactory diagnostic performances were presented for all comparison groups (Figure 3E–H).
 |
Table 3 Diagnostic Parameters of AKR1B10, AFP, CEA, and CA19-9 to Distinguish HCC Group from Control Groups |
Diagnostic Performance of Serum AKR1B10 in AFP-Negative HCC
To fully understand the clinical application values of indicators, we calculated the positive rates of AKR1B10, AFP, CEA, and CA19-9 based on clinical reference interval in HCC cohort. The results revealed that serum AKR1B10 had the highest positive rate with respect to other tumor markers (Table 4). Remarkably, AFP, the most widely used biomarker for HCC today, was within the reference range (0–20 ng/mL) in nearly half of the HCC cases in this study. Moreover, we evaluated the diagnostic performance of serum AKR1B10 in AFP-negative HCC. The results of ROC analysis implied that serum AKR1B10 exhibited favorable diagnostic effectiveness for AFP-negative HCC in all comparison groups (Figure 4 and Table 5).
 |
Table 4 Positive Rates of Serum AKR1B10, AFP, CEA, and CA19-9 in HCC Patients |
 |
Table 5 Diagnostic Parameters of Serum AKR1B10 to Distinguish AFP-Negative HCC Group from Control Groups |
Clinical Follow-Up Analysis of Serum AKR1B10
Serum AKR1B10 levels in 44 HCC patients who underwent resection at pre-surgery, 1–2 days after surgery, and 3–4 days after surgery were displayed in Figure 5A. A gradual decrease in serum AKR1B10 could be observed in HCC patients after undergoing surgical resection, suggesting that serum AKR1B10 levels may be influenced by the presence or absence of tumors. When the serum AKR1B10 levels were categorized as negative or positive according to cut-off value provided in the instruction, the proportions of patients who were positive for serum AKR1B10 showed the same decreased trend after surgery (Figure 5B).
Discussion
Owing to the highly malignant nature of HCC, it has been a hot topic to explore new biomarkers for the diagnosis and prognosis for HCC in recent years. In this study, we evaluated the usefulness of serum AKR1B10 as a potential biomarker in a Beijing cohort with HCC. We found that serum AKR1B10 expression was higher in patients with HCC relative to healthy controls, benign liver disease controls, and other tumor controls. The association between serum AKR1B10 and clinical features of HCC was not observed. Serum AKR1B10 showed a high diagnostic performance for HCC, and when combined with AFP, the diagnostic effectiveness was significantly improved. Specifically, serum AKR1B10 showed superior diagnostic effectiveness for AFP-negative HCC. The clinical follow-up study indicated a gradual decrease in serum AKR1B10 after surgery. These findings could lay the foundation for further clinical applications of serum AKR1B10 in HCC.
There are numerous methods to detect the expression level of AKR1B10 and evaluate its application for HCC. Previous studies have confirmed that AKR1B10 was higher in HCC tissues than in non-cancerous tissues and was associated with prognosis.16,23,24 However, the use of immunohistochemical method to detect AKR1B10 expression in tissues is severely limited by the requirement of obtaining biopsy tissues and the difficulty of dynamically assessing the prognosis. It can also be noted that some studies have used reverse transcription‑quantitative polymerase chain reaction (RT-PCR) method to examine the expression of AKR1B10 in tissues.25,26 Nevertheless, its cumbersome operating procedures and the lack of standardization make it unsuitable for large-scale application. In this study, we measured serum AKR1B10 levels in subjects using commercially available kit. From the perspective of sample type, serum samples are easy to obtain non-invasively and can be used for prognostic monitoring. The commercial kit based on the TRFIA method offers the advantages of accuracy, convenience, standardization, high throughput, and rapid analysis. In the pre-experimental phase, we selected samples with high, medium, and low concentrations to evaluate the reproducibility of the kit. The results showed that the CV values of all three samples were less than 10%, reflecting the satisfactory reproducibility of the kit. It also provided the methodological basis for the broad clinical application of serum AKR1B10.
Serum AKR1B10 in patients with HCC exhibited higher level when comparing with HC, BLD and OC groups. This was consistent with some previous reports.9,14 Mechanistically, AKR1B10 can be induced by epidermal growth factor (EGF) and insulin through the activator protein-1 (AP-1) mitogenic signaling pathway in the progression of HCC.27 Zhang et al reported that AKR1B10 was an essential downstream target of AU-rich binding factor 1 (AUF1) and was critical for maintaining E2F1-AUF1-induced proliferation and drug resistance of HCC cells.28 Consistently with this, another study also demonstrated that silencing AKR1B10 could attenuate the malignant phenotype of HCC, including increased apoptosis, inhibition of colony formation, and enhanced cytoreductive response to doxorubicin chemotherapy.16 Taken together, these findings suggested that AKR1B10 could play an influential role in the oncogenicity of HCC. Furthermore, in this study, we also revealed that serum AKR1B10 level in patients with HCC did not correlate with laboratory indicators and clinicopathological parameters. It implied that serum AKR1B10 might be an independent laboratory indicator of HCC. Considering the limited number of cases and the restricted clinical data in this study, future large-scale multicenter studies are still warranted.
In assessing the diagnostic effectiveness of serological tumor markers in HCC, AUC values for AFP and AKR1B10 were higher than those for CEA and CA19-9. It suggested that AFP and AKR1B10 harbored great potential in the diagnosis of HCC. Interestingly, when using the OC group as the reference, the diagnostic performance of AKR1B10 was more superior than AFP. This finding highlighted the potential advantage of AKR1B10 in distinguishing HCC from other cancers. In a meta-analysis conducted by Wang et al, serum AKR1B10 had a pooled sensitivity of 0.80 (95% CI: 0.70–0.86), a pooled specificity of 0.87 (95% CI: 0.77–0.93), and a pooled AUC value of 0.89 (95% CI: 0.86–0.92) in the diagnosis of HCC.29 This is consistent with our results. Regarding sensitivity and specificity, our study revealed that serum AKR1B10 possessed high specificity but relatively low sensitivity whereas serum AFP showed the opposite trend based on the cut-off values from ROC curves. We speculate that there are several reasons that might explain this observation. From the perspective of data distribution, the measured values of AKR1B10 were highly skewed in this study. Notably, there were numerous subjects in the HC group who also had elevated serum AKR1B10. Nevertheless, they all derived from a population with normal results of medical examinations, and there was no definitive evidence of liver diseases. This means that other physiological or pathological factors may exist which could affect the secretion or metabolism of AKR1B10. On the other hand, the diagnostic parameters of AFP in the present study seem to be higher than previously reports.30,31 This may be related to the unique clinical features of the subjects we studied. Healthy individuals in the HC group had normal results for most laboratory parameters, including AFP. Most patients in the BLD group did not have severe liver injury and did not have an indication of developing HCC. And, most of patients in the OC group did not have liver metastases from their primary tumors. Furthermore, when we tried to combine AFP with AKR1B10 for the diagnosis of HCC, we were surprised to discover that the diagnostic effectiveness was greatly improved relative to the individual indicators (all AUC >0.9). It reflected that the combined use of AFP and AKR1B10 for the diagnosis of HCC in the real-world may help to increase the detection rate of HCC, thereby contributing to early intervention and personalized treatment.
AFP is the most widely used tumor marker for HCC in clinical practice at present. However, it was reported that only 33–65% of patients with tumors <3cm in size had elevated AFP levels, whereas approximately 30% of patients with HCC were within the normal reference range for AFP.32 In line with the above observation, our study also found nearly half of the cases showed AFP levels below the clinical cut-off value (20 ng/mL). In this case, if the diagnosis is made solely on the serum AFP, it would result in a significant portion of patients with HCC being underdiagnosed and losing the opportunity for early intervention. Fortunately, we discovered that serum AKR1B10 exhibited a comparatively high diagnostic value for AFP-negative HCC. These results further confirmed the fact that serum AKR1B10 can serve as a powerful complement to AFP for the diagnosis of HCC.
We also found that serum AKR1B10 levels tended to be decreased after surgical resection in 44 patients who were followed up. When serum AKR1B10 was qualitatively analyzed at the cut-off value recommended by the instruction, the positive rates of AKR1B10 decreased significantly after surgical resection. It suggested that serum AKR1B10 levels may be influenced by the presence or absence of tumors. In other words, serum AKR1B10 levels may be decreased when the tumor is cleared. On this basis, serum AKR1B10 seems to have a potential correlation with the prognosis of HCC patients. As for the role of serum AKR1B10 in the prognostic assessment of HCC, previous reports were contradictory. Some studies9,14–16 pointed out that high expression of serum AKR1B10 predicted poor outcome while several investigations20,21 supported that high expression of AKR1B10 was beneficial for survival in HCC. A recent study that included 273 patients with primary HCC followed for 2 years concluded that serum AKR1B10 was an independent biomarker of unfavorable prognosis for HCC.17 Thus, more studies covering various populations and different follow-up periods are still needed to clarify this aspect.
This study confirmed the superior diagnostic effectiveness of serum AKR1B10 in the diagnosis of HCC, especially for AFP-negative HCC, based on a Chinese cohort. Serum AKR1B10 could be used in combination with AFP in the future to improve the detection rate of HCC. Meanwhile, clinical follow-up analysis also implied a correlation between serum AKR1B10 levels and tumor clearance. These interesting findings suggested the potential of serum AKR1B10 as a prospective biomarker for HCC. However, there are still some knowledge gaps regarding AKR1B10 at present. For example, the biological functions and molecular regulatory mechanisms of AKR1B10 in HCC are still not fully elucidated. Previous studies have shown that multi-omics techniques can be used to explore key molecular pathways and genetic signatures.33,34 Thus, these issues could be investigated in the future through subtle cellular and animal experiments combined with multi-omics studies. Furthermore, it is difficult to explain that a proportion of patients with HCC do not have increased levels of serum AKR1B10. The co-morbidities and genomic variants of these patients deserve further study. Based on the complexity and aggressiveness of HCC as well as the heterogeneity among patients, the current clinical tumor markers appear to be underpowered. We believe that serum AKR1B10 is desirable for diagnostic and prognostic evaluation of HCC in the next few years along with other biomarkers. On the one hand, faster detection, higher throughput, and lower costs could be achieved through methodological innovations. On the other hand, multicenter studies covering larger populations, mixed ethnicities, and various clinical stages should be conducted to adequately evaluate the clinical value of serum AKR1B10.
Inevitably, there were several limitations in our study. The number of HCC cases included in this study was relatively limited. Future multicenter studies with large samples could contribute to further subgroup analyses to shed light on the applied value of serum AKR1B10 for the diagnosis and prognosis of HCC. In addition, the follow-up period after surgery was relatively short due to the limited conditions. Follow-up studies with long-term and multiple time points would be helpful to fully estimate the prognostic value of serum AKR1B10. Furthermore, the diagnostic value of serum AKR1B10 for HCC in early-stage was not evaluated. The utility of serum AKR1B10 in the recurrence and metastasis of HCC has also not been explored.
Data Sharing Statement
The de-identified data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
This study was performed in compliance with Declaration of Helsinki and approved by the Ethics Committee of Peking Union Medical College Hospital (approval number: HS2017071). The Ethics Committee agreed to waive the informed consent in this clinical study. The reason for the waiver is that this study would not interfere with the diagnosis or treatments of the patients. All data involving patients in this study are strictly confidential in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFC2406500) and the Beijing Key Clinical Specialty for Laboratory Medicine Excellent Project (No. ZK201000).
Disclosure
Zhe Cao is an employee of Hunan Light of Life Biotechnology Co., Ltd. The other authors declare that they have no competing interests.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
2. Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Inst. 2022;2(1):1–9. doi:10.1016/j.jncc.2022.02.002
3. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi:10.1038/nrdp.2016.18
4. Rizzo A, Ricci AD, Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Future Oncol. 2020;16(32):2587–2589. doi:10.2217/fon-2020-0669
5. Mollica V, Rizzo A, Marchetti A, et al. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin Exp Med. 2023;23(8):5039–5049. doi:10.1007/s10238-023-01159-1
6. Rizzo A, Ricci AD, Brandi G. Trans-arterial chemoembolization plus systemic treatments for hepatocellular carcinoma: an update. J Pers Med. 2022;12(11):1788. doi:10.3390/jpm12111788
7. Santoni M, Rizzo A, Mollica V, et al. The impact of gender on the efficacy of immune checkpoint inhibitors in cancer patients: the MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596. doi:10.1016/j.critrevonc.2022.103596
8. Lu Q, Li J, Cao H, Lv C, Wang X, Cao S. Comparison of diagnostic accuracy of midkine and AFP for detecting hepatocellular carcinoma: a systematic review and meta-analysis. Biosci Rep. 2020;40(3). doi:10.1042/bsr20192424
9. Ye X, Li C, Zu X, et al. A large-scale multicenter study validates aldo-keto reductase family 1 member B10 as a prevalent serum marker for detection of hepatocellular carcinoma. Hepatology. 2019;69(6):2489–2501. doi:10.1002/hep.30519
10. Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer. 2019;18(1):114. doi:10.1186/s12943-019-1043-x
11. Cao D, Fan ST, Chung SS. Identification and characterization of a novel human aldose reductase-like gene. J Biol Chem. 1998;273(19):11429–11435. doi:10.1074/jbc.273.19.11429
12. Zhong L, Liu Z, Yan R, et al. Aldo-keto reductase family 1 B10 protein detoxifies dietary and lipid-derived alpha, beta-unsaturated carbonyls at physiological levels. Biochem Biophys Res Commun. 2009;387(2):245–250. doi:10.1016/j.bbrc.2009.06.123
13. Gallego O, Ruiz FX, Ardèvol A, et al. Structural basis for the high all-trans-retinaldehyde reductase activity of the tumor marker AKR1B10. Proc Natl Acad Sci U S A. 2007;104(52):20764–20769. doi:10.1073/pnas.0705659105
14. Zhu R, Xiao J, Luo D, Dong M, Sun T, Jin J. Serum AKR1B10 predicts the risk of hepatocellular carcinoma - a retrospective single-center study. Gastroenterol Hepatol. 2019;42(10):614–621. doi:10.1016/j.gastrohep.2019.06.007
15. Murata A, Genda T, Ichida T, et al. Pretreatment AKR1B10 expression predicts the risk of hepatocellular carcinoma development after hepatitis C virus eradication. World J Gastroenterol. 2016;22(33):7569–7578. doi:10.3748/wjg.v22.i33.7569
16. Matkowskyj KA, Bai H, Liao J, et al. Aldoketoreductase family 1B10 (AKR1B10) as a biomarker to distinguish hepatocellular carcinoma from benign liver lesions. Hum Pathol. 2014;45(4):834–843. doi:10.1016/j.humpath.2013.12.002
17. Xie C, Ye X, Zeng L, Zeng X, Cao D. Serum AKR1B10 as an indicator of unfavorable survival of hepatocellular carcinoma. J Gastroenterol. 2023;58(10):1030–1042. doi:10.1007/s00535-023-02011-9
18. Han C, Gao L, Bai H, Dou X. Identification of a role for serum aldo-keto reductase family 1 member B10 in early detection of hepatocellular carcinoma. Oncol Lett. 2018;16(6):7123–7130. doi:10.3892/ol.2018.9547
19. Han C, Gao L, Zhao L, et al. Immunohistochemistry detects increased expression of aldo-keto reductase family 1 member B10 (AKR1B10) in early-stage hepatocellular carcinoma. Med Sci Monit. 2018;24:7414–7423. doi:10.12659/msm.910738
20. Schmitz KJ, Sotiropoulos GC, Baba HA, et al. AKR1B10 expression is associated with less aggressive hepatocellular carcinoma: a clinicopathological study of 168 cases. Liver Int. 2011;31(6):810–816. doi:10.1111/j.1478-3231.2011.02511.x
21. Ha SY, Song DH, Lee JJ, Lee HW, Cho SY, Park CK. High expression of aldo-keto reductase 1B10 is an independent predictor of favorable prognosis in patients with hepatocellular carcinoma. Gut Liver. 2014;8(6):648–654. doi:10.5009/gnl13406
22. Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer. 2018;7(3):235–260. doi:10.1159/000488035
23. Wu CY, Jan YJ, Ko BS, Wu YJ, Wu YJ, Liou JY. Prognostic significance of 14-3-3ε, aldo-keto reductase family 1 B10 and metallothionein-1 in Hepatocellular carcinoma. Anticancer Res. 2018;38(12):6855–6863. doi:10.21873/anticanres.13060
24. Tsuzura H, Genda T, Sato S, et al. Expression of aldo-keto reductase family 1 member b10 in the early stages of human hepatocarcinogenesis. Int J Mol Sci. 2014;15(4):6556–6568. doi:10.3390/ijms15046556
25. Sonohara F, Inokawa Y, Hishida M, et al. Prognostic significance of AKR1B10 gene expression in hepatocellular carcinoma and surrounding non-tumorous liver tissue. Oncol Lett. 2016;12(6):4821–4828. doi:10.3892/ol.2016.5240
26. Torres-Mena JE, Salazar-Villegas KN, Sánchez-Rodríguez R, et al. Aldo-keto reductases as early biomarkers of Hepatocellular carcinoma: a comparison between animal models and human HCC. Dig Dis Sci. 2018;63(4):934–944. doi:10.1007/s10620-018-4943-5
27. Liu Z, Yan R, Al-Salman A, et al. Epidermal growth factor induces tumour marker AKR1B10 expression through activator protein-1 signalling in hepatocellular carcinoma cells. Biochem J. 2012;442(2):273–282. doi:10.1042/bj20111322
28. Zhang T, Guan G, Zhang J, et al. E2F1-mediated AUF1 upregulation promotes HCC development and enhances drug resistance via stabilization of AKR1B10. Cancer Sci. 2022;113(4):1154–1167. doi:10.1111/cas.15272
29. Wang Z, Pei Y, Li W, Zhang J, Liu J, Abdel Ghafar MT. Clinical value of AKR1B10 in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2022;17(12):e0279591. doi:10.1371/journal.pone.0279591
30. Xu D, Su C, Sun L, Gao Y, Li Y. Performance of serum glypican 3 in diagnosis of Hepatocellular carcinoma: a meta-analysis. Ann Hepatol. 2019;18(1):58–67. doi:10.5604/01.3001.0012.7863
31. Pang BY, Leng Y, Wang X, Wang YQ, Jiang LH. A meta-analysis and of clinical values of 11 blood biomarkers, such as AFP, DCP, and GP73 for diagnosis of hepatocellular carcinoma. Ann Med. 2023;55(1):42–61. doi:10.1080/07853890.2022.2153163
32. Liu Z, Pu Y, Bao Y, He S. Investigation of potential molecular biomarkers for diagnosis and prognosis of AFP-negative HCC. Int J Gen Med. 2021;14:4369–4380. doi:10.2147/ijgm.S323868
33. Lu Z, Priya Rajan SA, Song Q, et al. 3D scaffold-free microlivers with drug metabolic function generated by lineage-reprogrammed hepatocytes from human fibroblasts. Biomaterials. 2021;269:120668. doi:10.1016/j.biomaterials.2021.120668
34. Triozzi PL, Stirling ER, Song Q, et al. Circulating immune bioenergetic, metabolic, and genetic signatures predict melanoma patients’ response to anti-PD-1 immune checkpoint blockade. Clin Cancer Res. 2022;28(6):1192–1202. doi:10.1158/1078-0432.Ccr-21-3114
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





