Back to Journals » International Journal of General Medicine » Volume 17
Serum ACTH and Cortisol Level is Associated with the Acute Gastrointestinal Injury Grade in ICU Patients
Authors Xu W, Qiu Y, Qiu H, Zhong M, Li L
Received 20 October 2023
Accepted for publication 11 January 2024
Published 16 January 2024 Volume 2024:17 Pages 127—134
DOI https://doi.org/10.2147/IJGM.S445741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Wen Xu,* Yuzhen Qiu,* Hongping Qiu, Ming Zhong, Lei Li
Department of Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Li; Ming Zhong, Department of Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China, Email [email protected]; [email protected]
Background: The relationship between acute gastrointestinal symptoms and cortisol or adrenocorticotropic hormone (ACTH) levels has rarely been reported. We hypothesized that the elevation of serum cortisol or ACTH levels may be correlated with the severity of the acute gastrointestinal injury grade (AGI).
Methods: This study was an observational study. All patients were admitted to the ICU between 2019.1.1 and 2020.1.1.. Serum ACTH and cortisol levels and clinical data were collected from the electronic medication records. The highest AGI grade during the ICU stay was the major endpoint to observe. The patient was treated in a standard procedure in the ICU.
Results: A total of 235 patients were included in our study, 132 of whom developed AGI. In univariate regression, cortisol level was found to be a risk factor for 28-day mortality. Serum cortisol and ACTH levels correlated with APACHE II, AGI grade, PCT, and CRP levels. Spearman analysis and partial correlation analysis indicated that cortisol and ACTH levels were correlated with AGI grade.
Conclusion: The ACTH and cortisol levels were positively correlated with the higher severity of AGI grade. The cortisol level may be a useful way to access the GI injury.
Keywords: acute gastrointestinal injury, cortisol, adrenocorticotropic hormone
Erratum for this paper has been published.
Introduction
Elevated serum corticosteroid levels have also been reported in critical illnesses.1 Corticosteroid insufficiency is believed to be a major phenotype caused by decreased cortisol clearance and target tissue resistance to cortisol.2 In 2007, critical illness-related corticosteroid insufficiency (CIRCI) was defined by the Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) task force. It refers to the dysregulation of the hypothalamic-pituitary-adrenal axis, altered cortisol metabolism, and peripheral tissue resistance to glucocorticoids. However, the association between cortisol levels and the degree of illness or clinical outcome is unclear according to previous studies.1,3 The adrenocorticotropic hormone (ACTH), whose function is to regulate cortisol levels, has also been reported to be associated with the severity of critical illness.1
Critically ill patients showed a high heterogeneity. The relationship of ACTH or cortisol and patient’s outcome is controversial in this population. The relationship between ACTH, cortisol, and mechanical ventilation (MV) has been well-studied, showing CIRIC occurred in 33~22% MV patients, and low cortisol level may be associated with difficult weaning and prolonged ICU stay.4,5 Higher plasma cortisol levels are associated with a higher mortality rate in COVID-19 patients; however, ACTH levels have not been reported.6 On the other hand, acute gastrointestinal injury (AGI) is also a common syndrome in critical illness. The degree of AGI affects the outcome of the disease.7 However, a relationship between acute gastrointestinal symptoms and cortisol or ACTH levels has rarely been reported.
How ACTH and cortisol levels are related to AGI grade is an important aspect of the characteristics of AGI and may have an impact on the therapy choice or outcome. We hypothesized that the elevation of serum cortisol or ACTH levels may be correlated with AGI severity. Therefore, we performed this study to explore the relationship between serum cortisol or ACTH levels and AGI and to provide a basis for glucocorticoid application in the treatment of critically ill patients.
Method
Patient Eligibility
This observational study was conducted in a 12-bed surgical ICU of a teaching hospital. All patients admitted between 2019.1.1, 2020.1.1 were enrolled in this study. The inclusion criteria were as follows: 1) ICU stay > 72 hours and 2) age between 18~90-year-old. This study was approved by the Ethical Committee of Ruijin Hospital, Shanghai Jiao Tong University approved the study. The study was conducted following the Declaration of Helsinki. Patients or their kins were verbally informed about the use of their data. The consent was signed accordingly.
Data Collection
All the attending doctors in the department were trained to diagnose patients with AGI, following the 2012 ESICM recommendations.8 The AGI grades can be briefly described as follows: AGI grade I, the function of the GI was partially impaired, symptom was self-limiting. AGI grade II, GI dysfunction required interventions and could be improved after then. AGI grade III, the GI dysfunction was not improving despite interventions with the deterioration of other organ. AGI grade IV, AGI became directly life-threatening, with worsening of Multi-organ Dysfunction Syndrome (MODS).8
AGI grade and gastrointestinal symptoms were evaluated daily during the ICU stay. AGI grades were defined according to the highest AGI grade during the ICU stay. The need for cortisol or ACTH testing was regularly tested on admission, as determined by the doctor in charge of the patient.
Clinical data collected from the electrical medication records included demographic data, Acute Physiology and Chronic Health Evaluation (APACHE) II score, duration of ICU stay, Sequential Organ Failure Assessment (SOFA) score, C-reactive protein (CRP), procalcitonin (PCT), cortisol level, ACTH level, 28-day mortality (28 days after ICU admission), and ICU mortality. The primary endpoint was the highest AGI grade during ICU stay. The patient was treated in a standard procedure in the ICU.
Statistical Analyses
SPSS version 22.0 (IBM) was used for statistical analyses. All calculation data are presented as frequencies and percentages, means and standard deviations, or median and 25th/75th percentiles. Fisher’s exact test and the Kruskal–Wallis test were used to compare categorical and continuous variables, respectively. Spearman correlation analysis, and partial correlation analysis were used to investigate the correlation. All significance tests were 2-sided, with statistical significance set at P < 0.05.
Result
A total of 235 patients were included in our study, 276 of whom were admitted to ICU. The patient characteristics are listed in Table 1. The time of the first cortisol and ACTH levels were all within 36 h of ICU admission.
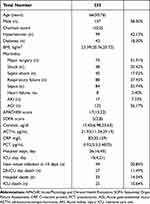 |
Table 1 Characteristics of Patients and Baseline Information |
We use 28-day mortality as outcome parameter (Table 2). The death group had higher cortisol and ACTH levels [Death vs survival: Cortisol 24.43 (15.15–38.62) vs 15.32 (9.63, 23.43) P<0.01; ACTH 33.04 (18.74,61.40) vs 11.15 (11.15,36.97) P=0.01].
 |
Table 2 Patient’s Characteristics for Survival in 28 Days |
Logistic regression was used to identify risk factors for 28-day mortality (Table 3). In univariate regression, cortisol level was found to be a risk factor for 28-day mortality (OR 0.96 95% CI 0.93–0.99, P<0.01). SOFA (OR 0.78 95% CI 0.71–0.86, P<0.01), AGI grade (OR 0.68 95% CI 0.49–0.95, P=0.02), PCT (OR 0.98 95% CI 0.97–0.99, P=0.01) also indicated to be a risk factor in univariate regression. Variables with P-values<0.05 were included in the multivariable logistic regression. In multivariate regression analysis, cortisol level, AGI grade and PCT were not risk factor for 28-day mortality, while SOFA was (OR 0.82 95% CI 0.72–0.89, P<0.01).
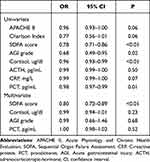 |
Table 3 Logistic Regression for 28-Day Mortality of the Patients |
Spearman analysis was used to determine the correlation between cortisol and ACTH levels and the other parameters (Table 4A). Both cortisol and ACTH levels were correlated with APACHE II (correlation coefficient: cortisol 0.29 95% CI0.16–0.41, P<0.01; ACTH 0.14 95% CI 0.01–0.27, P=0.03), AGI grade (correlation coefficient: cortisol 0.37 95% CI0.25–0.48, P<0.01; ACTH 0.21 95% CI0.08–0.32, P<0.01), PCT (correlation coefficient: cortisol 0.27 95% CI 0.15–0.39, p<0.01; ACTH 0.18 95% CI 0.05–0.30, P<0.01), and CRP (correlation coefficient: cortisol 0.43 95% CI0.32–0.53, P<0.01; ACTH 0.21 95% CI 0.08–0.33, P<0.01) levels.
 |
Table 4 (A) Correlation of Cortisol or ACTH and Other Parameters. (B) Correlation of AGI and Other Parameters, Unadjust. (C) Correlation of AGI Grade and Other Parameters, Adjusted |
We use the AGI grade as the outcome, the grade of AGI is correlated with the cortisol and ACTH level (Table 4B, correlation coefficient: cortisol 0.31 95% CI0.18–0.42, p<0.01; ACTH 0.21 95% CI0.08–0.33, p<0.01). We found a correlation between ACTH or cortisol levels and the SOFA, PCT, CRP, and APAHCHE II scores. We used partial correlation analysis to adjust for the effects of SOFA, PCT, CRP, and APACHE II scores; the correlation of cortisol levels still existed (Table 4C, correlation coefficient: cortisol 0.05 95% CI0.01–0.58, p=0.04; ACTH 0.03 95% CI0.01–0.26, p=0.03).
Discussion
AGI and ACTH-Cortisol Level
In this study, we found that ACTH and cortisol levels were associated with the degree of AGI, which is in accordance with our hypothesis.
Gastrointestinal symptoms are associated with hypothalamic-pituitary-adrenal (HPA) function. In healthy individuals, more diarrhea and early satiety were found in those with high or low post-dexamethasone cortisol levels than those with intermediate post-dexamethasone cortisol levels.9 Patients with major depression exhibited more GI symptoms than healthy population after dexamethasone suppression test.10 The relationship between GI symptoms and cortisol or ACTH levels in ICU patients has rarely been reported. Our study showed that ACTH and cortisol levels were associated with the degree of AGI in ICU population, which is accordance with the above studies. The AGI grade includes a wide range of GI symptoms, and the relationship between these symptoms and cortisol or ACTH levels should be investigated in future studies. In a healthy population, combined training increases GI symptoms as well as cortisol, interleukin-6 level.11 These results, together with ours, indicate a potential causality between systemic inflammation and GI symptoms. GI symptoms may be a good indicator of response between cortisol levels and the acute inflammation level.
In acute stress model, GI mucosa injury/bleeding is associated with increased ACTH and cortisol level.12 Alain-Pascal et al reported that high level of cortisol and ACTH increased the risk of GI bleeding in patients with traumatic brain injury.13 Kang et al reported higher GI bleeding incidence in septic patients treating with steroid.14 High cortisol level might also be a contributing factor for gastric ulcer complications by slowing down the ulcer healing process.15 In our study, it is difficult to correlate GI bleeding to high cortisol level or ACTH level, as patients usually underwent major GI surgery. But it is reasonable that their risk of bleeding was higher in patients with higher cortisol levels. Thus, in AGI patients, aggressive strategy should be adapted to prevent stress ulcer especially in those with high level of cortisol or ACTH.
Cortisol and ACTH Level in Critical Illness
Cortisol and ACTH levels reflect the degree of stress in many population.16 Increased cortisol and ACTH levels have been observed in critically ill patients or after a major surgery.1 In critical illness patients, the metabolism of ACTH and cortisol is complex. In the hyperacute phase, the HPA-axis is centrally activated, and a fast decline in cortisol carrier proteins, albumin and cortisol-binding globulin, and cortisol metabolism in liver and kidney further increase the amount of free cortisol in the circulation. Meanwhile, the therapeutic measures such as fluid therapy, vasopressors and hydrocortisone usage had great impact on cortisol and ACTH measuring, leading to difficulties in interpreting the result.17 In our study, the survival group had higher ACTH and cortisol levels on the first day, which is in accordance with the pattern in acute state.
In the critically ill population, a decrease in ACTH and increase in cortisol levels were found to be associated with patient mortality in some studies.18,19 The hospital mortality was higher in ICU patients diagnosed with ACTH-cortisol dissociation.20 The mechanism of dissociation is not clear. According to Van den Berghe et, dissociation mainly happens in subacute or prolonged phase of critical illness and proopiomelanocortin-mediated stimulation of the adrenal cortex may play an important role17 Long stayed ICU patients (>14 days) may develop Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PICS), and may be characterized by increasing in serum cortisol and decreasing in serum ACTH levels (ACTH-cortisol dissociation).21 In our study, very few patients stayed long (>14 days) in ICU. But we will pay further concern on long stayed ICU patients in future.
The relationship between cortisol level and stress or inflammation is complex. ACTH and cortisol levels are important for revealing not only the function of the HPA, but also the actual level of endocrine function in a particular state of patients. Thus, a set of tests to show the whole picture of ACTH, cortisol and HPA axis in ICU patients is essential, even though many factors make interpretation difficult. Particularly, to better understanding the metabolism of cortisol, cortisol carrier proteins, such as albumin and cortisol-binding globulin, should also be tested. Regular tests (ie every week) are especially necessary in AGI patients or long-stayed ICU patients. It is recommended to use a stimulation test to evaluate HPA function to diagnose CIRCI. However, studies have shown that the relationship between test results and patient outcomes is unclear,22 while the risk of GI bleeding increases even after low dose of dexamethasone admission in the test. So the stimulation test should only do when CIRCI was highly suspected.
Limitation
This study has several limitations. First, this study was conducted at a single center, and most patients were admitted after surgery. Therefore, it may be difficult to represent other ICU populations, such as those in internal medicine or emergency ICU. Second, this was an observational study; therefore, it could not show causality between AGI and cortisol or ACTH levels. However, further studies on this mechanism are required. Third, we did not include biomarkers (such as citrulline, intestinal fat acid-binding protein, and intestinal alkaline phosphatase) for GI function. Additionally, the intestinal microbiome plays an important role in the GI response to cortisol or ACTH.23,24 However, these tests are expensive and difficult to interpret. Further investigations are warranted to combine biomarkers and microbiome change.
Conclusion
Our study indicated that higher ACTH or cortisol levels were correlated with a higher severity of AGI. A regular test of cortisol and ACTH levels may be helpful in assessing GI injuries in ICU patients, especially in AGI patients. Further studies are required to confirm these results.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author, Ming Zhong, upon reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by the Ruijin Hospital Ethics Committee of Shanghai Jiaotong University School of Medicine, China. Formal informed consent was obtained from the patients or their next of kin.
Consent for Publication
Consent for publication was obtained from all the authors.
Acknowledgments
The study was conducted thanks to the helpful contributions of all ICU staff and the understanding and love of all the family members.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Gibbison B, Keenan DM, Roelfsema F, et al. Dynamic pituitary-adrenal interactions in the critically ill after cardiac surgery. J Clin Endocrinol Metab. 2020;105(5):1327–1342. doi:10.1210/clinem/dgz206
2. Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017 [published correction appears in Intensive Care Med. 2018 Feb 23]. Intensive Care Med. 2017;43(12):1751–1763. doi:10.1007/s00134-017-4919-5
3. Marik PE. Critical illness-related corticosteroid insufficiency. Chest. 2009;135(1):181–193. doi:10.1378/chest.08-1149
4. Huang CJ, Lin HC. Association between adrenal insufficiency and ventilator weaning. Am J Respir Crit Care Med. 2006;173(3):276–280. doi:10.1164/rccm.200504-545OC
5. Bagate F, Bedet A, Tomberli F, et al. Critical illness-related corticosteroid insufficiency during difficult weaning from mechanical ventilation. Ann Intensive Care. 2021;11(1):65. doi:10.1186/s13613-021-00852-2
6. Tan T, Khoo B, Mills EG, et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8(8):659–660. doi:10.1016/S2213-8587(20)30216-3
7. Zhong M, Xu W, Qiu Y, et al. Association of changes in acute gastrointestinal injury grade with prognosis in critically ill patients: a Prospective, Single-Center, Observational Study. J Multidiscip Healthc. 2021;14:279–286. doi:10.2147/JMDH.S291883
8. Reintam Blaser A, Malbrain ML, Starkopf J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38(3):384–394. doi:10.1007/s00134-011-2459-y
9. Karling P, Norrback K-F, Adolfsson R, et al. Gastrointestinal symptoms are associated with hypothalamic-pituitary-adrenal axis suppression in healthy individuals. Scand J Gastroenterol. 2007;42(11):1294–1301. doi:10.1080/00365520701395945
10. Karling P, Wikgren M, Adolfsson R, et al. Hypothalamus-pituitary-adrenal axis hypersuppression is associated with gastrointestinal symptoms in major depression. J Neurogastroenterol Motil. 2016;22(2):292–303. doi:10.5056/jnm15064
11. Li X, Kan EM, Lu J, et al. Combat-training increases intestinal permeability, immune activation and gastrointestinal symptoms in soldiers. Aliment Pharmacol Ther. 2013;37(8):799–809. doi:10.1111/apt.12269
12. Fan F, Ai Y, Sun T, et al. The role of inflammatory cytokines in anemia and gastrointestinal mucosal injury induced by foot electric stimulation. Sci Rep. 2021;11(1):3101. doi:10.1038/s41598-021-82604-7
13. Alain-Pascal BB, Wei HJ, Chen X, Zhang JN. Evaluation of stress hormones in traumatic brain injury patients with gastrointestinal bleeding. Chin J Traumatol. 2010;13(1):25–31.
14. Kang J, Han M, Hong S-B, et al. Effect of adjunctive corticosteroid on 28-day mortality in neutropenic patients with septic shock. Ann Hematol. 2019;98(10):2311–2318. doi:10.1007/s00277-019-03785-w
15. Hoshino C, Satoh N, Narita M, et al. Another ‘Cushing ulcer’. BMJ Case Rep. 2011;2011:bcr0220113888. doi:10.1136/bcr.02.2011.3888
16. Lyu N, Zhao Q, Fu B, et al. Hormonal and inflammatory signatures of different mood episodes in bipolar disorder: a large-scale clinical study. BMC Psychiatry. 2023;23(1):449. doi:10.1186/s12888-023-04846-1
17. Van den Berghe G, Téblick A, Langouche L, et al. The hypothalamus-pituitary-adrenal axis in sepsis- and hyperinflammation-induced critical illness: gaps in current knowledge and future translational research directions. EBioMedicine. 2022;84:104284. doi:10.1016/j.ebiom.2022.104284
18. Jacobs A, Derese I, Vander Perre S, et al. Dynamics and prognostic value of the hypothalamus-pituitary-adrenal axis responses to pediatric critical illness and association with corticosteroid treatment: a prospective observational study. Intensive Care Med. 2020;46(1):70–81. doi:10.1007/s00134-019-05854-0
19. Salluh JI, Shinotsuka CR, Soares M, et al. Cortisol levels and adrenal response in severe community-acquired pneumonia: a systematic review of the literature. J Crit Care. 2010;25(3):
20. Song JH, Kim JH, Lee S-M, et al. Prognostic implication of adrenocortical response during the course of critical illness. Acute Crit Care. 2019;34(1):38–45. doi:10.4266/acc.2018.00339
21. Zhang J, Luo W, Miao C, et al. Hypercatabolism and anti-catabolic therapies in the persistent inflammation, immunosuppression, and catabolism syndrome. Front Nutr. 2022;9:941097. doi:10.3389/fnut.2022.941097
22. Shaikh S, Nagendra L, Shaikh S, et al. Adrenal failure: an evidence-based diagnostic approach. Diagnostics. 2023;13(10):1812. doi:10.3390/diagnostics13101812
23. Bennett CJ, Henry R, Snipe RMJ, et al. Is the gut microbiota bacterial abundance and composition associated with intestinal epithelial injury, systemic inflammatory profile, and gastrointestinal symptoms in response to exertional-heat stress? J Sci Med Sport. 2020;23(12):1141–1153. doi:10.1016/j.jsams.2020.06.002
24. Kato-Kataoka A, Nishida K, Takada M, et al. Fermented milk containing lactobacillus casei strain shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl Environ Microbiol. 2016;82(12):3649–3658. doi:10.1128/AEM.04134-15
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
