Back to Journals » Infection and Drug Resistance » Volume 16
Seroprevalence of SARS-CoV-2 Antibodies in Denmark: Results of Two Nationwide Population-Based Surveys, February and May 2021
Authors Krogsgaard LW, Espenhain L, Tribler S, Sværke Jørgensen C , Hansen CH, Møller FT , Glode Helmuth I, Sönksen UW, Vangsted AM, Ullum H, Ethelberg S
Received 26 July 2022
Accepted for publication 14 December 2022
Published 15 January 2023 Volume 2023:16 Pages 301—312
DOI https://doi.org/10.2147/IDR.S383491
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Lene Wulff Krogsgaard,1 Laura Espenhain,1 Siri Tribler,1 Charlotte Sværke Jørgensen,2 Christian Holm Hansen,1 Frederik Trier Møller,1 Ida Glode Helmuth,1 Ute Wolff Sönksen,3,4 Anne-Marie Vangsted,4 Henrik Ullum,5 Steen Ethelberg1,6
1Department of Infectious Disease Epidemiology and Prevention, Statens Serum Institut, Copenhagen, Denmark; 2Department of Virus & Microbiological Special Diagnostics, Statens Serum Institut, Copenhagen, Denmark; 3Department of Bacteria, Parasites and Fungi, Statens Serum Institut, Copenhagen, Denmark; 4TestCentre Denmark, Statens Serum Institut, Copenhagen, Denmark; 5Division of Infectious Disease Preparedness, Statens Serum Institut, Copenhagen, Denmark; 6Department of Public Health, University of Copenhagen, Copenhagen, Denmark
Correspondence: Steen Ethelberg, Department of Infectious Disease Epidemiology and Prevention, Statens Serum Institut, 5 Artillerivej, 2300 Copenhagen, Denmark, Tel +45 3268 3545, Email [email protected]
Background: Seroprevalence studies can be used to measure the progression of national COVID-19 epidemics. The Danish National Seroprevalence Survey of SARS-CoV-2 infections (DSS) was conducted as five separate surveys between May 2020 and May 2021. Here, we present results from the two last surveys conducted in February and May 2021.
Methods: Persons aged 12 or older were randomly selected from the Danish Population Register and those having received COVID-19 vaccination subsequently excluded. Invitations to have blood drawn in local test centers were sent by mail. Samples were analyzed for whole Immunoglobulin by ELISA. Seroprevalence was estimated by sex, age and geography. Comparisons to vaccination uptake and RT-PCR test results were made.
Results: In February 2021, we found detectable antibodies in 7.2% (95% CI: 6.3– 7.9%) of the invited participants (participation rate 25%) and in May 2021 in 8.6% (95% CI: 7.6– 9.5%) of the invited (participation rate: 14%). Seroprevalence did not differ by sex, but by age group, generally being higher among the < 50 than 50+ year-olds. In May 2021, levels of seroprevalence varied from an estimated 13% (95% CI: 12– 15%) in the capital to 5.2% (95% CI: 3.4– 7.4%) in rural areas. Combining seroprevalence results with vaccine coverage, estimates of protection against infection in May 2021 varied from 95% among 65+ year-olds down to 10– 20% among 12– 40 year-olds. In March–May 2021, an estimated 80% of all community SARS-CoV-2 infections were diagnosed by RT-PCR and captured by surveillance.
Conclusion: Seroprevalence estimates doubled during the 2020– 21 winter wave of SARS-CoV-2 infections and then stabilized as vaccinations were rolled out. The epidemic affected large cities and younger people the most. Denmark saw comparatively low infections rates, but high test coverage; an estimated four out of five infections were detected by RT-PCR in March–May 2021.
Keywords: seroepidemiological studies, COVID-19 serological testing, SARS-CoV-2, population register, questionnaire, ELISA
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) and clinical symptoms range from asymptomatic infection to severe disease and death. Infections are generally detected by use of RT-PCR or antigen tests, however, silent infections and limitations in test availability reduce the proportion of cases detected in the community, which in many settings have challenged both surveillance and control of the epidemic.
Depending on the given SARS-CoV-2 testing strategy and capacity, a varying proportion of all infections in the community will go unrecognized. Thus, it is difficult to estimate the true prevalence of previously infected persons based primarily on RT-PCR or antigen test results, for which reason seroprevalence studies have become an important tool in monitoring the spread and progression of national COVID-19 epidemics.1 A number of serological surveys of SARS-CoV-2 have been conducted in several countries using various study designs.2–11 In Denmark, the Danish National Seroprevalence Survey of SARS-CoV-2 infections (DSS) was initiated in the spring of 2020 by Statens Serum Institut.12 Initially, DSS was performed over three rounds: In May 2020 (DSS-I), August 2020 (DSS-II) and November 2020 (DSS-III).13 In winter 2020–2021, Denmark was affected by a large wave of SARS-CoV-2 infections and as a result went into lock-down from 17 December 2020, gradually reopening in early April 2021.14 The national vaccination program was initiated on 27 December 2020 and in the following months Danish residents were personally invited for vaccination by a prioritization scheme.15 First, people of particular risk of severe disease and hospital staff with high exposure to COVID-19 infected patients were invited, followed by the oldest age group and downward. By 1 March 11% and by 1 July 70% of the population above the age of 20 years had received the first vaccination dose. In this situation, two further rounds of the seroprevalence survey were performed in February 2021 (DSS-IV) and May 2021 (DSS-V), with the aim of monitoring the progression of the epidemic at population level, nationally, regionally and by age group.
Here, we present the results of these two seroprevalence surveys conducted in the non-vaccinated population in 2021. We present the overall and geographically distributed progression in seroprevalence before and after the 2020–21 winter wave of SARS-CoV-2 infection. Additionally, we present estimates of the difference between the estimated number of true cases and the number of cases detected by RT-PCR in the spring of 2021.
Methods
Study Design
From February to May 2021, we conducted two separate rounds of nationwide population-based cross-sectional studies, each using a random representative sample of residents in Denmark aged 12 years and older. Full details on the design of the study have been described previously.13
SARS-CoV-2 Testing in Denmark
The Danish test strategy was based on publicly offered, widespread, free-for-all RT-PCR tests to detect infected cases, later extended to also include antigen-tests of varying types – though results from the latter were not included in public surveillance within the study period. The testing strategy and coverage of the population changed substantially during the pandemic. In the early days of the pandemic (February, 2020), RT-PCR tests were offered primarily to symptomatic cases. From 20 May 2020 and onwards, RT-PCR tests were also offered free of charge to non- or mild symptomatic persons as part of the national surveillance of the pandemic.16 Test capacity was gradually extended and by mid-2021 public test stations served by the publicly owned TestCenter Denmark (TCDK) were operating and covering all parts of the country (geographical size: 43,000 km2). In May 2021, where testing intensity peaked, 11 million personal official RT-PCR tests were performed (population size: 5.8 million inhabitants).17
Data Sources
In Denmark, all persons are registered in the Danish Civil Registration System (CRS) with a civil registration number.18 The CRS contains information on age, sex and residency and was used to draw random population samples for invitations to the study. The Danish National Microbiology Database (MiBa) contains all test results from clinical microbiology departments19 as well as all results from TCDK20 and the reference laboratories at the Danish national center for infectious disease control, the Statens Serum Institut (SSI). The national Danish Vaccination register (DDV) contains information about given vaccinations on an individual level, including all COVID-19 vaccinations.15,21 From DDV we extracted information on date of first COVID-19 vaccination. The civil registration number was used to link personal information on address, age and sex with antibody test results and results from RT-PCR tests from MiBa as well as information on COVID-19 vaccination status, retrieved from DDV.
Study Setting and Population
In the last week of February and the first week of May, random samples of 125,000 residents of Denmark ≥12 years old were retrieved from the CRS. Persons already vaccinated with at least one vaccine dose against COVID-19 were removed from the lists (Figure 1). Invitation letters were then sent via the Danish digitalized postal system (e-Boks) to unvaccinated adults (18+ years old) and adolescents (12–17 years old); persons without e-Boks received the invitation by the ordinary mail system. Letters of invitation contained information about the aim and study design, the antibody test (how to interpret and understand the test result), and how to participate. The invitation letters also included information about the selection procedure, risks associated with participation, data security issues, and legal rights, including the right to withdraw from the study. Participation was voluntary and consent was given by booking a timeslot for the test, using a personal login (NemId) at a secure website. Parents booked test, and thereby consented, on their child’s behalf. Invitation letters were available in Danish, English and Arabic language versions. In both study rounds, invitations were sent out once per week over a 4-week period, in week numbers 9–12 (1–22 March) and 19–22 (10–31 May), 2021, respectively.
 |
Figure 1 Flowchart from random sample to selected study participants in the Danish National Seroprevalence Survey of SARS-CoV-2 infections Round 4 (DSS-IV) and Round 5 (DSS-V). |
Blood sampling for antibody testing was provided to participants of the study through 22, nationally distributed, designated test TCDK facilities. For the purpose of this study, these (PCR) test facilities were equipped to also perform blood sampling for antibody testing. The study participants were informed in the invitation letter on how to book a timeslot for antibody testing via a secure webpage. Participants were asked to transport themselves to the nearest test facility at their own cost. A five mL blood sample (BD Vacutainer® Serum tubes) was taken by trained personnel and transported to the reference laboratory at SSI for analysis. Total serum concentration of anti-SARS-CoV-2 immunoglobulin was measured by use of the Wantai SARS-CoV-2 Ab ELISA (Beijing Wantai Biological Pharmacy Enterprise, 53 Beijing, China) according to the manufacturer’s instructions.22 The sensitivity and specificity of the test are 0.967 (95% CI: 0.924–0.986) and 0.995 (95% CI: 0.987–0.998), respectively.22
Statistical Analyses
The seroprevalence was estimated as the proportion of included individuals with a positive antibody test. All non-vaccinated individuals with a conclusive antibody test result within 10 weeks of the invitation date (to allow for participants signing up late) were included; if the individual had been vaccinated in-between receiving the invitation and being tested for antibodies, the person was not included in the seroprevalence estimation (Figure 1). Since testing of the invited participants was performed from 1 day and up to 6 weeks after the invitation date, we used the median sample date for each of the two surveys to anchor them to a single point in time (2 March and 27 May, 2021, respectively). Calculations were adjusted for the sensitivity and specificity of the Wantai SARS-CoV-2 Ab ELISA test using the Rogan-Gladen estimator and the 95% confidence intervals using Lang and Reiczigel’s method.23
The seroprevalence estimates are presented by sex, age group and geographical residence (using Statistic Denmark classification of type of municipalities (five levels: capital, metropolitan, provincial, commuter, rural); by the five Danish administrative ‘regions’, the 11 administrative ‘provinces’ and additionally the four largest cities in Denmark).
We further estimated the overall proportion of immunity, either caused by previous infection or vaccination, in the population ≥12 years as a+b*(1-a), where a is the proportion vaccinated (retrieved from DDV) and b is the above estimated seroprevalence in the unvaccinated population. Finally, we compared this estimated total number of infected persons in the population with those testing positive by RT-PCR as recorded in MiBa up to and between the two survey dates.
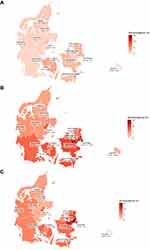
For context, in addition to the results of the DSS-IV and V survey rounds, we present results from all rounds of DSS, stratified by age group in order to visualize the course of the epidemic over time. Finally, we visualize the seroprevalence among the 18–49 year-olds on a choropleth map by the 11 Danish provinces and four largest cities by August 2020 (DSS-II),13 February 2021 (DSS-IV) and May 2021 (DSS-V).
Ethical Considerations
The DSS was performed as a national disease surveillance project under the Danish Health Act § 222, registered with the Danish Data Protection Agency and approved regarding legal, ethical and cyber-security issues by the SSI Compliance department in conjunction with the Danish governmental law firm. All participants provided informed consent for the serological testing. The holder of parental authority provided informed consent for participants under the age of 18 years. The manuscript complies with the Declaration of Helsinki.
Results
The number of tested participants in the two surveys, DSS-IV and DSS-V, were 11.381 (25% of the invited) and 5.584 (14% of the invited), respectively (Figure 1). In both survey periods, a higher proportion of females participated (Table 1). Relatively more residents from the Capital Region and in the North Denmark Region participated. In DSS-IV, higher participation rates were generally observed for people ≥40 years old, compared to younger age groups. In DSS-V, relatively more people with ages between 30 and 64 years participated than did 12 to 19 and 65+ olds (Table 1).
 |
Table 1 Participation and Seroprevalence in the Danish National Seroprevalence Survey of SARS-CoV-2 Infections Round 4 (DSS-IV) and Round 5 (DSS-V), by Sex, Age Group and Residency Category |
The proportion of participants with detectable SARS-CoV-2 antibodies was estimated at 7.2% (95% CI: 6.6–7.6%) in February 2021 increasing to an estimated 8.6% (95% CI: 7.6–9.5%) in May 2021 (Table 1). In February 2021, the highest proportions of antibodies were detected in the age groups 12–29 years and 40–49 years. In May 2021, no significant increase in antibody levels was observed in any age group, except for the 50–64 year-olds (of whom many had already been vaccinated once), where the proportion with antibodies had increased from 6.4% (5.5–7.3%) to 11% (8.7–14.0%). Small numbers of participants in the youngest and oldest age-bands in May 2020 led to wide confidence intervals and may have affected the precision of the point estimates.
Because invitations for both surveys were sent out in four portions over the course of a 4-week period, changes in vaccination coverage over the study period were taken into account in order to calculate seroprevalence by age group, excluding those already vaccinated at least once. During DSS-IV (weeks 9–12, 2021), the vaccination coverage among people 65+ years old increased from approximately 20% to 30% while among the 18–64 year-olds, the vaccination uptake was stable at a level of 5–10% (Figure 2). In contrast, during DSS-V, approximately 95% of those aged 65+ years had received the first COVID-19 vaccination. In those aged 50–64 years, vaccination coverage increased from 30% to 87%. Halfway into the DSS-V invitation period, the 40–49 year-olds were offered vaccination with vaccination coverage increasing from 13% in the first week to 42% in the last week of the DSS-V study period (Figure 2). The overall pattern in the seroprevalence was similar in all age groups (Table 1). Figure 3 shows the development in the seroprevalence estimates divided into three major age groups, including the three first survey rounds of 2020 for context.13 The 12–17 years olds were included in the seroprevalence surveys from July 2020, where the seroprevalence was estimated to 1.0% (95% CI: 0.0–2.4%) and during winter in February 2021 increased to 8.3% (6.1–11%). Among the 18–49-year-olds, the seroprevalence increased from 1.8% (0.7–2.8%) to 3.0% (2.1–3.8%) in the period May–October 2020. During the winter, the seroprevalence increased to 10.9% (9.1–12.5%). In spring (March–May 2021), the seroprevalence stabilized in both age groups. In the age group 50+ a very low seroprevalence of 0.5% (0.0–1.3%) was estimated in May 2020. The seroprevalence increased to 1.5% (0.7–2.1%) in October 2020. During winter, the seroprevalence increased to 6.6% (4.9–7.9%) and in spring 2021, the majority of the 50+ year old had received their first vaccine dose.
The seroprevalence of SARS-CoV-2 in the Danish population differed markedly by geography (Figure 4). In general, higher seroprevalence estimates were found in Copenhagen and the Copenhagen surroundings whereas lower seroprevalence estimates were found in the western parts of the country, away from the capital, in North, West and East Jutland. During winter 2020/2021, the seroprevalence increased in all provinces, however larger increases were observed in Zealand, particularly in Copenhagen with 13.3% (95% CI: 11.3–15.5%), the Copenhagen surroundings with 11.4% (8.5–14.9%) and in the province East Zealand with 9.1% (6.7–12.0%). From February to May 2021, none or little increase in the seroprevalence estimates were observed, except from in the “Copenhagen area” with an estimated prevalence of 15.4% (11.7–19.3%).
A total of 863 of the participants in both surveys were registered with a positive RT-PCR test result prior to the antibody test. Of those, 836 persons had the antibody test performed two weeks or later after the positive RT-PCR result and SARS-CoV-2 antibodies were detected in 780 persons (93–100% when adjusting for the sensitivity and specificity of the test) whereas 56 did not have detectable antibodies. The median time in days between the positive RT-PCR result and the positive antibody test result was 120.5 days [range: 17–461] for the 780 seropositive persons and 127.5 days [range: 18–312] for the 56 seronegative persons.
The protection against infection in the Danish population in May 2021 was estimated (Table 2). It increased with age, ranging from 11% in the youngest age group to 94% in the 65+. The seroprevalence obtained from past infection was relatively low in all age groups and the predominant contribution to the estimated total protection was due to COVID-19 vaccinations. The difference in total immunity across age groups primarily mirrored the rollout of the vaccines after a prioritized scheme according to age groups.
 |
Table 2 Estimated Total Protection Against SARS-CoV-2 Infection in the Danish Population by Age Group in May 2021 |
We estimate that 8.6% (7.5–9.6%) of the population above the age of 12 had been infected by late May 2021. This would correspond to 437,000 (381,000–488,000) persons. In comparison, by 27 May 2021, 251,369 persons above the age of 12 years had been diagnosed with a SARS-CoV-2 infection by RT-PCR, indicating that 1.7 persons were infected with SARS-CoV-2 for every person who had been diagnosed with RT-PCR from the beginning of the pandemic in February 2020 until May 2021. Between the surveys done in February (DSS-IV) and May 2021 (DSS-V), the seroprevalence point estimates in the as yet unvaccinated population rose from 7.2% to 8.6%. This would correspond to 71,111 persons having been infected over the period. In the same time, 57,508 persons in the age group tested positive by RT-PCR. This indicated that in the spring of 2021, four in five infections were diagnosed.
Discussion
In this national population-based study, we present measurements from the late winter and spring/early summer of 2021, on the prevalence of antibodies against SARS-CoV-2 among the non-vaccinated Danish population aged 12 years or older as a marker of past SARS-CoV-2 infection. In February 2021 (DSS-IV), approximately one year after the start of the epidemic in Denmark, we found detectable antibodies in 7.2% (95% CI: 6.3–7.9%) of the participants. This was almost twice the measured seroprevalence of 4.1% (95% CI: 3.1–4.9) three months earlier in the beginning of December 202013 but mirrors the epidemic curve as obtained by national RT-PCR testing of individuals, showing a wave of infections in December 2020. In response to this wave of infections, a national lockdown, including restrictions on normal social activity, was set from mid December 2020 until April 2021, when restrictions were gradually lifted. Accordingly, the seroprevalence only increased with 1.4 percentage points from February to May 2021, where an overall seroprevalence of 8.6% (95% CI: 7.5–9.6%) was estimated.
The seroprevalence had increased in all provinces in Denmark after the 2020–21 winter wave of SARS-CoV-2 infections. However, there were considerable geographical differences in seroprevalence over time. At all times, the highest prevalence was observed in the capital Copenhagen and the surrounding provinces, whereas the lowest prevalence was observed in those parts of the country placed furthest away from the capital. Approximately 1/3 of the Danish population lives in Copenhagen and its surroundings. Because the population density is high and many large workplaces, education facilities, etc. are located in and around the capital, the possibility of virus transmission is particularly high here. In addition, the population of Copenhagen is younger compared to the rest of the country. Overall, higher seroprevalence estimates were observed in the age groups younger than 30 years and the lower seroprevalence was found for the oldest. Thus, a higher prevalence of SARS-CoV-2 infections in Copenhagen and surroundings was expected.
No statistically significant difference in the seroprevalence was found between men and women; however, the point estimate was higher for males. More women are working as healthcare-providers; they were exposed to infections with COVID-19 to a higher degree due to their close contact to hospitalized patients or vulnerable and elderly. Being infected early during the pandemic, healthcare-professionals might potentially have participated in the seroprevalence study to a lesser degree, knowing that they already had been infected and thus would be positive. Further, this group was among the first to be offered COVID-19 vaccination and therefore would have less often been included in the study population in the second round.
A limitation of this study was the relatively low participation rate in both survey rounds, somewhat lower than in the 2020 survey rounds (which varied from 26% to 48%).13 Though participation did not appear to be particularly skewed measured by parameters such as sex, age and geography of residence, we cannot exclude that certain population segments would have been under-represented in the study. Reassuringly however, our results align well with another large Danish seroepidemiological study done in parallel to ours. In that study, targeting blood donors, an overall seroprevalence of 7.2 (95% CI: 6.3–7.8) was observed in February 2021.24 Also, similar geographical distributions were observed in both studies, with raised seroprevalence levels in Copenhagen (11.6%; 95% CI: 10.4–12.6) in the blood donor study.24
Blood donors are generally healthier than the background population and the almost identical seroprevalence estimates in the two studies could indicate that the same may be the case in our study, for instance disabled or those with chronic disease morbidity might have been underrepresented. Also, the COVID-19 vaccination program was enrolled for the oldest and most vulnerable citizens during DSS-IV and DSS-V and as vaccinated persons were excluded from the evaluation of the seroprevalence, the study participants might on average have been healthier than the background population. Participation might also have been affected by other not evaluated factors such as distance to the nearest test facility, socioeconomic factors and ethnic background. In a Danish study specifically addressing people living in social housing areas, a three times higher seroprevalence, compared to the general population, was found.25 If subgroups living in social housing areas are underrepresented in our study, the seroprevalence rates might be underestimated, especially in and around the larger cities such as Copenhagen and surrounding areas.
In general, one should be cautious to compare the results from serological studies performed in different countries because estimates will be sensitive to the exact timing of such studies relative to infection waves, the study protocol and the national preventive measures launched in each country to reduce transmission at the given time. Many seroprevalence studies have investigated specific sub-groups, such as health-care workers and frontline personnel.2 However, compared to population-based surveys done in other European countries at a comparable time period, the seroprevalence seems to have been relatively low in Denmark.1,10,26–31 This supports the notion that Denmark was among the countries, which took a relatively benign course through the first one and an half year of the epidemic. Possible explanations for this include 1) the effect of the initial and hard lock-down in early March 2020, when only low numbers of infected cases had been reported, 2) the overall high compliance towards societal restrictions and to advice given by health authorities in the population,32 3) the integrated surveillance of SARS-CoV-2 infections using the very advanced societal digitalization resources 4) the tight cooperation between different health authorities, including comprehensive contact tracing, where close contacts were contacted by the Danish Patient Safety Authority with recommendation of self-isolation, and not least 5) the availability of massive testing capacity. To put the latter in perspective, in this study we could estimate that in the spring of 2021, four out of five infections with SARS-CoV-2 were diagnosed by PCR and captured in national surveillance. Though this estimate was based on a simple comparison of numbers, and might therefore not be precise, we nevertheless believe the conclusion, that the majority of community infections in Denmark were in fact captured by PCR-based surveillance, to be true.
In Denmark, there are extensive national registers. A strength of the study was the use of the national civil register to obtain a random sample of all residents older than 11 years. Also, during DSS-IV and DSS-V, the COVID-19 vaccination program was enrolled in Denmark, and we used the Danish Vaccination Register for information on COVID-19 vaccination status. The register is considered to have a high validity, with all given vaccinations registered by administration date.21 Thus, we were able to investigate the seroprevalence in the non-vaccinated population, without relying on the participators recall of time of vaccination and overcoming the challenge of vaccinations being rolled out while the surveys were running. Another strength was the use of the already established set-up for RT-PCR test facilities in TCDK, for taking the blood samples in our survey. Thereby, most citizens already had knowledge about the test facilities and booking system, and most would have had easy access to a test facility. Also, we took the precision of the Wantai SARS-CoV-2 Ab ELISA test into account when calculating the seroprevalence estimates. The test detected 93% of the known PCR confirmed prior infections, regardless of time since infection within the period.
In conclusion, our study provides estimates of the cumulative level of SARS-CoV-2 infections in the non-vaccinated population in Denmark during winter and spring/early summer of 2021. The winter wave in 2020–21 led to infection of almost the same number of persons as in the entire previous epidemic period (10 months), supporting that in the spring of 2021 the majority of the population was still susceptible to infection. The contribution of previous infections, and even more so of vaccinations, led to a high level of protection in the population in the summer of 2021 and a very low number of infections registered through national surveillance. We further found that the SARS-CoV-2 epidemic predominantly affected metropolitan areas, in particular Copenhagen and its surroundings, and also affected young and middle-aged people more than seniors. In the first part of 2021, only an estimated 1 in 5 SARS-CoV-2 infections were not captured by national surveillance, and the cumulative number of infections as estimated by serology were low compared to that of other European countries.
Data Sharing Statement
This work is carried out under the Surveillance auspices of the SSI as regulated in paragraph 222 of the Danish Communicable Disease Act, using personal identifiable information collected with individual consent for the purpose of the present study only. Data cannot be made publicly available for ethical and legal reasons as this would compromise violation of the rights of the participants as defined upon entry into the study.
Acknowledgments
We would like to warmly thank everyone who participated in this study by giving blood. We are very grateful to the staff in the test stations for taking blood samples. We thank all staff in TestCenter Denmark, the involved SSI departments and the expert groups that advised on the design of the study. We thank Oliver McManus for making the maps using R software.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
In April 2020, the Danish Parliament called for the SSI to conduct a “representative population survey”.33 The study was set-up and carried out by the SSI independent of the Parliament/Government which also had no role in the study design which was chosen following advice made by a group of independent, specifically appointed Danish scientific experts in April 2020.34 This work was supported by an ad hoc grant from the Danish Government (§16.11.73 on the National budget 2021). The funders also had no role in data collection, data analysis, interpretation or writing of the report.
Disclosure
The authors have declared that no competing interests exist in this work.
References
1. Bergeri I, Whelan M, Ware H, et al. Global epidemiology of SARS-CoV-2 infection: a systematic review and meta-analysis of standardized population-based seroprevalence studies. PLOS Medicine. 2022;(11):e1004107. doi:10.1371/JOURNAL.PMED.1004107
2. Arora RK, Joseph A, Van Wyk J, et al. SeroTracker: a global SARS-CoV-2 seroprevalence dashboard. Lancet Infect Dis. 2021;21:e75–e76. doi:10.1016/S1473-3099(20)30631-9
3. Rostami A, Sepidarkish M, Leeflang MMG, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27(3):331–340.
4. Rostami A, Sepidarkish M, Fazlzadeh A, et al. Update on SARS-CoV-2 seroprevalence: regional and worldwide. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27(12):1762–1771.
5. Bobrovitz N, Arora RK, Cao C, et al. Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. PLoS One. 2021;16(6):e0252617. doi:10.1371/journal.pone.0252617
6. Lewis H, Ware H, Whelan M, et al. SARS-CoV-2 infection in Africa: a systematic review and meta-analysis of standardised seroprevalence studies, from January 2020 to December 2021. BMJ Global Health. 2022;(7):e008793. doi:10.1136/bmjgh-2022-008793
7. Murhekar MV, Bhatnagar T, Thangaraj JWV, et al. Seroprevalence of IgG antibodies against SARS-CoV-2 among the general population and healthcare workers in India, June–July 2021: a population-based cross-sectional study. PLoS Med. 2021;18(12):e1003877. doi:10.1371/journal.pmed.1003877
8. Jones JM, Stone M, Sulaeman H, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations. J Am Med Assoc. 2021;326(14):1400–1409. doi:10.1001/jama.2021.15161
9. Leclercq V, Van den Houte N, Gisle L, et al. Prevalence of anti-SARS-CoV-2 antibodies and potential determinants among the Belgian adult population: baseline results of a prospective cohort study. Viruses. 2022;14(5):920. doi:10.3390/v14050920
10. Gornyk D, Harries M, Glöckner S, et al. SARS-CoV-2 Seroprevalence in Germany. Dtsch Arztebl Int. 2021;118:48.
11. Madhi SA, Kwatra G, Myers JE, et al. Population immunity and covid-19 severity with omicron variant in South Africa. N Engl J Med. 2022;386(14):1314–1326. doi:10.1056/NEJMoa2119658
12. COVID-19 seroprevalence study [Prævalensundersøgelse af covid-19]; 2022. Danish. Available from: https://covid19.ssi.dk/overvagningsdata/undersoegelser/praevalensundersogelsen.
13. Espenhain L, Tribler S, Sværke Jørgensen C, Holm Hansen C, Wolff Sönksen U, Ethelberg S. Prevalence of SARS-CoV-2 antibodies in Denmark: nationwide, population-based seroepidemiological study. Eur J Epidemiol. 2021;36(7):715–725. doi:10.1007/s10654-021-00796-8
14. Munch PK, Espenhain L, Hansen CH, Krause TG, Ethelberg S. Case-control study of activities associated with SARS-CoV-2 infection in an adult unvaccinated population and overview of societal COVID-19 epidemic counter measures in Denmark. PLoS One. 2022;17(11):e0268849. doi:10.1371/journal.pone.0268849
15. Gram MA, Nielsen J, Schelde AB, et al. Vaccine effectiveness against SARS-CoV-2 infection, hospitalization, and death when combining a first dose ChAdOx1 vaccine with a subsequent mRNA vaccine in Denmark: a nationwide population-based cohort study. PLoS Med. 2021;18(12):e1003874. doi:10.1371/journal.pmed.1003874
16. Michlmayr D, Hansen CH, Gubbels SM, et al. Observed protection against SARS-CoV-2 reinfection following a primary infection: a Danish cohort study among unvaccinated using two years of nationwide PCR-test data. Lancet Reg Heal Eur. 2022;20:100452. doi:10.1016/j.lanepe.2022.100452
17. COVID-19 [Internet]; 2022. Available from: https://en.ssi.dk/covid-19.
18. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi:10.1007/s10654-014-9930-3
19. Schønning K, Dessau RB, Jensen TG, et al. Electronic reporting of diagnostic laboratory test results from all healthcare sectors is a cornerstone of national preparedness and control of COVID-19 in Denmark. APMIS. 2021;129:438–451. doi:10.1111/apm.13140
20. Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397(10280):1204–1212. doi:10.1016/S0140-6736(21)00575-4
21. Grove Krause T, Jakobsen S, Haarh M, Molbak K. The Danish vaccination register. Euro Surveill Bull Eur Sur Les Mal Transm. 2012;17:17.
22. Harritshøj LH, Gybel-Brask M, Afzal S, et al. Comparison of 16 serological SARS-CoV-2 immunoassays in 16 clinical laboratories. J Clin Microbiol. 2021;59(5). doi:10.1128/JCM.02596-20
23. Lang Z, Reiczigel J. Confidence limits for prevalence of disease adjusted for estimated sensitivity and specificity. Prev Vet Med. 2014;113(1):13–22. doi:10.1016/j.prevetmed.2013.09.015
24. Kaspersen KA, Hindhede L, Boldsen JK, et al. Estimation of SARS-CoV-2 infection fatality rate by age and comorbidity status using antibody screening of blood donors during the COVID-19 epidemic in Denmark. J Infect Dis. 2022;225(2):219–228. doi:10.1093/infdis/jiab566
25. Fogh K, Eriksen ARR, Hasselbalch RB, et al. Seroprevalence of SARS-CoV-2 antibodies in social housing areas in Denmark. BMC Infect Dis. 2022;22(1). doi:10.1186/s12879-022-07102-1
26. Ward H, Cooke G, Whitaker M, et al. REACT-2 Round 5: increasing prevalence of SARS-CoV-2 antibodies demonstrate impact of the second wave and of vaccine roll-out in England. medRxiv. 2021;2021:21252512.
27. Anda EE, Braaten T, Borch KB, et al. Seroprevalence of antibodies against SARS-CoV-2 virus in the adult Norwegian population, winter 2020/2021: pre-vaccination period. medRxiv. 2021. doi:10.1101/2021.03.23.21253730
28. Contini C, Pearson R, Wang L, et al. Bottom-up evolution from disks to high-genus polymersomes. ChemRxiv. 2018. doi:10.26434/chemrxiv.6108467.v1
29. Björkander S, Du L, Zuo F, et al. SARS-CoV-2–specific B- and T-cell immunity in a population-based study of young Swedish adults. J Allergy Clin Immunol. 2022;149(1):65–75.e8. doi:10.1016/j.jaci.2021.10.014
30. Piler P, Thon V, Andrýsková L, et al. Nationwide increases in anti-SARS-CoV-2 IgG antibodies between October 2020 and march 2021 in the unvaccinated Czech population. Commun Med. 2022;2:1. doi:10.1038/s43856-022-00080-0
31. Bühler K-M, Echeverry-Alzate V, Calleja-Conde J, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies: relationship with COVID-19 diagnosis, symptoms, smoking, and method of transmission. IJID Reg. 2022;4:10–16. doi:10.1016/j.ijregi.2022.05.007
32. Jørgensen F, Bor A, Petersen MB. Compliance without fear: individual-level protective behaviour during the first wave of the COVID-19 pandemic. Br J Health Psychol. 2021;26(2):679–696. doi:10.1111/bjhp.12519
33. Agreement on extension of the first phase of a controlled societal reopening - The Prime Minister. [Aftale vedrørende udvidelse af den første fase af en kontrolleret genåbning – statsministeriet]; 2022. Danish. Available from: https://www.stm.dk/statsministeriet/publikationer/aftale-vedroerende-udvidelse-af-den-foerste-fase-af-en-kontrolleret-genaabning/.
34. Statens Serum Institut: expert committee report; Design of national representative population survey, 7 May 2020. [Statens Serum Institut: rapport fra ekspertudvalget: stikprøvedesign til en løbende repræsentativ undersøgelse af befolkningen - 7. maj 2020]; 2022. Copenhagen. Danish. Danish: https://files.ssi.dk/Ekspertudvalgsrapportvedrstikprvedesign07052020revideret13052020bg20.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.