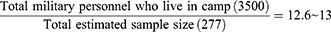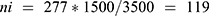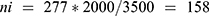Back to Journals » Infection and Drug Resistance » Volume 17
Seroprevalence of Hepatitis B and C Viruses and Their Associated Factors Among Military Personnel at Military Camps in Central Gondar, Ethiopia: A Cross-Sectional Study
Authors Abebe AD , Assefa M , Belete D , Ferede G
Received 18 December 2023
Accepted for publication 5 April 2024
Published 11 April 2024 Volume 2024:17 Pages 1407—1417
DOI https://doi.org/10.2147/IDR.S455562
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi Ruan
Ayanaw Dinku Abebe, Muluneh Assefa, Debaka Belete, Getachew Ferede
Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Muluneh Assefa, Tel +251944900600, Email [email protected]
Background: Globally, viral hepatitis is a leading cause of death and is highly prevalent in Ethiopia. Military personnel are more vulnerable to hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, and there are no data on such populations in the study area. Therefore, this study aimed to determine the seroprevalence of HBV and HCV infections and their associated factors among military personnel in military camps in Central Gondar, Ethiopia.
Materials and Methods: This institutional-based cross-sectional study was conducted with 277 military personnel from April to August 2022 at military camps in Central Gondar, Ethiopia. A systematic random sampling technique was used to select the study participants. Sociodemographic and other relevant data were collected using a structured questionnaire. Five milliliters of venous blood were collected using a vacutainer tube and tested for hepatitis B surface antigens and anti-hepatitis C virus antibodies using an enzyme-linked immunosorbent assay. Data were analyzed using STATA version 14 software and logistic regression models were used to determine the association between HBV/HCV infection and risk factors.
Results: Out of 277 participants, the overall seroprevalence of HBV and HCV infections was 19 (6.9%) and 9 (3.3%), respectively. The rate of HBV and HCV co-infection was 2 (0.7%). Having multiple sexual partners (p = 0.048), frequent alcohol use (p = 0.034), hospitalization (p = 0.014), and history of receiving injections from traditional practitioners (p = 0.040) were significant predictors of HBV infection. In contrast, a history of blood transfusion (p = 0.048) and sexually transmitted infections (p = 0.039) were significant risk factors for HCV infection.
Conclusion and Recommendations: An intermediate prevalence of HBV and HCV infections was observed among the military personnel. Continuous screening, adherence to healthcare service guidelines, and strengthening of vaccination are crucial for preventing HBV and HCV infections.
Keywords: hepatitis B virus, hepatitis C virus, military personnel, Ethiopia
Background
Hepatitis is an inflammation of the liver caused by an infectious agent and is a leading public health problem. Common viral agents of hepatitis are hepatitis B virus (HBV) and hepatitis C virus (HCV), which can cause acute and chronic forms of liver disease.1 Globally, HBV and HCV infections are major causes of chronic liver disease, eventually leading to cirrhosis, hepatocellular carcinoma (HCC), and even death.2,3 Hepatitis B and C viruses share significant similarities, including high global prevalence, modes of transmission, hepatotropism, and capacity to induce chronic infection.4,5
The World Health Organization (WHO) estimates that 354 million people live with HBV and HCV.6 Worldwide, approximately 296 million people are currently living with HBV, and the burden disproportionately affects sub-Saharan Africa and East Asia.7 Indeed, preliminary statistics indicate that if the current state of underdiagnosis and undertreatment persists, it is predicted that global HBV-related mortality will rise by 39%, from 850,300 deaths in 2015 to 1,109,500 deaths in 2030.8 More than 60% of chronic liver disease and 80% of HCC are caused by chronic HBV and HCV infections.9 The economic impact of HBV and HCV infections is vast because HCC has a high fatality rate in Africa, including Ethiopia, and usually affects economically productive age groups.9
Moreover, the WHO report also described Ethiopia as a country with a lack of diagnostic facilities and appropriate national surveillance strategies, poor prevention and control, and a lack of a coordinated and systematic national response to chronic viral hepatitis, even though the country is classified under geographical regions with intermediate to hyper-endemic viral hepatitis infections.10,11 A meta-analysis study in Ethiopia reported 7.4% and 3.1% pooled prevalence rates of HBV and HCV infections, respectively.12
Although the potential for transmission varies, intravenous drug use, tooth extraction, tattooing, sharing of utensils with infected persons, age, and unsafe sexual practices were found to be common risk factors for HBV and HCV infections.13–15 Military personnel are at a higher risk because they share items such as hairbrushes, combs, razors, and toothbrushes that increase exposure and facilitate virus transmission.14 Furthermore, the military is frequently mobile from place to place for different professional reasons and stays longer without their family, which may cause them to have multiple sex partners and increase their exposure to different sexually transmitted infections (STIs).16
Several studies have reported the prevalence of HBV and HCV infections among different segments of the population in the study area17–24 and there is a single report of armed forces in Bahir Dar, Amhara Regional State of Ethiopia.16 No data were described the prevalence of viral hepatitis among military personnel in the study area. Therefore, this study aimed to determine the seroprevalence and associated risk factors of HBV and HCV infections among military personnel at the Central Gondar military camps, Ethiopia.
Materials and Methods
Study Design, Period, and Setting
This institutional-based cross-sectional study was conducted from April to August 2022 at the Azezo and Seraba military camps. Military camps are located in the Central Gondar Administrative Zone, Amhara National Regional State, approximately 750 km northwest of Addis Ababa, the capital city of Ethiopia.
Study Population
Military personnel found in military camps during the data collection period and those who consented to participate and provided blood samples were included. However, military personnel who were mentally or physically ill and unable to participate in the interviews were excluded from this study.
Sample Size and Sampling Technique
The sample size was determined by considering the 95% confidence interval (CI), 80% power from a previous study,16 and 5% margin of error. A total of 277 participants were selected using systematic random sampling. Based on the information obtained from Central Gondar Zone military camps an estimated 3500 militaries were found in the two camps (Azezo and Seraba) each consisting of 1500 and 2000 militaries, respectively. The sampling interval value was determined as follows:
Based on the order in which the military personnel were present at camp, the study participants were selected every 13 person patterns after determining the starting unit, which was three. To avoid recycling, special marks were used on the thumb and supported by verbal confirmation of whether to participate or not in the previous time during sample collection. The number of samples to be collected from each of the two camps was determined by using the proportional allocation formula: 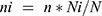 , where N = Total population, Ni = the total population of the subgroup, n = the total sample size, and ni = the total sample size in the subgroup.
, where N = Total population, Ni = the total population of the subgroup, n = the total sample size, and ni = the total sample size in the subgroup.
Thus, 119 and 158 samples were collected from Azezo and Seraba, respectively, giving a total of 277 participants in our study.
Study Variables
The dependent variable was seroprevalence of HBV and HCV infections. The independent variables were sex, age, marital status, residence, educational status, work experience, family history of liver disease, previous hospitalization, history of HBV or HCV infection, war-related injury, dental extraction, surgical history, history of blood transfusion, history of body fluid contact, STI, cigarette smoking, alcohol use, tattooing, number of sexual partners, nose piercing, ear piercing, sharing sharp materials, history of drug use, receiving an injection from a traditional practitioner, and use of a condom.
Data Collection
Data on sociodemographic characteristics and risk factors for HBV and HCV infections were collected by trained health officers and senior nurses using a structured questionnaire after obtaining written informed consent. A pre-tested structured questionnaire was used for data collection.
Blood Sample Collection and Transportation
Five milliliters of venous blood was collected from each study participant by a trained laboratory technologist using vacutainer tubes. Whole blood samples were centrifuged at 3000 rpm for 10 minutes to separate the serum. Serum samples were transported to the Gondar Blood Bank Services laboratory using a cold box and stored at −20°C until use in an enzyme-linked immunosorbent assay (ELISA).
Laboratory Method
Hepatitis B surface antigen (HBsAg) and anti-HCV antibodies from the serum of each military personnel were determined using commercially available ELISA kits according to the manufacturer’s instructions (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd., Beijing, China).25 We used HBsAg ELISA kit, with a sensitivity of 100% and a specificity of 99.92%, and anti-HCV ELISA kit, with a sensitivity of 100% and a specificity of 99.55%.
Data Management and Quality Control
Data were collected using a pretested questionnaire before the study period among 5% of military personnel at the Weleka submilitary camp in Gondar Town. One day of training was provided for data collectors on the aim of the study and the content of the questionnaire and regular supervision. Standard operating procedures were strictly followed during the blood sample collection, storage, and analytical processes. The storage conditions and expiration dates of the reagents were checked. The quality of the ELISA test was ensured by known positive and negative controls
Data Analysis and Interpretation
Data were checked for completeness and consistency, and coded manually. The data were entered into Epi Data version 3.1 and exported to the STATA software package (version 14) for analysis. Descriptive statistics such as percentages, means, and standard deviations were calculated. The data output is summarized and presented in tables. Logistic regression models were used to determine the association between outcome variables (HBV and HCV) and explanatory variables (risk factors). All variables in the bivariate logistic regression model whose P-value < 0.2 were passed into the multivariable analysis. Finally, the degree of association was assessed using an adjusted odds ratio (AOR), precision with a 95% confidence interval, and a p-value ≤ 0.05 was considered statistically significant.
Ethical Consideration
Ethical approval was obtained from the School of Biomedical and Laboratory Sciences, College of Medicine and Health Science, University of Gondar, Ethical Review Committee (Ref. No. SBMLS/243; March 18, 2022). Official permission was obtained from the Central Gondar Zone military camp higher management. Written informed consent was obtained from each study participant, and confidentiality was maintained at each level of the study using code numbers for identification. The study participants who tested positive for HBsAg and anti-HCV antibodies were linked to a physician for clinical management. All laboratory procedures in this study were conducted following the amended Declaration of Helsinki.26
Results
Sociodemographic Characteristics of the Study Participants
A total of 277 military personnel participated in this study. Out of these participants, 203 (73.3%) were males. The mean age of the study participants was 27.39 ± 8 years. More than half (67.2%) of participants were below the age of 29 years, followed by 57 (20.6%) were 30 to 39 years and 34 (12.3%) were over 40 years old. About 119 (43.0%) of the study participants were single. Most of the participants, 156 (56.3%) were recruited from urban areas. Half of the study participants (139) attained 1 to 8 grade, 106 (38.3%) were secondary school students, and only 22 (7.9%) had a college diploma and above. A large proportion of military personnel 246 (88.8%) had less than 10 years of work experience (Table 1).
 |
Table 1 Socio-Demographic and Related Variables of Military Personnel with HBV and HCV Infections at Military Camps in Central Gondar, Ethiopia (n = 277) |
Seroprevalence of HBV and HCV Infections
Out of 277 study participants, the overall seroprevalence of HBV and HCV was 19 (6.9%) (95% CI: 4.1–10.5%) and 9 (3.3%) (95% CI: 1.5–6.1%), respectively. The co-infection rates of HBV and HCV were 2 (0.7%) (95% CI: 0.09–2.6%). The prevalence of HBV was almost similar in male and female military with 6.9% and 6.8%, respectively. However, HCV was higher in males (3.9%). The proportion of both HBV and HCV infection was higher in older study participants, with 11.8% and 5.9%, respectively. Hepatitis C virus infection was high in military personnel unable to read and write (10.0%), those having a history of blood transfusion (9.0%), and past smokers (8.0%). The highest prevalence of both viruses was found in participants from urban residences (7.7% of HBV) and (4.5% of HCV).
The proportion of HBV infection was higher in military personnel who had work experience of greater than or equal to 10 years (19.4%), and a history of hospitalization (19.2%). The presence of HBV and HCV history responded during interview increased their prevalence, which was 12.0% and 17.4%, respectively. Both HBV and HCV were higher in participants having a history of STIs (11.5% each). Additionally, the highest seroprevalence of HBV was also observed in military personnel receiving injections from a traditional practitioner (17.4%), having multiple sexual partners (12.0%), having a history of frequent alcohol use (11.7%), and not using condoms (8.7%) (Table 1).
Factors Associated with HBV and HCV Infections Among Military Personnel
Multiple logistic regression models showed that HBV infection was significantly associated with hospitalization (AOR: 3.78, 95% CI: 1.31–10.9, p = 0.014), frequent alcohol consumption history (AOR: 5.35, 95% CI: 1.13–2.35, p = 0.034), multiple numbers of sexual partners (AOR: 5.55, 95% CI: 1.01–28.3, p = 0.048), and receiving an injection from a traditional practitioner (AOR: 4.34, 95% CI: 1.1–17.76, p = 0.040) (Table 2). Similarly, history of blood transfusion (AOR: 5.9, 95% CI: 1.02–33.8, p = 0.048) and STI (AOR: 6.6, 95% CI: 1.1–39.9, p = 0.039) were statistically significant with HCV infection (Table 3).
 |
Table 2 Bivariate and Multivariable Analysis of Factors Associated with HBV Infection Among Military Personnel at Military Camps in Central Gondar, Ethiopia (n = 277) |
 |
Table 3 Bivariate and Multivariable Analysis of Factors Associated with HCV Infection Among Military Personnel at Military Camps in Central Gondar, Ethiopia (n = 277) |
Discussion
Ninety-six percent of all viral hepatitis-related deaths are caused by hepatocyte-specific hepatitis B and C virus infections.16 According to the WHO classification of countries, HBV prevalence >8% can be graded as high, 2–8% is intermediate, and <2% is considered as low.27 Ethiopia is grouped under geographic regions with an intermediate prevalence of viral hepatitis infections.28 In this study, the seroprevalence of HBV among military personnel was intermediate (6.9%). This finding is comparable to previous studies conducted in Bahir Dar, Ethiopia (4.2%)16 and Iran (4.8%).29 However, a higher prevalence of HBV was reported in Senegalese military personnel on mission to Darfur (Sudan) (14.2%),30 Senegal (10.8%),31 and Northern Ethiopia (13.0%).32 Our finding is higher than reports in Lithuania (1.97%),33 Pakistan (3.24%;34 2.8%35), China (0.44%),36 Greece (0.32%),37 Caribbean country (4.0%),38 Brazil (0.22%),39 UK (0.37%),40 and India (1.25%).41 The discrepancy between our findings and the studies conducted elsewhere might be due to variations in sample size, sampling technique, socioeconomic status, availability of medical services, study period, vaccination status, type of risk exposure to HBV infection, and diagnostic techniques.
The overall seroprevalence of HCV infection in this study was 3.3%. This finding is consistent with studies conducted in Pakistan (3.4%)35 and India (1.5%).42 The lower prevalence of HCV was reported in Bahir Dar, Ethiopia (0.2%),16 Brazil (0.7%;43 0.28%39), Iran (0.7%),29 Afghanistan (0.82%),44 UK (0.06%),40 and Turkey (0.46%). In contrast, our finding is lower than studies conducted in Pakistan (3.69%),35 Rwanda (16.0%),45 and Caribbean countries (36.7%).38 The difference might be due to the potential variability of the diagnostic test kit employed, sample size, geographic location, awareness of transmission methods of HCV, and exposure to risk factors.
In this study, the co-infection of HBV and HCV was 0.7%. This implies that HBV and HCV could share a similar route of transmission and risk factors. This is supported by some studies, in which HBV and HCV co-infection is common due to the sharing of transmission routes, especially in endemic areas and among subjects at risk of parenteral transmissible infections such as blood transfusion and injection drug use.46,47 In contrast, no co-infection of these viruses was detected in studies conducted in Ethiopia and elsewhere.16,38,41 The absence and/or low prevalence of HBV and HCV co-infection is related to the replication inhibition mechanism of both viruses. A mutual inhibition of viral replication occurs in HBV and HCV co-infection, with HCV exerting a negative effect on HBV replication and HBsAg carriers with concurrent HCV infection having low-level HBV viremia, low titers of HBsAg in serum, and low-levels of intracellular HBsAg.48 Although the detailed mechanism remains unclear, viral interference involves complex integration between virus replication properties and the host immune system. The HBV interference with HCV replication results in a lower HCV RNA titer in patients with concurrent HBV infection.49
Regarding factors for HBV infection, having a history of multiple sexual partners was significantly associated with acquiring HBV infection, which is supported by certain studies.39,44,45 This is due to experiencing multiple contacts with HBV carriers through unprotected sex. The HBV infection was higher in study participants who consumed alcohol. This finding is consistent with a study conducted in Afghanistan.44 Behavioral factors like alcohol consumption can increase sexual desire and put people at increased risk of HBV transmission.50 A study reported that more frequent episodes of alcohol intoxication were associated in a dose-dependent manner with an increased risk of having more sexual partners.51 Besides, alcohol increases HBV replication and oxidative stress, suppresses the immune response, promotes liver damage, and increases the likelihood of developing cirrhosis. There is also an increased risk of HCC in HBV-infected patients who consume a lot of alcohol.52,53
Hepatitis B virus is highly contagious, a hundred times more infectious than human immunodeficiency virus (HIV), and stays outside the bloodstream longer than HIV.54 In this study, a history of receiving injections from a traditional practitioner was associated with the acquisition of HBV infection. This finding is consistent with a study conducted in India.55 This is due to unsafe injection practices, such as the use of one syringe for more than one individual, injections prepared in an unclean area, and re-use of single-use disposable needles and syringes.56 Low awareness of HBV and the sharing of needles in the study participants may also increase its transmission. Moreover, military personnel with a history of hospitalization were significantly associated with HBV infection similar to a study in Iran.14 This could be due to poor infection control practices in health facilities, improper sterilization of medical equipment, absence of protective equipment, and low awareness of the hospital population. Since HBV is stable on environmental surfaces for at least one week, indirect inoculation of HBV can also occur via inanimate objects.57
In our study, risk factors such as previous history of blood transfusion and STI were significantly associated with HCV infection. In our setting, HCV testing from blood and blood products using ELISA may not be able to accurately detect the virus in the window period and occult hepatitis, which requires advanced laboratory techniques. In other studies, a history of STI was a possible risk factor for HCV infection.16,39 Sexually transmitted infections present a continuing challenge to the efforts to prevent diseases in the military.31 The risk of acquiring HCV infection increased among heterosexual persons with pre-existing HIV, particularly among those engaging in high-risk sexual behaviors and having unprotected sex with multiple sexual partners.58 Additionally, HCV RNA can be present in the menstruation of a female, increasing the possibility of blood-to-blood contact and the transmission of the virus.59
The study has the following limitations: there is a lack of information about the type of infection, such as acute, chronic, or recovery/immunity. We only performed HBsAg and anti-HCV antibody tests for detecting current HBV and HCV infections. Because of resource limitations, molecular analysis and serotyping of HBV and HCV were not performed. Despite its drawbacks, this study added new information regarding HBV and HCV infections among military personnel for the first time in the study area.
Conclusion and Recommendations
This study demonstrated an intermediate prevalence of both HBV and HCV infections among military personnel. The previous history of hospitalization, frequent alcohol consumption, multiple sexual partners, and receiving injections from traditional practitioners were significantly associated with HBV infection. However, a history of blood transfusion and STI were significant risk factors for HCV infection. Continuous screening of military personnel, improving adherence to healthcare service guidelines, and strengthening vaccination of those high-risk individuals are key strategies to prevent HBV and HCV infections and decrease the hepatitis virus spread in the community.
Abbreviations
AOR, Adjusted Odds Ratio; COR, Crude Odds Ratio; ELISA, Enzyme-Linked Immune Sorbent Assay; HBsAg, Hepatitis B surface Antigen; HBV, Hepatitis B Virus; HCC, Hepatocellular Carcinoma; HCV, Hepatitis C Virus; HIV, Human Immunodeficiency Virus; HRP, Horse Radish Peroxidase; STI, Sexually Transmitted Infections; WHO, World Health Organization.
Data Sharing Statement
All relevant information is included in the manuscript and will be provided upon reasonable request to the corresponding author.
Acknowledgments
The authors would like to thank the management of the Seraba and Azezo military camps for all their assistance and support throughout the data collection process. Again, we would like to express our gratitude to the study participants and Gondar District Blood Bank Laboratory Staff for their support.
Author Contributions
All authors made a significant contribution to this manuscript; took part in the conception, drafting, revising, or critically reviewing the article; have agreed on the journal to which the article has been submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There was no funding for this study.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Ayele AG, Gebre-Selassie S. Prevalence and risk factors of hepatitis B and hepatitis C virus infections among patients with chronic liver diseases in public hospitals in Addis Ababa, Ethiopia. Int Scholarly Res Notices. 2013;2013:1–7.
2. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–1342. doi:10.1002/hep.26141
3. Ott J, Stevens G, Groeger J, Wiersma S. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. doi:10.1016/j.vaccine.2011.12.116
4. Malhotra R, Soin D, Grover P, Galhotra S, Khutan H, Kaur N. Hepatitis B virus and hepatitis C virus co-infection in hemodialysis patients: a retrospective study from a tertiary care hospital of North India. J Nat Sci Biol Med. 2016;7(1):72. doi:10.4103/0976-9668.175076
5. Caccamo G, Saffioti F, Raimondo G. Hepatitis B virus and hepatitis C virus dual infection. World J Gastroenterol. 2014;20(40):14559. doi:10.3748/wjg.v20.i40.14559
6. World Health Organization. [cited 2024 February 19]. Available from: https://www.who.int/health-topics/hepatitis#tab=tab_1.
7. Hsu Y-C, Huang DQ, Nguyen MHJNRG. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. 2023;20(8):1–14.
8. Razavi-Shearer D, Blach S, Gamkrelidze I, et al. The disease burden of hepatitis B and hepatitis C from 2015 to 2030: the long and winding road. J Hepatol. 2022;77:S43.
9. Bane A, Patil A, Khatib M. Healthcare cost and access to care for viral hepatitis in Ethiopia. Int J Innov Appl Stud. 2014;9(4):1718.
10. World Health Organization. Global policy report on the prevention and control of viral hepatitis in WHO member states; 2013.
11. Shiferaw F, Letebo M, Bane A, Idohou-Dossou N, Sow PS, Wade S. Chronic viral hepatitis: policy, regulation, and strategies for its control and elimination in Ethiopia. BMC Public Health. 2016;16(1):1–13. doi:10.1186/s12889-015-2639-8
12. Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016;16(1):1–14. doi:10.1186/s12879-016-2090-1
13. Owiti JA, Greenhalgh T, Sweeney L, Foster GR, Bhui KS. Illness perceptions and explanatory models of viral hepatitis B & C among immigrants and refugees: a narrative systematic review. BMC Public Health. 2015;15(1):1–17. doi:10.1186/s12889-015-1476-0
14. Alavian SM. Military personals should be vaccinated against hepatitis B infection. J Arch Mil Med. 2014;2(1). doi:10.5812/jamm.16450
15. Sobuh IM Risk factors of Hepatitis B transmission in north west bank: a case-control study; 2013.
16. Birku T, Gelaw B, Moges F, Assefa A. Prevalence of hepatitis B and C viruses infection among military personnel at Bahir Dar Armed Forces General Hospital, Ethiopia. BMC Res Notes. 2015;8(1):1–6. doi:10.1186/s13104-015-1719-2
17. Wondmagegn M, Wondimeneh Y, Getaneh A, Ayalew G. Seroprevalence of hepatitis B virus, hepatitis C virus, syphilis and associated factors among female sex workers in Gondar Town, Northwest Ethiopia. Infect Drug Resist. 2022;Volume 15:5915–5927. doi:10.2147/IDR.S380952
18. Million Y, Teklu T, Alemu S, Ferede A, Belachew T, Desta K. Hepatitis B and hepatitis C viral infections and associated factors among patients with diabetes visiting Gondar Referral Teaching Hospital, Northwest Ethiopia: a comparative cross-sectional study. J Hepatocell Carcinoma. 2019;Volume 6:143–150. doi:10.2147/JHC.S222609
19. Kasew D, Wondmagegn M, Bayleyegn B. Seroprevalence of hepatitis B and C virus among highly active antiretroviral therapy experienced children in Gondar, Ethiopia. Trop Med Health. 2022;50(1):1–9. doi:10.1186/s41182-022-00489-2
20. Getie B, Ayalew G, Amsalu A, Ferede G, Yismaw G, Tessema B. Seroprevalence and associated factors of hepatitis B and C virus among pulmonary tuberculosis patients attending health facilities in Gondar Town, Northwest Ethiopia. Infect Drug Resist. 2021;14:3599–3608. doi:10.2147/IDR.S327503
21. Tesfa H, Biadgo B, Getachew F, Tegegne K, Yismaw G, Muluye D. Seroprevalence of hepatitis B and C virus infection among patients attending serology laboratory of Gondar University Hospital. BMC Res Notes. 2013;6(1):1–4. doi:10.1186/1756-0500-6-164
22. Anagaw B, Shiferaw Y, Anagaw B, et al. Seroprevalence of hepatitis B and C viruses among medical waste handlers at Gondar town health institutions, Northwest Ethiopia. BMC Res Notes. 2012;5(1):1–10. doi:10.1186/1756-0500-5-55
23. Tigabu A, Engda T, Mekonnen F. Seroprevalence of transfusion transmissible viral infections (HIV, HBV and HCV) among voluntary blood donors at university of Gondar comprehensive specialized hospital, Gondar; Northwest Ethiopia. BMC Infect Dis. 2019;19(1):1–8. doi:10.1186/s12879-019-3950-2
24. Patel NH, Meier-Stephenson V, Genetu M, et al. Prevalence and genetic variability of occult hepatitis B virus in a human immunodeficiency virus positive patient cohort in Gondar, Ethiopia. PLoS One. 2020;15(11):e0242577. doi:10.1371/journal.pone.0242577
25. Beijing wantai biological pharmacy enterprise co. L, Beijing, China; 2020 Available from: https://www.ystwt.cn/hepatitis-b/.
26. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194.
27. Evlampidou I, Hickman M, Irish C, et al. Low hepatitis B testing among migrants: a cross-sectional study in a UK city. Br J Gen Pract. 2016;66(647):e382–e91. doi:10.3399/bjgp16X684817
28. Lazarus J, Safreed-Harmon K, Sperle I Global policy report on the prevention and control of viral hepatitis in WHO member states; 2013.
29. Alavian S, Rajai M, Arab M, et al. Viral hepatitis in Iranian armed forces: prevalence of HBV and HCV in the wounded-in-action (WIA). Hepat Mon. 2005;5(4):129–131.
30. Diop M, Diouf A, Seck SM, et al. Prevalence of hepatitis B surface antigen and its associated factors in Senegalese military personnel sent on mission to Darfur. Pan Afr Med J. 2017;26:154. doi:10.11604/pamj.2017.26.154.11594
31. Ndiaye AA, Fall IS, Lo G, et al. HBsAg seroprevalence among Senegalese militaries. Mil Med Res. 2015;2(1):1–5.
32. Tsega E, Krawczynski K, Hansson BG, et al. Outbreak of acute hepatitis E virus infection among military personnel in northern Ethiopia. J Med Virol. 1991;34(4):232–236. doi:10.1002/jmv.1890340407
33. Kupcinskas L, Petrauskas D, Petrenkiene V, Saulius K. Prevalence of hepatitis B virus chronic carriers and risk factors for hepatitis B virus infection among Lithuanian army soldiers. Mil Med. 2007;172(6):625–627. doi:10.7205/MILMED.172.6.625
34. Mirza IA, Mirza SH, Irfan S, Siddiqi R, Tariq WUZ, Janjua AN. Seroprevalence of hepatitis B and C in young adults seeking recruitment in armed forces. Pakistan Armed Forces Med J. 2006;56(2):192–197.
35. Sherif TB. Seroprevalence of hepatitis B and C in healthy adult male recruits. Pak J Med Sci. 2006;17(4).
36. Wang T, Dai Y, Lu W, et al. An epidemiological survey of HBV infection and low-level HBsAg in military camps in eastern China. Medicine. 2018;97(38):e12201.
37. German V, Giannakos G, Kopterides P, Liaskonis K, Falagas ME. Serologic indices of hepatitis B virus infection in military recruits in Greece (2004–2005). BMC Infect Dis. 2006;6(1):1–6. doi:10.1186/1471-2334-6-163
38. O’Connor SM, Mixson-Hayden T, Ganova-Raeva L, et al. Integrated HIV surveillance finds recent adult hepatitis B virus (HBV) transmission and intermediate HBV prevalence among military in uncharacterized Caribbean country. PLoS One. 2019;14(10):e0222835. doi:10.1371/journal.pone.0222835
39. Da Motta LR, Adami ADG, Sperhacke RD, et al. Hepatitis B and C prevalence and risk factors among young men presenting to the Brazilian Army: a strobe-compliant national survey-based cross-sectional observational study. Medicine. 2019;98(32):e16401. doi:10.1097/MD.0000000000016401
40. Brown AE, Ross DA, Simpson AJ, et al. Prevalence of markers for HIV, hepatitis B and hepatitis C infection in UK military recruits. Epidemiol Infect. 2011;139(8):1166–1171. doi:10.1017/S0950268810002712
41. Suryam V, Kathuria S, Karunakaran S. A study of seroprevalence of hepatitis B and hepatitis C among troops in armed forces. Med J Armed Forces India. 2011;67(3):257. doi:10.1016/S0377-1237(11)60053-1
42. Puri P, Sharma P, Nagpal A. Viral hepatitis in India: armed forces perspective. Med J Armed Forces India. 2016;72(3):201. doi:10.1016/j.mjafi.2016.07.004
43. Villar LM, Scalioni LP, Cruz HM, et al. Prevalence of hepatitis B and C virus infections among military personnel. Braz J Infect Dis. 2015;19(3):285–290. doi:10.1016/j.bjid.2015.02.002
44. Todd CS, Nasir A, Mansoor GF, et al. Cross-sectional assessment of prevalence and correlates of blood-borne and sexually-transmitted infections among Afghan National Army recruits. BMC Infect Dis. 2012;12(1):1–9. doi:10.1186/1471-2334-12-196
45. Umumararungu E, Ntaganda F, Kagira J, Maina N. Prevalence of hepatitis C virus infection and its risk factors among patients attending Rwanda military hospital, Rwanda. Biomed Res Int. 2017;2017:1–7. doi:10.1155/2017/5841272
46. Taye M, Daka D, Amsalu A, Hussen S. Magnitude of hepatitis B and C virus infections and associated factors among patients scheduled for surgery at Hawassa University comprehensive specialized hospital, Hawassa City, Southern Ethiopia. BMC Res Notes. 2019;12(1):1–6. doi:10.1186/s13104-019-4456-0
47. Konstantinou D, Deutsch M. The spectrum of HBV/HCV coinfection: epidemiology, clinical characteristics, viralinteractions and management. Ann Gastroenterol. 2015;28(2):221.
48. Yu G, Chi X, Wu R, et al. Replication inhibition of hepatitis B virus and hepatitis C virus in co-infected patients in Chinese population. PLoS One. 2015;10(9):e0139015. doi:10.1371/journal.pone.0139015
49. Angtuaco TL, Jensen DM. HBV and HCV co-infection. Chronic Viral Hepatitis. 2002;109–121.
50. Beckman LJ, Ackerman KT. Women, alcohol, and sexuality. Recent Dev Alcohol. 1995;12:267–285. doi:10.1007/0-306-47138-8_18
51. Thompson JC, Kao TC, Thomas RJ. The relationship between alcohol use and risk-taking sexual behaviors in a large behavioral study. Prev Med. 2005;41(1):247–252. doi:10.1016/j.ypmed.2004.11.008
52. Gitto S, Vitale G, Villa E, Andreone P. Update on alcohol and viral hepatitis. J Clin Transl Hepatol. 2014;2(4):228. doi:10.14218/JCTH.2014.00030
53. Ong A, Wong VWS, Wong GLH, Chan HLY. The effect of caffeine and alcohol consumption on liver fibrosis–a study of 1045 Asian hepatitis B patients using transient elastography. Liver Int. 2011;31(7):1047–1053. doi:10.1111/j.1478-3231.2011.02555.x
54. Abdulai MA, Baiden F, Adjei G, Owusu-Agyei S. Low level of hepatitis B knowledge and awareness among pregnant women in the Kintampo north municipality: implications for effective disease control. Ghana Med J. 2016;50(3):157–162. doi:10.4314/gmj.v50i3.7
55. Singh M, Kotwal A, Gupta R, Adhya S, Chatterjee K, Jayaram J. Sero-epidemiological and behavioural survey of HIV, HBV and HCV amongst Indian armed forces trainees. Med J Armed Forces India. 2010;66(1):50–54. doi:10.1016/S0377-1237(10)80093-0
56. Gupta E, Bajpai M, Sharma P, Shah A, Sarin S. Unsafe injection practices: a potential weapon for the outbreak of blood borne viruses in the community. Ann Med Health Sci Res. 2013;3(2):177. doi:10.4103/2141-9248.113657
57. Kengibe PY, Makulo J-R-R, Nlandu YM, et al. Response to single dose hepatitis B vaccine in Congolese non-HIV hemodialysis patients: a prospective observational study. Pan Afr Med J. 2019;34. doi:10.11604/pamj.2019.34.122.19603
58. Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52(4):1497–1505. doi:10.1002/hep.23808
59. Silverman AL, Puccio JE, Kulesza GW, McCray DG, Gordon SCJAJo G. HCV RNA is present in the menstrual blood of women with chronic hepatitis C infection. Am J Gastroenterol. 1994;89(8).
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.