Back to Journals » International Journal of General Medicine » Volume 13
Seroprevalence of Helicobacter pylori Infection and Associated Factors Among Adult Dyspeptic Patients in Public Health Facilities, Mizan Aman Town, Southwest, Ethiopia: Institutional-Based Cross-Sectional Study
Authors Belay AS , Abateneh DD , Yehualashet SS
Received 23 July 2020
Accepted for publication 22 August 2020
Published 10 September 2020 Volume 2020:13 Pages 577—585
DOI https://doi.org/10.2147/IJGM.S273523
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Alemayehu Sayih Belay,1 Dejene Derseh Abateneh,1,2 Sisay Shewasinad Yehualashet1,3
1Mizan Tepi University, College of Health Sciences, Department of Nursing, Mizan Aman, Ethiopia; 2Kotebe Metropolitan University, Menelik II College of Medicine and Health Sciences, Department of Medical Laboratory Sciences, Addis Ababa, Ethiopia; 3Debre Berhan University, Institute of Health Sciences, Debre Berhan, Ethiopia
Correspondence: Alemayehu Sayih Belay
Mizan Tepi University, College of Health Sciences, Department of Nursing, P.O. Box: 260, Mizan Teferi, Ethiopia
Tel +251-911669861
Email [email protected]
Background: Helicobacter pylori infection is a public health problem associated with chronic gastritis, peptic ulcer, and gastric cancer. It is endemic in developing countries like Ethiopia. This study was aimed to assess seroprevalence of H. pylori infection and associated factors among adults’ dyspeptic patients in public health facilities of Mizan Aman Town, Southwest Ethiopia.
Methods: Cross-sectional study was conducted in public health facilities of Mizan Aman Town, from April 1, 2018, to June 30, 2018. A total of 208 adult dyspeptic patients were included in the study. A structured questionnaire was used to collect data. Serum was tested for anti-H. pylori antibody using a commercial test strip. Data were entered using Epi info 6.04 and exported to SPSS 21 for analysis. Bivariate and multivariate logistic regression was employed and OR with 95% CI was retrieved. P-value of less than 0.05 was considered as statistically significant.
Results: A total of 208 participants were interviewed. The mean age of respondents was 31.70 (SD ± 9.123) years. Seroprevalence of H. pylori infection was 89 (42.8%). Presence of domestic animals (AOR = 13.33, 95% CI = (2.203– 80.692)), sources of drinking water (AOR = 0.011, 95% CI = (0.001– 0.110)), toilet type (AOR = 11.236, 95% CI = (1.921– 65.73)), shared beds with siblings (AOR = 7.775, 95% CI = (1.676– 36.082)), family size (AOR = 0.015, 95% CI = (0.003, 0.089)), storing and reusing water (AOR =0.014, 95% CI = (0.002– 0.103)) and occupational status (AOR = 23.33, 95% CI = (2.034– 67.661)) were variables significantly associated with seroprevalence of H. pylori.
Conclusion: Seroprevalence of H. pylori infection is relatively high in Ethiopia. Family size, shared bed, presences of domestic animals, storage and reuse of water, toilet type, sources of drinking water, and occupation were significant factors associated with H. pylori infection. The possible identified modifiable risk factors should be addressed through effective health education.
Keywords: H. pylori, seroprevalence, dyspepsia, Ethiopia
Introduction
Helicobacter pylori infection is a global public health problem and associated with chronic gastritis, and strongly linked to peptic ulcer diseases and gastric cancer. The bacterium is endemic in Africa and Asia.1 Its prevalence is highly variable in relation to geographical area, age, and socioeconomic factors; which is high in developing countries. Globally, different strains of H. pylori appear to be associated with differences in virulence, and the resulting interplay with host and environmental factors leads to subsequent differences in the epidemiology of infection.2,3
It has been well established that H. pylori is the cause of almost all duodenal ulcers and chronic benign gastric ulcers.4 The infection is found to be associated with gastritis, non-ulcer dyspepsia, duodenal ulcer, gastric ulcer, gastric cancer, and gastric lymphoma of mucosa-associated lymphoid tissue.5 Gastric cancer is a major global health threat and is the third leading cause of cancer deaths worldwide causing an estimated >720,000 deaths per year globally.6,7 Chronic H. pylori infection can also contribute to gastric mucosal instability by reducing gastric acid secretion.8
An epidemiological survey reveals that H. pylori infection is significantly higher in developing countries where the prevalence rate ranges between 70% and 90%, as compared to 20–50% in developed countries. The association between H. pylori infection and dyspeptic symptoms has long been established with H. pylori infection having high population attributable risk for dyspepsia.9 In Nigeria, various studies on H. pylori show prevalence rates between 73.0% and 94.5% among patients with dyspepsia10–12.
In developing countries, 70–90% of the population harbor H. pylori, which is mostly acquired during childhood.13 Transmission of H. pylori is largely through oral–oral or fecal-oral routes. A lack of proper sanitation, safe drinking water, basic hygiene, as well as poor diets and overcrowding, play a role in determining the overall prevalence of infection. Transmission of the bacterium is pronounced through poor environmental sanitation and crowding conditions including fecal contamination of water sources.2,14 In addition, the continuing emergence of resistance to the conventional anti-bacterial drugs used to treat H. pylori infections is challenging the eradication of H. pylori in developing countries.15,16
In Ethiopia, studies documented a high prevalence of H. pylori infection among adults in various localities.17,18 Planning and prevention measures that reduce the public health impact of H. pylori infection are critically needed. In this regard, investigating the epidemiology of the infection among the different segments of population is required to design effective intervention measures. Hence, the current study was aimed at the assessment of seroprevalence of H. pylori infection and associated factors among patients with dyspepsia in selected public health facilities in southwest Ethiopia.
Methods
Study Design and Setting
An institutional-based cross-sectional study was conducted in Mizan Tepi University Teaching Hospital (MTUTH) and Mizan health center, Bench Maji Zone, Southwest Ethiopia. The health facilities are located in Southwestern part of Ethiopia, Mizan Aman town, 561 km far from the capital city Addis Ababa. The town has a latitude and longitude of 7°0ʹN 35°35ʹE/7.000°N35.583°E, respectively, with an elevation of 1451 meters above sea level. The health facilities are expected to provide medical services for about 1 million people in Southwest Ethiopia and surroundings. The study was conducted from April 1, 2018 to June 30, 2018.
Source and Study Population
Adult dyspeptic patients aged ≥18 years based on ROME III criteria were included in the study. Patients with history of gastrectomy and who were critically ill and unable to give responses were excluded.
Sample Size Determination and Procedure
The minimum sample size (n) was determined by using single population proportion formula [n = (Z α/2)2 P (1-P)/d2], where Zα/2 = the value under standard normal table at 95% level of confidence which is 1.96, expected prevalence P, set at 85.6%, a previous study conducted in Gondar hospital, Ethiopia,19 d= precision which was set at 5%. Including 10% non-response rate, the final sample size was 208 dyspeptic patients.
The sample size was proportionally allocated to the two health facilities based on previous flow of dyspeptic patients through six months record review. Proportionally, a total of 139 dyspeptic patients from MTUTH and 69 dyspeptic patients from Mizan health center were selected consecutively.
Data Collection Tool and Procedure
Structured questionnaire which was adopted and modified from different reviewed literatures was used.19–21 Socio-demographic data included sex, age, ethnicity, and socio-economic factors and data on risk factors of H. pylori included smoking, diet, and alcohol consumption. The questionnaire was pretested among 5% of the sample size in other health institution before the actual data collection period. The coherence and skipping pattern of the questionnaire were corrected after the pretest.
Specimen Collection and Laboratory Procedure
Upon completion of the questionnaire, 3–5 mL of the venous blood sample was aseptically collected by trained laboratory professionals and serum sample was tested for anti-H. pylori antibody using a commercially available H. pylori serology test strip (HEXAGON H. PYLORI, Germany), following manufacturer’s instructions and interpreted accordingly. The kit has 97% sensitivity and 95% specificity compared with ELISA (HEXAGON H. PYLORI, Germany). Those participants tested positive results for the H. pylori serology test were asked to produce the stool when coming for the H. pylori serology test and the results were confirmed by the stool antigen test. The questionnaire was pre-tested and adequate supervision during data collection was made. The quality of the laboratory results was guaranteed by applying quality control measures during specimen collection and testing.
Operational Definitions
H. pylori Positive: Those participants whose serum result was reactive for the anti-H. pylori antibody test.
H. pylori Negative: Those participants whose serum result was non-reactive for an anti-H. pylori antibody test.
Adult: a human being whose age is 18 years and above who is expected to become sexually mature (According to Ethiopian National law).
Dyspepsia: Pain or discomfort centered in the central portion of the upper abdomen associated with a variety of symptoms such as fullness in the upper abdomen, early satiety, bloating or nausea or vomiting (ROME III criteria).
Educational status: refers to the highest level of education attended by the respondent during the time of the survey.
Income: for rural study participants calculated in kind the crop and cattle changed into monetary forms.
Data Processing and Analysis
Data were entered by using Epi info version 6.04 and exported into SPSS version 21 for cleaning, categorization, and analysis. The results were summarized using descriptive statistics including frequencies and proportions. Bivariate analysis was conducted to identify the association between each independent variable with the outcome variables. Multivariate analysis was employed to identify independent predictors associated with the outcome variables. In the multivariate analysis, adjusted odds ratio (AOR) and corresponding 95% confidence intervals were retrieved. Those variables with a p-value of less than 0.05 were considered as statistically significant.
Results
Socio-Demographic Characteristics of the Respondents
A total of 208 eligible participants were included in the study from selected public health institutions. The mean age of the respondents was 31.70 (SD ±9.123) years. Most of the participants, 87 (41.8%) were in the age range of 25–34 years. Of the total respondents, 123 (59.1%) were rural residents. Eighty-three (39.9%) of participants were not able to read and write (Table 1).
 |
Table 1 Socio-Demographic Characteristics of the Respondents (n=208), Southwest Ethiopia, 2018 |
Seroprevalence of Helicobacter pylori Infection
The overall seroprevalence of H. pylori infection was 89 (42.8%) through antibody detection. Of the positives, 24/89 (26.96%) and 19/89 (21.34%) were occupational housewives and farmers, respectively. A high proportion of H. pylori seropositive cases, 49/89 (55.05%), 59/89 (66.29%), and 82/89 (92.13%) were reported among participants with a household family size of ≥6, who have domestic animals, and who does not treat water before use, respectively (Table 2).
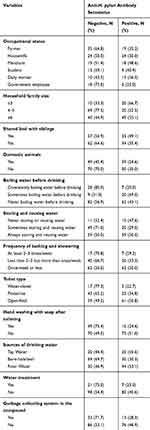 |
Table 2 Distribution of Seroprevalence of H. pylori Infection (n=208), Southwest Ethiopia, 2018 |
Risk Factors of H. pylori Infection
A number of variables like; occupational status, household family size, shared bed with siblings, presence of domestic animals, boiling water before drinking, storing and reusing water, frequency of bathing and showering, toilet type, hand washing with soap after toileting, sources of drinking water, water treatment and garbage collecting system in the compound were the factors found to be significantly associated with the participants' current serostatus of anti-H. pylori antibody by bivariate analysis. However, after adjusting for possible confounders with multivariate analysis, only variables like; occupational status, household family size, shared a bed with siblings, presence of domestic animals, storing and reusing water, toilet type, and sources of drinking water were found to be significantly associated.
Those participants whose family size was 4 to 5 per household were less likely to be positive for anti-H. pylori antibody than those whose family size was ≥6 members per household (AOR = 0.015, 95% CI = (0.003, 0.089)). Respondents who shared a bed with siblings were 7 times more likely to be positive for anti-H. pylori antibody than their counterparts (AOR = 7.775, 95% CI = (1.676–36.082)) and participants who respond for the presence of domestic animals were 13 times more likely to be positive for anti-H. pylori antibody than their counterparts (AOR = 13.33, 95% CI = (2.203–80.692)). Participants whose sources of drinking water were bore-hole/well were less likely to be positive for the current status of anti-H. pylori antibody than those who used river water (AOR = 0.011, 95% CI = (0.001–0.110)).
Those whose educational status were students were more likely to be positive for the current status of anti-H. pylori antibody than government worker (AOR =23.33, 95% CI= (2.034–67.661)). Those who sometimes store, and reuse water were less likely to be positive for the current status of anti-H. pylori antibody that those who always store and reuse the water (AOR =0.014, 95% CI= (0.002–0.103)).
Participants who use the open field for toilet were 11 times more likely to be positive for the current status of anti-H. pylori antibody than those who use water pit toilet (AOR =11.236, 95% CI= (1.921–65.73)). Respondents who use well water were less likely to be positive for the current status of anti-H. pylori antibody than those who use river water (AOR =0.011, 95% CI= (0.001–0.110)) (Table 3).
 |
Table 3 Determinant Factors for Participant’s Current Status of Anti-H. pylori Antibody (n=208), Southwest Ethiopia, 2018 |
Discussion
In the current study, the seroprevalence of H. pylori infection was 42.8% (95% CI (36%; 50%)) through the detection of anti-H. pylori antibody in the serum samples. More than half of the world’s populations in developing countries are infected with H. pylori infection.
In this study, the seroprevalence of H. pylori infection was much higher than a study conducted in North Jakarta 22.5%20 and comparable with a study conducted in Tanzania, 39% (95% CI: 32.3 −45.7).22 Whereas the result is lower compared to the studies done in Kano Nigeria, 81.7%,23 Bahrain, 54%,24 Nigeria, 73.6%,21 South Africa, 66.1%,25 and in Northwest Ethiopia, 85.6%19 and Addis Ababa St. Paul hospital, 64.2%.26 In comparison with the other studies conducted in different areas of the world, it was also found to be lower as compared with the systematic review and meta-analysis conducted in Ethiopia from 1990–2017, where the overall pooled prevalence of H. pylori infection was 52.2%.27 The difference could be attributed due to the socioeconomic difference, study period difference, and laboratory methods used by the individual studies, in which some of the above studies used histological technique, and urea breath test to determine the prevalence of H. pylori infection. Another reason could be due to the difference in sample size as that of this study is relatively lower.
Different predictor variables were found to be significant with serostatus of H. pylori infection.
In the current study, having high family size per household is significantly associated with H. pylori infection. The condition related to crowdedness which favors the transmission of the bacterium between individuals. Similar studies from Iraq,28 North Jakarta,29 Isreal30 and in Nigeria,21,31 documented high crowding index was a significant predictor H. pylori infection. Furthermore, this result is supported by the studies conducted in China32 and Sweden,33 documented that family size is a predictor variable for H. pylori infection in which high family members per household will play a significant role for interfamilial transmission of the bacterium during childhood which results in high seroprevalence of H. pylori in the general population too. In addition, a shared bed with siblings is a predictor variable associated with high prevalence of H. pylori infection in the current study. A similar study reported that the prevalence of H. pylori infection increased with the increment of sibling number (20% in those with none sibling versus 63% with eight or more siblings) in a household.34
The presence of domestic animals was found to be significantly associated with serostatus of H. pylori infection. A similar finding was documented from a study in Butajira, Ethiopia.35 The probability of acquiring the bacterium could be increased due to the presence of domestic animals like cats, dogs, and cattle’s which could contribute to the contamination of food and drinking utensils and could also directly or indirectly contaminate food items and water as well.36
In the current study, sources of drinking water from the river and storing and reusing water frequently were significantly associated with seroprevalence of H. pylori infection compared to those who use tap water. In addition, the toilet type of participants was a factor associated with seroprevalence of H. pylori infection. A similar related finding was reported from a study done in Sana’a,37 and North Jakarta20 which reported poor hand hygiene, hand washing habit, low clean water, and low sanitation index as factors significantly associated with seroprevalence of H. pylori infection. This indicated that poor personal hygiene and environmental sanitation are the source of transmission for infectious diseases causative agents including H. pylori. Whereas, a contradicted finding was reported from a study conducted in Rural Beninase,38 which indicated seroprevalence of H. pylori infection does not have an association with the source of drinking water. This difference with the current study could be attributed to the difference in sources of water used because nearly one-third of the participants of this study were used river water whereas the study subjects in Benine used tap water and well water only. River water is easily contaminated by animal and human waste products, and drinking such water was a high-risk factor for H. pylori infection.39
The result of the current study needs to be interpreted cautiously in which the study has some limitations. The first limitation is, we only studied people aged 18 years or above and did not assess the prevalence in children. Secondly, the inclusion of patients receiving antibiotics for other infections or who have been treated for H. pylori infection in the past may affect the diagnostic accuracy of the test result. Thirdly, it was conducted in a health institution setting and may not be a true representative of the prevalence of H. pylori among dyspeptics in the general population of South-West Ethiopia. A community-based study including all age group therefore needs to be considered by future researchers.
Conclusions
In conclusion, the observed seroprevalence of H. pylori infection among adult dyspeptic patients is relatively high in the study area. Family size shared a bed with siblings, presence of domestic animals, frequent storage and reuse of water, toilet type, and sources of drinking water were significant factors associated with the overall seroprevalence of H. pylori infection. The result can further suggest that the bacterium is still a public health problem that requires further study and effective interventions. Hence, the possible modifiable risk factors should be addressed through effective health education on personal hygiene and sanitation, water handling, toilet usage, handling of domestic animals. Dyspeptic patients should be tested for H. pylori serologically and future broad studies with advanced laboratory procedures should be conducted to identify further associated factors and socioeconomic impact of H. pylori infection.
Data Sharing Statement
All relevant data are included within the manuscript, but any additional data required are available from the corresponding author upon request. Email: [email protected].
Ethical Considerations
This study was conducted in accordance with the Declaration of Helsinki and after ethical clearance was obtained from the Institute of Research, Community Development and Support (IRCDS) office of the Mizan Tepi University (Ref No: MTU/CHS/91/421/21/10). After explaining the objectives of the study, written consent was obtained from each study participant. For the participants who were unable to read and write, trained interviewers have carefully explained the purpose, benefits, and potential risks before consent was obtained. In this case, fingerprint was used as a signature. The interview with study participants was conducted with strict privacy and confidentiality. The test was also performed following the manufacturer’s instructions and interpreted accordingly. Then, for those study participants who resulted positive, all necessary information was informed and finally linked to the health facilities for further investigation (histology, rapid urease test (RUT), microbiological culture, and polymerase chain reaction (PCR), endoscopy) and treatment.
Acknowledgments
The authors wish to express their earnest gratitude to Mizan-Tepi University Teaching Hospital and Mizan Health Center for their kind cooperation during data collection and laboratory work. In addition, we would like to express our honorable gratitude to study participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
We declare that we do not have any conflict of interests.
References
1. Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21(2):205–214. doi:10.1016/j.bpg.2006.10.005
2. Hunt R, Xiao S, Megraud F, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis. 2011;20(3):299–304.
3. Sabbi T, De Angelis P, Dall’Oglio L. Helicobacter pylori infection in children: management and pharmacotherapy. Expert Opin Pharmacother. 2008;9(4):577–585. doi:10.1517/14656566.9.4.577
4. Saad R, Chey WD. A clinician’s guide to managing Helicobacter pylori infection. Cleve Clin J Med. 2005;72(2):109–110. doi:10.3949/ccjm.72.2.109
5. Rogha M, Nikvarz M, Pourmoghaddas Z, Shirneshan K, Dadkhah D, Pourmoghaddas M. Is Helicobacter pylori infection a risk factor for coronary heart disease? ARYA Atheroscler. 2012;8(1):5.
6. Leung WK, Wu M, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9(3):279–287. doi:10.1016/S1470-2045(08)70072-X
7. Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the human development index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi:10.1016/S1470-2045(12)70211-5
8. Machado AMD, Figueiredo C, Touati E, et al. Helicobacter pylori infection induces genetic instability of nuclear and mitochondrial DNA in gastric cells. Clin Cancer Res. 2009;15(9):2995–3002. doi:10.1158/1078-0432.CCR-08-2686
9. Ugwuja EI, Ugwu NC. Helicobacter pylori in uninvestigated dyspepsia in primary cares in Abakaliki, Nigeria. Online J Health Allied Sci. 2007;6:1.
10. Holcombe C, Umar H, Lucas S, Kaluba J. Low incidence of clinically significant gastroduodenal pathology despite a high incidence of Helicobacter pylori infection. Trans R Soc Trop Med Hyg. 1994;88(5):569–571. doi:10.1016/0035-9203(94)90166-X
11. Ndububa D, Agbakwuru A, Adebayo R, et al. Upper gastrointestinal findings and incidence of Helicobacter pylori infection among Nigerian patients with dyspepsia. West Afr J Med. 2001;20(2):140–145.
12. Otegbayo J, Oluwasola O, Yakubu A, Odaibo G, Olaleye O. Helicobacter pylori serology and evaluation of gastroduodenal disease in Nigerians with dyspepsia. Afr J Clin Exp Microbiol. 2004;5(1):131–138. doi:10.4314/ajcem.v5i1.7366
13. Saad RJ, Chey WD. Persistent Helicobacter pylori infection after a course of antimicrobial therapy—what’s next? Clin Gastroenterol Hepatol. 2008;6(10):1086–1090. doi:10.1016/j.cgh.2008.05.009
14. Khedmat H, Karbasi-Afshar R, Agah S, Taheri S. Helicobacter pylori infection in the general population: a middle eastern perspective. Caspian J Intern Med. 2013;4(4):745.
15. Schmitz A, Josenhans C, Suerbaum S. Cloning and characterization of the Helicobacter pylori flbA gene, which codes for a membrane protein involved in coordinated expression of flagellar genes. J Bacteriol. 1997;179(4):987–997. doi:10.1128/JB.179.4.987-997.1997
16. Cammarota G, Martino A, Pirozzi G, et al. High efficacy of 1‐week doxycycline‐and amoxicillin‐based quadruple regimen in a culture‐guided, third‐line treatment approach for Helicobacter pylori infection. Aliment Pharmacol Ther. 2004;19(7):789–795. doi:10.1111/j.1365-2036.2004.01910.x
17. Kibru D, Gelaw B, Alemu A, Addis Z. Helicobacter pylori infection and its association with anemia among adult dyspeptic patients attending Butajira Hospital, Ethiopia. BMC Infect Dis. 2014;14(1):656. doi:10.1186/s12879-014-0656-3
18. Tadesse E, Daka D, Yemane D, Shimelis T. Seroprevalence of Helicobacter pylori infection and its related risk factors in symptomatic patients in southern Ethiopia. BMC Res Notes. 2014;7(1):834. doi:10.1186/1756-0500-7-834
19. Moges F, Kassu A, Mengistu G, et al. Seroprevalence of Helicobacter pylori in dyspeptic patients and its relationship with HIV infection, ABO blood groups and life style in a University Hospital, Northwest Ethiopia. World J Gastroenterol. 2006;12(12):1957. doi:10.3748/wjg.v12.i12.1957
20. Darnindro N, Syam AF, Fauzi A, Rumende CM. Seroprevalence and socio-demographic factors of Helicobcater pylori infection in patients with dyspepsia in Kalibaru primary health care north Jakarta. Acta Med Indones. 2015;47(4):297–303.
21. Ajiboye TT, Jatau ED, Inabo HI. Seroprevalence of Helicobacter pylori infection in patients with nonulcer dyspepsia in Zaria metropolis. Int J Sci Res Publ. 2016;6(5).
22. Jaka H, Mushi MF, Mirambo MM, et al. Sero-prevalence and associated factors of Helicobacter pylori infection among adult patients with dyspepsia attending the gastroenterology unit in a tertiary hospital in Mwanza, Tanzania. Afr Health Sci. 2016;16(3):684–689. doi:10.4314/ahs.v16i3.7
23. Bello AK, Umar AB, Borodo MM. Prevalence and risk factors for Helicobacter pylori infection in gastroduodenal diseases in Kano, Nigeria. Afr J Med Health Sci. 2018;17(1):41. doi:10.4103/ajmhs.ajmhs_36_17
24. Kamath R, Al-Qamish J, Yousif A, Fakro AR, John S. Prevalence of Helicobacter pylori among dyspeptic patients in Bahrain. Bahrain Med Bull. 1995;17(2).
25. Tanih N, Okeleye B, Ndip I, et al. Helicobacter pylori prevalence in dyspeptic patients in the Eastern Cape Province–race and disease status. S Afr Med J. 2010;100(11):734–737. doi:10.7196/SAMJ.4041
26. Teka B, Gebre-Selassie S, Abebe T. Sero-prevalence of Helicobacter pylori in HIV positive patients and HIV negative controls in St. Paul’s general specialized hospital, Addis Ababa, Ethiopia. Science. 2016;4(5):387–393.
27. Melese A, Genet C, Zeleke B, Andualem T. Helicobacter pylori infections in Ethiopia; prevalence and associated factors: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):8. doi:10.1186/s12876-018-0927-3
28. Baqir HI, Abdullah A, Al-Bana A, Al-Aubaidi H. Sero-prevalence of Helicobacter pylori infection in unselected adult population in Iraq. IJGE. 2002;1(3):22–29.
29. El Dine SS, Mubarak M, Salama R, et al. Low seroprevalence of anti-cagA antibodies inspite of high seroprevalence of anti-H. pylori antibodies in rural Egyptian community. Res J Med Sci. 2008;3(2):118–123.
30. Muhsen K, Athamna A, Athamna M, Spungin-Bialik A, Cohen D. Prevalence and risk factors of Helicobacter pylori infection among healthy 3-to 5-year-old Israeli Arab children. Epidemiol Infect. 2006;134(5):990–996. doi:10.1017/S0950268806006030
31. Smith S, Jolaiya T, Fowora M, et al. Clinical and socio-demographic risk factors for acquisition of Helicobacter pylori infection in Nigeria. Asian Pac J Cancer Prev. 2018;19(7):1851.
32. Shi R, Xu S, Zhang H, et al. Prevalence and risk factors for Helicobacter pylori infection in Chinese populations. Helicobacter. 2008;13(2):157–165. doi:10.1111/j.1523-5378.2008.00586.x
33. Tindberg Y Helicobacter pylori Infection Among Children in Sweden. Institutionen Södersjukhuset/Karolinska Institutet, Stockholm Söder Hospital; 2001.
34. Ford AC, Forman D, Bailey AG, Goodman KJ, Axon AT, Moayyedi P. Effect of sibling number in the household and birth order on prevalence of Helicobacter pylori: a cross-sectional study. Int J Epidemiol. 2007;36(6):1327–1333. doi:10.1093/ije/dym201
35. Lindkvist P, Enquselassie F, Asrat D, Muhe L, Nilsson I, Giesecke J. Risk factors for infection with Helicobacter pylori – a study of children in rural Ethiopia. Scand J Infect Dis. 1998;30(4):371–376. doi:10.1080/00365549850160666
36. Mohamed AA, El-Gohari A Epidemiological aspects of Helicobacter pylori infections as an emergence zoonotic disease: animal reservoirs and public health implications (a review article).
37. Sana’a Y, Al-Shamahy HA. Seroprevalence of Helicobacter pylori among children in Sana’a, Yemen. Ann Saudi Med. 2005;25(4):299–303. doi:10.5144/0256-4947.2005.299
38. Aguemon B, Struelens M, Massougbodji A, Ouendo EM. Prevalence and risk‐factors for Helicobacter pylori infection in urban and rural Beninese populations. Clin Microbiol Infect. 2005;11(8):611–617. doi:10.1111/j.1469-0691.2005.01189.x
39. Nurgalieva ZZ, Malaty HM, Graham DY, et al. Helicobacter pylori infection in Kazakhstan: effect of water source and household hygiene. Am J Trop Med Hyg. 2002;67(2):201–206. doi:10.4269/ajtmh.2002.67.201
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
