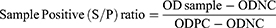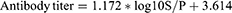Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Seroprevalence and Associated Risk Factors of Infectious Bursal Disease in Chickens Managed Under Intensive and Backyard Production Systems in Western Oromia, Ethiopia
Authors Abdeta D, Tamiru Y , Amante M , Abebe D, Kenei F , Shiferaw J , Tefera M
Received 11 November 2021
Accepted for publication 12 January 2022
Published 25 January 2022 Volume 2022:13 Pages 39—46
DOI https://doi.org/10.2147/VMRR.S347373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Young Lyoo
Debela Abdeta,1 Yobsan Tamiru,1 Morka Amante,1 Dereje Abebe,1 Felmeta Kenei,1 Jirata Shiferaw,2 Misgana Tefera2
1Wollega University, Nekemte, Ethiopia; 2Addis Ababa University, College of Veterinary Medicine and Agriculture, Bushoftu, Ethiopia
Correspondence: Debela Abdeta, Wollega University, P. O. Box 394, Nekemte, Oromia, Ethiopia, Email [email protected]
Background: Infectious bursal disease (IBD) is a highly contagious viral disease challenging poultry industry throughout the world. It is also among the main obstacles in the different chicken production system in Ethiopia. This study was conducted with the aim of determining seroprevalence and associated risk factors of IBD in backyard and intensively managed chickens of the study area.
Methods: A cross-sectional study was conducted in selected districts of western Oromia on intensive and backyard production systems to investigate seroprevalence of IBD and associated risk factors from September 2020 to August 2021. A total of 384 chickens of either sex, 128 from each district, were included from three randomly selected peasant associations (PAs). In addition, 180 individual poultry keepers, of both sexes, and different educational backgrounds, were included for questionnaire survey (Supplementary Material). Data were analyzed using SPSS Version-20, and results were presented as percentages. The samples were processed by using (ProFLOKIBV, USA) indirect enzyme-linked immunosorbent assay (I-ELISA) kits.
Results: Out of 384 serum samples tested, 66.93% (n = 257) were found positive by indirect ELISA. The highest prevalence was recorded from Sasiga district Balo Bareda PA. Prevalence of IBD showed significant statistical association (p < 0.05) among owner education level, chicken rearing practice, origin and breed. Questionnaire survey results showed the majority of respondents lack sufficient knowledge about IBD and other chicken diseases. The practice of backyard chicken producers on vaccination and treatment of sick birds is limited.
Conclusion: In conclusion, IBD is a major factor hindering production and health of birds. Proper vaccination programs coordinated with awareness creation of chicken owners on how to isolate and treat sick birds should be implemented.
Keywords: backyard, chick, ELISA, production, seroprevalence
Introduction
Ethiopia has the largest livestock population in Africa with nearly 61 million chickens in 2018.1 Of the total population of chickens in Ethiopia, 99% are reared under the traditional backyard system of management, while 1% is under intensive management system. This backyard extensive production system has a significant role in livelihood of farmers.2 These flocks provide a yearly output of 72,300 metric tons of meat and 78,000 metric tons of eggs. Chickens are widespread in Ethiopia and are important to subsistence, economic and social livelihoods of a large human population. Chickens are especially important to women, children and aged individuals, who are the most vulnerable members of the society in terms of under-nutrition and poverty.3,4
Many biological and socio-economic factors are incriminating for the decrease of poultry population in Ethiopia, of which disease and poor animal health practices are the two most important responsible factors worth mentioning here. Among the different diseases causing losses in the poultry production in the country, infectious bursal disease is the top one.5
Infectious bursal disease (IBD) is a highly contagious immunosuppressive disease caused by a virus of the genus Avibirnavirus of the family Birnaviridae.6 It has been described that IBD and its socio-economic significance is recognized worldwide,7 occurring in more than 95% of member countries of OIE.8 Currently from those cited viral diseases, IBD is the most important threat to poultry production in Ethiopia.5,9,10
The first report of IBD in Ethiopia was in 2005 involving 20–45-day-old broiler and layer chickens from commercial farms.9 Subsequently, IBD has become a priority problem in commercial and backyard poultry production systems despite regular vaccination practices (in some cases) using attenuated infectious bursal disease virus (IBDV) D78 vaccine and improved biosecurity measures.
The reports from some parts of Ethiopia showed high prevalence of the disease. In the study conducted by Jenbreie et al on 2597 chickens a prevalence of 83.1% was recorded at Mekelle and Gondar districts using ELISA test. However, it is very difficult to indicate the general figure of the disease due to lack of, or uneven, reports from different parts of the country.11 The study conducted so far in the country is not enough to show the exact picture of the disease. Although the disease is one of the main poultry health constraints in terms of viral diseases, responsible for marked economic losses in the backyard production system of a country, there is limited well documented information on the seroprevalence and associated risk factors of IBD. So far no published report has been found on the backyard chicken production system from Western Ethiopia, especially Jimma Arjo, Sasiga and Nedjo districts, even though production loss and chicken mortality reportedly occur in these districts.
The objectives of this paper were
- To determine seroprevalence of Infectious Bursal Disease in backyard and intensively managed chickens of the study area.
- To assess the associated risk factors of IBD in both intensive and backyard managed chickens of the study area.
Materials and Methods
Study Area
A cross-sectional study was conducted from September 2020 to August 2021 in apparently healthy backyard chickens from Jimma Arjo, Nedjo and Sasiga Districts of Oromia regional state, Ethiopia. Three peasant associations (PAs) were randomly selected from each district in consultation with the respective districts’ Livestock and Fisheries Development and Resource Office experts.
Jimma Arjo district is located at 379 km to the west of Addis Ababa, the capital city of Ethiopia. According to a Jimma Arjo district administration office report, in 2017 the population of the district was estimated to be 114,175. There are 20 rural kebeles and 2 town administrations in the district. Arjo town is found in Oromia regional state, eastern Wollega Zone 377 km from Addis Ababa and 48 km from Nekemte Zonal city. It is located at an altitude of 1500–2400 meters above sea level. The district has annual rainfall of 1800–2700 mm with temperatures of 15–24°C.
Nedjo district is found in West Wollega located 515 km away west of the country’s capital city. The altitude of the district is 1600–1900 m above sea level, the minimum and maximum annual rainfall of the district is 1000 and 1600 mm, and the temperature varies from 12°C to 26°C. The total livestock population is estimated to be 291,081 heads of cattle, 47,460 heads of sheep, 32,209 heads of goats, 15,866 heads of equines and 62,864 heads of poultry.12
Sasiga district has 26 rural PAs and one urban PA, with an estimated population of 13,145. The area has different vegetation types, livestock and wild game such as crocodiles, hippos, buffalos, kudu, bush pigs and warthogs. Since the area is fertile land the community’s livelihood mainly depends on agriculture with a mixed farming system. Livestock production mainly acts as a fundamental part for these mixed livestock production and agricultural land farming systems.
Ethical Approval
Ahead of starting data collection, ethical clearance was received from the research ethics committee of the School of Veterinary Medicine, Wollega University dated 23/07/2020, minute no. SVM.RERC/019. Data were collected after getting the permission from animal owners to collect samples from animals. During the course of data collection, information received was nameless, and confidentiality of data was secured.
Study Population
Chickens kept under extensive traditional husbandry form were included. In this study, age, breed, sex, origin and history of vaccination were taken as potential risk factors. The age of the chicken was classified as adult (>12 months) and young (6–12 months). The age was determined subjectively based on the size of crown, length of spur and flexibility of the xiphoid cartilage13 together with information from the chicken owners.
Study Design
A cross-sectional study was conducted from October 2020 up to August 2021 to determine the seroprevalence and its associated risk factors in the study area. Blood samples were collected from randomly selected, apparently healthy chickens, aged above 6 months, of randomly selected households in the PAs. Number of samples per district, PAs and household were determined and distributed proportionally. Three PAs were selected from each district, and the household was randomly selected by simple random sampling technique. A semi-structured questionnaire interview of the selected household owner was used to assess factors associated with the occurrence of IBDV. Age, sex, breed, flock size, hygiene condition, types of production, ways to treat sick chickens, types of housing and feces disposal mechanism were topics included in interview questions.
Sample Size Determination
The total number of animals required for this study was determined using the formula given by Thrusfield,14 for simple random sampling method. Required sample size (N) =1.962 Expected Prevalence (1- P Expected Prevalence)/d2, where d is the desired absolute precision at 95% confidence level and 5% absolute precision. Since there was no previous report from the same area, a 50% expected prevalence was assumed. The total sample size for blood collection was 384.
Sampling and Laboratory Investigation
For serological detection sterile 3 mL disposable syringes were used to collect blood samples from the brachial vein of the chickens. The whole blood collected from chickens was labeled and allowed to clot under normal atmospheric temperature in the slant position within the syringe. Then, the clear serum was harvested into labeled vial processes for laboratory testing. The serological tests were performed by indirect ELISA test using a commercial kit specific to IBDV (ProFLOKIBV, USA). Appropriate positive and negative controls included in the test kit were added to each plate run. The test serum and ELISA test reagents were brought to room temperature. Prior to being assayed, a 1:50 dilution of the sample was prepared with the manufacturer's diluents in a 2-step process. Then 100 µL of each diluted sample was pipetted into the appropriate well on the antigen-coated plate.15
One hundred microliters (100 µL) of undiluted positive and negative controls was added in their appropriate wells in duplicate. The plate was incubated for 30 minutes at room temperature (21–24°C). The plates were then manually washed 3 times with deionized water and blotted dry on laboratory tissue paper (towel) after washing. One hundred microliters of conjugate was added to all wells, and the plate was incubated at room temperature for 30 minutes. Then wash and dry were repeated as described above. One hundred microliters of substrate was added to all the wells and incubated at room temperature for 15 minutes. To stop the reaction, 100 µL of stop solution was added to all the wells. The optical density (OD) absorbance value of each sample on the test plate was measured with ELISA reader, wavelength 405 nm.
To check the validity of IBD ELISA results, a validity test was done. In the valid IBD ELISA result, the mean optical density (OD) value of positive control serum is greater than 0.250, and the ratio of the mean value of the positive and negative control (ODPC and ODNC) is greater than 3. For the interpretation of the result, serum sample positive (SP) control ratio was required. If the SP value was >0.3, the IBD antibody status was considered to be positive, but a value of ≤0.3 was taken as negative. For each sample, we calculated the SP ratio and antibody titer as follows;
Questionnaire Survey
In all study PAs, selected chicken owners were interviewed face to face in Afan Oromo language, which takes approximately 20 minutes only. Demographic details like age, sex, residential place and educational status of the study participants were covered by the questionnaire. Accordingly, the age of the respondents was categorized as: 15–29 years (young), 30–45 years (adult) and >45 years (old).16 It was pretested and organized well to be easy and understandable after the test in a randomly picked PA of one district. Generally, based on chicken population and number of households per PAs, 180 semi-structured questionnaire interviews were collected from households.
Data Management and Analysis
Data collected from the field and the serological test were coded and stored in MS Excel spreadsheet and transferred to SPSS version 20 software package. In order to determine the overall prevalence, the number of I-ELISA positive animals was divided by the total number of animals tested. Dependence variables (seropositivity) were compared against independent variables such as breed, age, type of production and management issues. The chi-square test was used to determine the association between the disease and potential risk factors. In all cases, 95% confidence intervals and p<0.05 were set for significance.
Data Quality Control and Quality Assurance
The quality of the data was assured via careful development of the sample collection format and questionnaire format for data collection after a thorough literature review. The questionnaire was prepared firstly in English, translated into Afan Oromo language (the local language) and re-translated into English to check its consistency. The questionnaire format was validated using a pre-test on 5% of the sample size that was randomly selected, and appropriate modifications were made.
Results
Seroprevalence of IBD Virus Infection
In the present study, a total of 384 blood samples were collected from unvaccinated chickens to determine the serological status of IBD infection and its associated risk factors in backyard and intensively reared poultry farms in the study area. The current study revealed that out of 384 serum samples tested 66.93% were found positive by indirect ELISA. From the seropositive sample on I-ELISA, the highest prevalence (80.47%) was from the Sasiga area (Table 1).
 |
Table 1 Seroprevalence of IBD in each Study Area |
Potential Risk Factors of IBD
Seroprevalence of IBD infection was analyzed across different associated risk factors. According to the sex-wise analysis of the disease, it was found to be significantly (p-value <0.05) higher in female chickens. Likewise, comparing the prevalence of the infection in different management systems, it was significantly higher in intensively managed chickens (Table 2).
 |
Table 2 Seroprevalence of IBD for Different Potential Risk Factors |
Seroprevalence of IBD at Peasant Associations
Different seroprevalence rates of IBD were observed among 9 PAs of the three districts with highest prevalence from Balo Bareda (92.85%) and Goori (90.48%) PA. There is significant statistical association (p<0.05) between PA and disease occurrence from Sasiga and Nedjo districts (Table 3).
 |
Table 3 Sero-Prevalence of IBD at the Level of Peasant Associations (PAs) |
Socio-Demographics of the Respondents
From a total of 180 study participants, greater numbers of the respondents 37.7% (n=68) were in the range 30–45 years of age (adult). The majority of the respondents participating in the study were females 61.67% (n=111). Educational background of respondents indicated 25% (n=45) could write and read, 31.67% (n=57) had completed 1–8th grade schooling, 30.56% (n=55) 9–12th grade and 13.89% (n=25) of them were graduates of different specialties engaged in poultry production at the backyard level (Table 4).
 |
Table 4 Socio-Demographic Status of Respondents by Districts (n=180) |
From the total respondents, 66.7% (n=120) used various traditional methods such as smoking before taking their chickens to the clinic. Regarding vaccination practices, none of the backyard chicken producers take their animals to the clinic for vaccination purposes. It was also found that, in contrast to the intensive producers, the backyard poultry producers clean only in the morning. The source of birds for backyard practice is from markets, whereas intensive producers get young chicks from commercial chick producers.
Discussion
Poultry production is a significant source of high-quality animal protein. Nonetheless, the high prevalence of infectious diseases in poultry poses a significant threat to productivity and survival.17 The current study, which was carried out on both intensively farmed and backyard chickens in three districts of the Western Oromia, discovered the presence of IBDV-specific antibodies in the absence of vaccination, indicating that the chickens were exposed to the virus in the field. In the current study, 384 chickens from various management systems were tested using an indirect ELISA with an IBDV-specific antibody. The area had a relatively higher seroprevalence (66.93%) of the disease, indicating that the virus is spreading at a faster rate.
On the other hand the current study is relatively comparable to the previous prevalence studies of Tesfaheywet et al,18 Swai et al19 and Kassa and Molla,20 who reported a seroprevalence of 82.2%, 82.8% and 73.5%, respectively. However, it was found to be higher than the earlier studies reported in Ethiopia.21–23 The observed differences in prevalence of the disease status in the current study could be attributed to the differences in management practices, sensitivity and specificity of diagnostic tests used and differences in the status of the chickens in the study.24
In the present study, sex, origin and management system were found to be the potential risk factors, and statistically significant differences were observed (p<0.05). Lower prevalence of IBD was significantly detected in male chickens as compared to the female chickens. This result was not consistent with earlier findings of Kassa and Molla20 and Mazengia.21 On the other hand, Reta25 reported the absence of influence of sex on the prevalence of the disease. Variation in seroprevalence of the disease was also observed across location. The highest prevalence was identified in the Sasiga area. The difference existing between different locations of the study area may be attributed to the difference in management practices and environment conditions. Moreover, the result showed that the highest seroprevalence of the disease was observed in backyard-managed chickens. This result is similar to the finding of Swa et al,19 Tesfaheywet et al18 and Dey et al.26 High prevalence of the disease in backyard chickens might be due to frequent exposure to immunosuppressive factors such as heat stress, deprivation of water, and poor nutrition, which results in a suppression of the immune system in backyard chickens, as reported by Hassan et al.27
The report of introduction and existence of IBD in Ethiopia has recently come with the IBD outbreak in Bishoftu large-scale poultry farms in 20–45-day-old broiler and layer chickens and indicated that the mortality rate of the disease is in the range 45–50%, and seroprevalence of 93.30% of IBD antibody was recorded.21 The case report study at Bahir Dar and Farta areas indicated an incidence rate of 29.40% and 21.70% in backyard chickens in an outbreak in Bishoftu.9 Moreover, a study conducted by Tadesse and Jenbere in Eastern Ethiopia indicated a prevalence rate of 82.2% in backyard chickens.28
Conclusion
In conclusion, infectious bursal disease is one important highly contagious acute infection of chickens and a big threat for chicken production in Ethiopia. So it is necessary to advise farmers to get their chickens vaccinated and reduce the prevalence, besides maintaining the hygienic condition of the environment in which the chickens are reared.
Abbreviations
ELISA, enzyme-linked immunosorbent assay; IBD, infectious bursal disease; IBDV, infectious bursal disease virus; OD, optical density.
Data Sharing Statement
The datasets that support the findings of this study are available from the principal author upon request.
Ethical Approval and Consent to Participate
All participants were informed about the purpose of this study, and signed written legal consent for participation was received prior to the commencement of the study. For participants under the age of 18 parental informed consent was obtained. Study design involves animals for blood samples and human participants for interviews. The survey protocol and animal handling ethics were approved by the Wollega University School of Veterinary Medicine Ethical Review Board. Support letters were also granted from each district's Livestock and Fishery Resource Office. Throughout the procedure of blood collection and animal handling best practices of veterinary care were followed, and animal owners provided informed consent for samples to be collected.
Acknowledgments
The authors acknowledge Wollega University and Addis Ababa University College of Veterinary Medicine and Agriculture.
Author Contributions
DA contributed to drafting of idea, supervision, data analysis and write-up. DA and FK contributed to data collection; YT, MA, JS and MT carried out laboratory work. The authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. FAO. FAOSTAT database; 2019. Available from: http://www.fao.org/faostat/en/.
2. Dawit A, Tamirat D, Setotaw T, Devesh R. Draft report on overview and background paper on Ethiopia’s poultry sector: relevance for HPAI research in Ethiopia, Addis Ababa, Ethiopia; 2008.
3. CSA (Central Statistics Authority). Agricultural sample survey 2009/10. Report on livestock and livestock characteristics, 2. Statistical Bulletin No. 468. Addis Ababa, Ethiopia; 2009.
4. Teklewold H, Dadi L, Yami A, Dana N. Adopting poultry breeds in the highlands of Ethiopia. Ethiopian Institute of Agricultural Research; 2006:26.
5. Food and Agricultural Organizations. Emergency Center for Transboundary Animal Diseases Socio Economics, Production and Biodiversity Unit, Poultry Sector Country Review of Ethiopia. France from acute outbreaks. J Vet Med B. 2008;39:683–691.
6. OIE. Manual of standards for diagnostic test and vaccines. Infectious bursal disease (Gumboro disease); 2008:549–565.
7. Muller H, Islam M, Raue R. Research on infectious bursal disease the past, the present and the future. Vet Microbiol. 2003;97(1–2):153–165. doi:10.1016/j.vetmic.2003.08.005
8. Van Den Berg TP. Acute infectious bursal disease in poultry: a review. Avian Pathol. 2000;29(3):175–194. doi:10.1080/03079450050045431
9. Zeleke A, Gelaye E, Sori T, Ayelet G, Sirak A, Zekarias B. Investigation on infectious bursal disease outbreak in Debre-Zeit, Ethiopia. Inter J Poult Sci. 2005;7:504–506.
10. Solomon W, Abebe W. Infectious bursal disease (Gumboro disease) case report of Andasa poultry farm Amahara region. Ethiop Vet J. 2007;11:151.
11. Jenbreie S, Ayelet G, Gelaye E, Kebede F, Lynch SE, Negussie H. Infectious bursal disease: seroprevalence and associated risk factors in major poultry rearing areas of Ethiopia. Trop Anim Health Prod. 2013;45(1):75–79. doi:10.1007/s11250-012-0176-3
12. BFED. Nedjo bureau of finance and economic development unpublished yearly report; 2016.
13. Magwisha H, Kassuku A, Kyvsgaard N, Permin A. A comparison of the prevalence and burdens of helminth infections in growers and adult free range chickens. Trop Anim Health Prod. 2002;34(3):205–214. doi:10.1023/A:1015278524559
14. Thrusfield M. Veterinary Epidemiology.
15. Alcorn M. How to Carry Out Filed Investigation: In Poultry Disease.
16. Guadu T, Shite A, Chanie M, Bogale B, Fentahun T. Assessment of knowledge, altitude and practices about rabies and associated factors: in the case of Bahir Dar Town. Glob Vet. 2014;13:348–350.
17. Adebiyi O, Ferdinand C, Kufre J, Okop A. A modified Delphi study towards developing a guideline to inform policy on fetal alcohol spectrum disorders in South Africa: a study protocol. BMJ. 2018;8:1–12.
18. Tesfaheywet Z, Hair-Bejo M, Rasedee A. Hemorrhagic and clotting abnormalities in infectious bursal disease in specific pathogen- free chicks. World Appl Sci J. 2012;16:1123–1130.
19. Swai E, Kessy M, Sanka P, Mtui P. A serological survey for infectious bursal disease virus antibodies in free-range village chickens in northern Tanzania. J S Afr Vet Assoc. 2011;82(1):32–35. doi:10.4102/jsava.v82i1.30
20. Kassa SA, Molla W. Seroprevalence of infectious bursal disease in backyard chickens of North West Ethiopia. Sci J Crop Sci. 2012;1:20–25.
21. Mazengia H. Review on major viral diseases of chickens reported in Ethiopia. J Infect Dis Immun. 2012;4(1):1–9.
22. Natnael T. Pathological and Seroprevalence Studies on Infectious Bursal Disease in Chickens in and Around Bahir Dar, North West, Ethiopia [MSc Thesis]. Bishoftu, Ethiopia: Faculty of Veterinary Medicine, Addis Ababa University; 2015:54.
23. Lemma F, Zeryehun T, Kebede A. Seroprevalence of Infectious Bursal Disease in non-vaccinated village chicken in Jigjiga and Harar Districts, Eastern Ethiopia. J Vet Sci Technol. 2019;10:572.
24. El-Yuguda A, Baba S. An outbreak of infectious bursal disease (IBD) in an eight-week old IBD vaccinated commercial poultry flock in Maiduguri, Nigeria. Trop Vet. 2004;22(3&4):93–98.
25. Reta T. Seroprevalence Study of Infectious Bursal Disease in Unvaccinated Backyard Chickens in Agro Ecological Areas of East Shoa Zone [DVM Thesis]. Bishoftu, Ethiopia: Addis Ababa University, Faculty of Veterinary Medicine; 2008.
26. Dey S, Dinesh C, Pathak N, Ramamurthy H, Kumar M, Madhan M. Infectious bursal disease virus in chickens: prevalence, impact, and management strategies. Vet Med. 2019;10:85.
27. Hassan BA, Prokopenko SN, Breuer S, Zhang B, Paululat A, Bellen HJ. Skittles, a Drosophila phosphatidylinositol 4-phosphate 5-kinase, is required for cell viability, germline development and bristle morphology, but not for neurotransmitter release. Genetics. 1998;150(4):1527–1537. doi:10.1093/genetics/150.4.1527
28. Tadesse B, Jenbere S. Sero-prevalence of Infectious Bursal disease in backyard chickens at selected Woredas of Eastern Ethiopia. J Biol Agric Healthc. 2014;4(17):2224–3208.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.