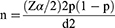Back to Journals » Infection and Drug Resistance » Volume 14
Seroprevalence and Associated Factors of Hepatitis B and C Virus Among Pulmonary Tuberculosis Patients Attending Health Facilities in Gondar Town, Northwest Ethiopia
Authors Getie B , Ayalew G , Amsalu A , Ferede G, Yismaw G, Tessema B
Received 1 July 2021
Accepted for publication 24 August 2021
Published 3 September 2021 Volume 2021:14 Pages 3599—3608
DOI https://doi.org/10.2147/IDR.S327503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Birhanu Getie,1 Getnet Ayalew,2 Anteneh Amsalu,2,3 Getachew Ferede,2 Gizachew Yismaw,4 Belay Tessema2
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia; 2Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3University of South Australia, Adelaide, SA, Australia; 4Amhara Public Health Institute, Bahir Dar, Ethiopia
Correspondence: Getnet Ayalew
Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, P.O. Box: 196, Gondar, Ethiopia
Email [email protected]
Background: Hepatitis B virus (HBV) and hepatitis C virus (HCV) are hepatotropic viruses whose primary replication occurs in the liver. Despite the significant clinical importance of early screening of hepatitis B and C virus infection in decreasing the hepatotoxicity effect of anti-tuberculosis drugs, screening of hepatitis B and C virus among tuberculosis (TB) patients before treatment has not been practiced in Ethiopia. Thus, this study was conducted to determine the seroprevalence and associated factors of HBV and HCV infections among pulmonary TB (PTB) patients attending health facilities in Gondar, Northwest Ethiopia.
Methods: A cross-sectional study was conducted among 145 bacteriologically confirmed PTB patients from January 1 to May 30, 2019. After obtaining a signed informed consent from each participant, data on socio-demographic, clinical, and associated factors were collected using a structured pre-tested questionnaire. Besides, a blood sample was collected to determine HBsAg and HCV antibodies by enzyme linked immune sorbent assay (ELISA). The data were entered and analyzed using SPSS version 21. A Fisher’s exact test was used to see the relationship between dependent and independent variables, and a p-value ≤ 0.05 was considered as statistically significant.
Results: Out of the 145 PTB patients screened, 5 (3.4%) patients tested positive for HBsAg, yet none of them were found to be positive for anti-HCV. Besides, the proportion of HIV-positive was 12 (8.3%). History of hospital admission (P= 0.005), tattooing (P= 0.009) and dental extraction (P=0.003) were significantly associated with HBsAg.
Conclusion: Although anti-HCV antibodies were not detected, the prevalence of HBV was relatively high in tuberculosis patients. This study highlights the need for the introduction of routine screening of viral hepatitis markers for all TB patients before anti-TB treatment for better management of patients. Likewise, further clinical and epidemiological studies are needed.
Keywords: hepatitis B virus, hepatitis C virus, seroprevalence, pulmonary tuberculosis
Background
Hepatitis B virus (HBV) and Hepatitis C virus (HCV) infections contribute to major disease mortality and morbidity worldwide. Hepatitis is an inflammation of the liver, most usually caused by a viral infection. There are five different types of hepatitis viruses (A-E) that are responsible for viral hepatitis. Of these viruses, hepatitis B and C viruses account for a substantial proportion of liver diseases and are responsible for liver damage ranging from minor disorders to liver cirrhosis and hepatocellular carcinoma (HCC).1,2
Hepatitis B virus infection is found worldwide, with prevalence rates varying markedly between countries. Chronic carriers constitute the main reservoir of infection in some countries, particularly in developing countries.3–5 In 80% to 90% of the cases, HBV infection runs a benign course with complete recovery and elimination from the body, but only 5% to 10% of infected people develop a chronic infection. A Chronic infection can result in the development of hepatocellular carcinoma (HCC) or cirrhosis of the liver, with the incidence varying widely from one geographic area to another.6,7 Hepatitis C virus (HCV) infection is of rising public health concern due to its substantial effect on morbidity and mortality,8,9 ie, it is a leading cause of cirrhosis, hepatocellular carcinoma (HCC), liver transplantation, and liver-related death worldwide.10,11 The disease’s health and economic burden on countries both from hepatic and extra hepatic complications imposes therapeutic and healing progress.12,13
Tuberculosis is an infectious bacterial disease caused by Mycobacterium tuberculosis complex.14 A relatively small proportion (5% to 15%) of the estimated 1.7 billion people infected with M. tuberculosis will develop TB disease during their lifetime. However, the probability of developing TB disease is much higher among people with risk factors such as living with HIV, under-nutrition, diabetes, smoking and alcohol consumption.15 Although currently, four first-line drugs: isoniazid (INH), rifampicin (REP), ethambutol (EMB) and pyrazinamide (PZA) are recommended for the treatment of drug-susceptible TB,14,16 drug-induced liver injury (DILI) with INH, RFP and PZA has been reported more frequently.17 Drug-induced liver injury (DILI) associated with anti-TB treatment is the most common adverse event which affects treatment success rate.18
The prevalence of HBV infection among tuberculosis patients is up to 25.6%.19 The HCV burden among tuberculosis patients ranges from 1.0% to 44.6%.20,21 In Ethiopia, about 8% of the population was infected with HBV and around 2% of the community was infected with HCV.22–24
Even though HBV and/or HCV infections are risk factors for DILI, other factors like elder age, female gender, alcohol abuse and malnutrition have been reported as significant contributors to the development of DILI during anti-TB treatment.25,26 Though chronic liver disease is known to increase the risk of hepatotoxicity, there are controversies about the effect of HBV and/or HCV infections on the development of DILI during anti-TB treatment.25,27 The association between HCV and/or HBV and TB infections has never been comprehensively studied. However, studies provide evidence that HCV infection may increase the risk of active TB disease through its immunomodulatory effects and the association is biologically plausible.28
In Ethiopia, the prevalence of HBV and HCV infections and associated risk factors among TB patients have not been well investigated. Thus, the current study is aimed at assessing the prevalence and associated risk factors of HBV and HCV infections among TB patients who were attending at Gondar town health facility, Northwest Ethiopia.
Materials and Methods
Study Setting
This study was conducted at outpatient TB clinics at Gondar town health facilities, namely, University of Gondar Teaching and Referral Hospital, Maraki Health Center, and Gondar Health Center. Gondar, which is a metropolitan city in the Amhara national regional state, is around 747 kilometers away from Addis Ababa, the capital city of Ethiopia, in the northwest direction, and 182 kilometers from Bahir Dar, which is the capital city of the Amhara regional state. There are 8 governmental health centers, 21 private clinics, one private primary hospital and one governmental tertiary teaching hospital (University of Gondar Teaching and Referral Hospital) in the town. The later hospital provides health-care services for more than 5 million people living in the North, South, and West Gondar Zones, as well as urban and rural kebeles surrounding the town. The hospital has a TB clinic which provides outpatient (OPD), direct observed treatment (DOTS) and Gene Xpert laboratory services.29
Study Design and Period
A health facility-based cross-sectional study was conducted among newly diagnosed PTB patients from January 1 to May 30, 2019 at Gondar town health facilities.
Study Participants and Selection Procedure
The study population included all newly diagnosed PTB patients aged >18 years and who were attending DOTS clinics in the study area during the study period. Using consecutive covenant sampling, all newly diagnosed PTB patients aged 18 years and above were included in the study. Patients who were less than 18 years of age, were disabled, could not respond to the interview and could not give blood samples were excluded from the study.
Sample Size
The sample size for this study was determined by the formula for single population proportion.
Where: z=standard score corresponding to 95% CI=1.96
p=assumed proportion of intention to use = 0.095
d=Margin of error/precision = 5%
The prevalence of HBV and/or HCV among TB patients was 9.5% in a study done by Nail et al.30 Adding 10% for a non-response rate, the total sample size was 145.
Definition of Terms
Acute infection: is an active infection that develops rapidly after exposure (less than six months of exposure). Chronic infection: is a persistent (long-term) infection developed after six months of exposure. Co-infection: is the presence of concurrent dual infections.31 Hepatitis B surface antigen (HBsAg): is a marker present in people who are infected with HBV (ie, people with both recent infection and chronic infection). Sexually transmitted infections (STIs): are infectious diseases which are passed on from one person to another through intimate sexual contact.7
Data Collection Procedures
Written informed consent was obtained from each participant by trained nurses. The clinical examinations were done by the clinicians and/or nurses. The English version of the pre-tested questionnaire contained socio-demographic characteristics (age, gender, marital status, area of residence, level of education attained, occupation) and risk factors (ear-piercing, tattooing, history of contact with an infected person, blood transfusion, tooth extraction, hospital admission). Data was also collected from the respondents by trained nurses.
Laboratory Testing for Viral Hepatitis Markers
Five mL of a venous blood sample was collected using vacationer tubes. The specimens were labelled appropriately using patients’ identification number. The collected blood specimens were then transported appropriately to the University of Gondar Microbiology Laboratory using a cold box and then allowed to clot at room temperature for 30 minutes. Serum was separated from cells by centrifugation at a speed of 35,000 revolutions per minute (rpm) for 5 minutes. Two mL of serum was transferred into Nunc tubes and kept in the refrigerator at −20∘ c until tested for HBV and HCV. The commercial enzyme-linked immunosorbent assay (ELISA) test kit (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd, Beijing, China) was used for the detection of HBsAg and anti-HCV antibodies following the manufacturers’ instructions. The study used the antibody “sandwich” ELISA method in which polystyrene microwell strips are pre-coated with monoclonal antibodies specific for HBsAg. The patient serum is added to the microwells. During incubation, the specific immunocomplex in case of the presence of HBsAg in the specimen is captured in the solid phase. Then the second antibody conjugated to the enzyme horseradish peroxidase (the HRP-conjugate) directed against a different epitope of HBsAg is added into the wells. Throughout the second incubation step, these HRP conjugate antibodies bind to any anti-HBs-HBsAg complexes previously formed during the first incubation, and the unbound HRP-conjugate is then removed by washing. Chromogen solutions containing tetra methyl-benzidine (TMB) and urea peroxidase are added to the wells. In the presence of the antibody-antigen-antibody (HRP) “sandwich” immunocomplex, the colorless chromogens are hydrolyzed by the bound HRP-conjugate into a blue-colored product. The blue color changes to yellow after stopping the reaction with sulfuric acid. The amount of color intensity is proportional to the amount of antigens captured in the wells, and to the amount in the specimen respectively. Wells containing a specimen negative for HBsAg remain colorless.32
Laboratory Testing for Tuberculosis and HIV
In Ethiopia, the diagnosis of TB and HIV is performed according to the national guidelines for comprehensive HIV prevention, care and treatment, and the national guidelines for TB, drug resistant TB, and Leprosy. These guidelines are based on the World Health Organization recommendations and all laboratory testing procedures are performed according to the Standard Operating Procedures (SOPs).33–36
Quality Control
Data collectors were trained for one day on the data collection procedure. The questionnaires were validated by pre-testing them on about 10% of the sample size on some randomly selected patients at Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia, and necessary modifications were made accordingly. After data collection, each questionnaire was given a unique code by the principal investigator and was rechecked to ensure the quality of the data.
For the ELISA test, Standard Operating Procedures (SOPs) were strictly followed and internal quality control materials were included and performed based on the manufacturers’ instructions. To assure the controlled performance of our testing procedures, an in-house control for both negative and positive control samples, in addition to controls provided by the manufacturers, was used. As a result, the reagents and the test method were assessed with those known positive and negative control materials used to evaluate the storage conditions of the reagents and the performance capability of the method, and all positive samples were repeated.
Ethical Approval
Ethical approval was obtained from the ethical review committee of School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar with a reference number SBMLS/2924/11. Likewise, the study was conducted in accordance with the ethical principles of the declaration of Helsinki on human subjects. All study participants were informed concerning the study verbally and a written consent was obtained from each participant. All information was treated as strictly confidential and used for this study only. Positive results were communicated to health-care providers. Patients were advised, monitored and followed by the clinicians during their anti-TB treatment to prevent complications.
Data Entry and Analysis
The data was entered into Statistical Package for Social Science (SPSS) version 21 for analysis. The descriptive statistics (means, percentages, or frequencies) were calculated. A Fisher’s Exact Test was used to see the relationship between dependent and independent variables. The association was assessed using p-value and a p-value ≤0.05 was considered as statistically significant.
Results
Socio-Demographic, Clinical and Behavioral Characteristics of Study Participants
During the study period, a total of 145 pulmonary TB patients were included in the study. Of those, 90/145 (62.1%) were male. The mean (+SD) age of the study participants was 31.58 years (+11.89) and the greatest number of the study participants (68/145 (46.9%)) was under the age category of 18 to 27 years, followed by 42/145 (29.0%) participants who were 28 to 37 years of age. Seventy-four (51.0%) TB patients were married, 63/145 (43.4%) were single, 6/145 (4.2%) were divorced, and 2/145 (1.4%) were widowed. Moreover, among the 145 TB patients, 102 (70.3%) were urban residents and the rest 43 (29.7%) were from rural settings. Of all the participants, 46/145 (31.7%) did not attend formal education while 41/145 (28.3%) attained primary and 31/145 (21.4%) secondary education. Regarding the occupation of study participants, 32/145 were students and accounted for 22.1%, followed by housewives (31/145 (21.4%)) and farmers (26/145 (17.9%)) (Table 1).
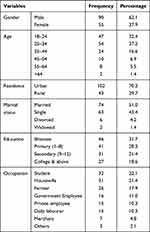 |
Table 1 Socio Demographic Characteristics of TB Patients Attending Gondar Town Health Facilities in Northwest Ethiopia, January to May, 2019 (N= 145) |
In this study, only one participant was vaccinated against HBV and 12 (8.3%) of the study participants had HIV infection. The majority of the study participants (78 (53.8%)) had a history of intravenous medication; 32 (22.1%) had tattooing on the body; 32 (22.1%) had ear-piercing and 28 (19.3%) of the participants had a history of hospital admission. Of the study participants, 3 (2.1%) had a history of blood transfusion, 7 (4.8%) had a history of contact with jaundiced patients, 7 (4.8%) had a history of Uvulectomy, 10 (6.9%) had a history of frequent alcohol consumption, 11 (7.6%) had a history of dental extraction, and 14 (9.7%) had a history of surgery (Table 2).
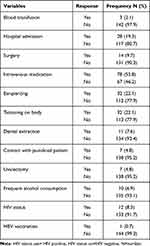 |
Table 2 Clinical and Behavioral Characteristics of TB Patients Attending Gondar Town Health Facilities in Northwest Ethiopia, January to May, 2019 (N= 145) |
Seroprevalence of Viral Hepatitis Markers
The overall prevalence of HBsAg was 5/145 (3.4%) (95% CI; 0.7 to 6.9). In our study, all the study participants tested negative for anti-HCV antibody. Though the association is not significant (p=0.142), the proportion of HBsAg positive cases was higher (3 (6.8%)) in the older age groups (greater than 34 years) when compared with the younger age groups (18 to 34 years) which was 2 (2.0%). Likewise, the proportion of HBsAg positive cases was higher among females than among males (2 (3.6%) vs 3 (3.4%)). Moreover, the prevalence of HBsAg was higher among urban residents, which was 4 (3.9%) compared to rural residents which was 1 (2.3%) (P = 0.534). The proportion of HBV infection among the literate study participants was 4 (4.0%), which was higher when compared to the illiterate (1 (2.2%)). Furthermore, sero-positivity of HBsAg was lower among unemployed study participants (2 (1.8%)) when compared to the employed (3 (9.7%)) study participants. Using Fisher’s Exact test, history of hospital admission (P=0.005), tattooing on the body (P=0.009) and dental extraction (P=0.003) were independent predictors for HBV infection (Table 3).
Discussion
Viral hepatitis is a serious global health problem. Liver infection, which is caused by Hepatitis B and C viruses, is increasing rapidly in the general population, particularly in TB patients. The directly observed therapy strategy (DOTS) is an effective way to treat patients with active TB.37 However, drug-induced hepatotoxicity (DIH) is one of the most frequent and serious side effects. Three of the first-line anti-TB agents: isoniazid (INH), rifampin (RIF), and pyrazinamide (PZA) are known for the development of DIH.38 Hepatotoxicity ptdevelopment during anti-TB treatment can be compounded by HCV and HBV infections.19,39,40 As a result, patients on anti-TB therapy with chronic HBV co-infection are more susceptible to developing liver failure and have poor outcomes during TB treatment.27 In view of that, the proportion of TB patients with viral hepatitis infection and who had died before TB treatment completion was higher than of TB patients without a viral hepatitis report (21% vs 9%).41
The HBsAg protein is the main marker indicating the presence or absence as well as endemicity of HBV infection in the general population of particular geographical areas. On the basis of the carrier rate of this marker, WHO categorized countries in to 3 regions of high (>8%), intermediate (2–7%) and low endemicity (<2%).42 Ethiopia is one of the sub-Saharan countries, which were labelled medium to high burden countries in the previous population-based study.24
The overall prevalence of HBV infection in this study was 3.4% and none of the participants was found to be positive for anti-HCV antibody. The seropositivity of HBV found in this study was relatively similar to the results reported in studies from London (2.6%),43 Georgia (4.3%),38 and Pakistan (5.5%).44 However, the prevalence of HBV in this study was higher than in the report from Iraq (1.8%).45 The reason for this discrepancy might be the difference in study subjects and/or the difference in local area HBV endemicity. For example, the UK has a low burden of HBV, but there is a high rate of HBV prevalence among migrant individuals living in the UK but were born abroad in countries, with intermediate (2% to –7%) or high HBV prevalence (≥8%) as defined by the WHO, such as China or Pakistan.46 Similarly, the rates of TB in non-United Kingdom born are approximately twenty-fold higher than those born in the UK.47 In the case of Georgia and Pakistan, the endemicity of HBV is intermediate and intermediate to high, respectively.48,49
On the other hand, the prevalence rate of HBV in our study was lower than the reports in Sudan (9.5%),30 (15.3%),20 Nigeria (14.9%)50 and Brazil (14.6%).19 This variation could be attributed to differences in the characteristics of the study population, geographical differences in prevalence and risk factors, sample size, sampling technique, and diagnostic techniques (kits and reagents) used, for advancement of diagnostic techniques makes a difference. The ELISA test used in this study to detect HBsAg is more specific and reliable than the immunochromatographic techniques used in the study conducted in Sudan. However, the study in Brazil used PCR for HBV DNA detection.
In this study, although HBV and HIV co-infection was shown in only one patient, it was found imperative to manage and monitor all patients with this condition. Despite the poor investigation in low resource countries like Ethiopia, there are studies that show the prevalence of high risk of hepatotoxicity among TB/HIV and HBV co-infected patients. The reports showed that the incidence of abnormal liver function tests in HIV-positive TB patients was higher than in HIV-negative TB patients (10.2% vs 9.9%). There was no statistical difference between them. However, HIV-positive TB patients had a higher incidence of DIH than HIV-negative TB patients (4.2% vs 1.0%, OR 4.348, 95% CI 1.935–9.769, p = 0.000). The incidences of abnormal liver function tests and DIH in the HIV/TB/HBV group were much higher than those in the HIV/TB group (40.7% vs 11.1%, OR 5.525, 95% CI 2.325–13.131, p = 0.000; 18.5% vs 2.5%, OR 9.015, 95% CI 2.545–31.930, p = 0.000). The incidence of abnormal liver function tests in the HIV/TB/HCV group was much higher than in the HIV/TB group (20.0% vs 11.1%, OR 2.009, 95% CI 1.057–3.820, p = 0.031), according to a study from China.40 Generally, the risk of developing DIH is higher among HIV-positive TB patients than among HIV-negative TB patients, which is evidenced by different studies.51–53 This is due to the fact that HIV-positive patients use different drugs for treatment of opportunistic infections related to AIDS or prophylaxis.
Out of all the study participants, 28 (19.3%) responded that they had a previous history of hospital admission, of which 4 (14.3%) were found to be positive for HBsAg, ie, a statistically significant association was observed between previous history of hospital admission and HBV infection (P = 0.005). Participants might acquire HBV infection in a hospital environment where they may come into contact with highly infectious carriers. This implies that all admitted patients need to be screened for HBV at their first visit and regularly during their treatment and follow-up, and preventive measures should be described.
About thirty-two (22.1%) study participants responded that they had a previous history of tattooing on their bodies, of which 4 (12.5%) were found to be positive for HBsAg. Hence, a statistically significant association was observed between previous history of body tattooing and HBV infection (P = 0.009). The possible reason might be related to social customs or culture. The high tendency for body tattooing with crosses, sun patterns, or other religious and cultural symbols on their arms, hands, necks, foreheads, and legs in this district (Gondar) has contributed to the spread of HBV infection. Thus, intervention such as awareness creation or health education about the virus and its modes of transmission is needed in the study area.
In relation to dental extraction, 11 (7.6%) of the study participants responded that they had a previous history of dental extraction, of whom 3 (27.3%) were found to be positive for HBsAg. Thus, a statistically significant association was observed between previous history of dental extraction and HBV infection (P = 0.003). This might be due to non-adherence to guidelines for infection control and the use of non-disposable equipment and the lack of sufficient sterilization technology.
In the present study, age, gender, marital status, area of residence, educational level, occupation, history of blood transfusion, history of intravenous medication, history of surgery, Uvulectomy, history of contact with jaundiced person, ear-piercing, frequent alcohol consumption and HIV status were not identified as statistically significant predictors of HBV infection. Though this study generated important data on HBV and HCV infections among PTB patients, it has limitations. The seroprevalence of HBV was not confirmed by polymerase chain reaction (PCR) (ie, HBsAg positive samples for HBV DNA and HBsAg negative samples for occult HBV infection). However, the diagnostic method used in this study (ELISA) is more reliable, sensitive and specific compared to the immunochromatographic techniques most frequently used for detection of viral hepatitis in most resource-limited countries.
Conclusions
Despite the fact that anti-HCV antibodies were not detected, the prevalence of HBV was relatively high in tuberculosis patients. History of hospital admission, tattooing on the body, and dental extraction were independently associated factors for HBV infection. This study highlights the need for the introduction of routine screening of HBV and HCV markers for all TB patients before anti-TB treatment so as to better manage patients. Further epidemiological and clinical studies with advanced diagnostic techniques like PCR are needed for a better understanding of the HBV and HCV infections’ effect on anti-TB treatment outcomes and to optimize the management of these clinical conditions.
Abbreviations
DILI, Drug induced liver injury; DIH, Drug induced hepatotoxicity; DNA, Deoxyribonucleic acid; HBsAg, Hepatitis B surface Antigen; HBV, Hepatitis B Virus; HCC, Hepatocellular Carcinoma; HCV, Hepatitis C Virus; HIV, Human immunodeficiency virus; PTB, pulmonary TB; SOPS, Standard Operating Procedures; SPSS, Statistical packages for social sciences; TB, Tuberculosis; WHO, World Health Organization.
Data Sharing Statement
All data generated and analysed during this study were included in the manuscript.
Acknowledgments
We thank the study subjects, data collectors in all DOTS clinics of the health facilities in Gondar town for their participation and the University of Gondar for funding this research work. Finally, we would like to extend our thanks to the department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, for the opportunity it created for us to conduct this research work.
Author Contributions
All authors have made a significant contribution to this study, all the way through the conception, study design, execution, acquisition of data, data analysis and interpretation to drafting, revising or critically reviewing stages of the article. The authors also gave final approval of the version to be published, agreed on the journal to which the article has been submitted, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Ayele AG, Gebre-Selassie S. Prevalence and risk factors of hepatitis B and hepatitis C virus infections among patients with chronic liver diseases in public hospitals in Addis Ababa, Ethiopia. ISRN Trop Med. 2013;2013:1–7. doi:10.1155/2013/563821
2. Lemoine M, Eholié S, Lacombe K. Reducing the neglected burden of viral hepatitis in Africa: strategies for a global approach. J Hepatol. 2015;62(2):469–476. doi:10.1016/j.jhep.2014.10.008
3. Ryan KJ, Ray CG, Sherris JC. Sherris Medical Microbiology: An Introduction to Infectious Diseases. New York: McGraw-Hill; 2004.
4. Pommerville JC, Alcamo IE. Alcamo’s Fundamentals of Microbiology. Sudbury, Mass.: Jones and Bartlett; 2011.
5. Harvey RA. Microbiology. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007.
6. Kayser FH. Medical microbiology. Stuttgart; New York, NY: Georg Thieme Verlag; 2005.
7. Carter JB, Saunders VA. Virology: Principles and Applications. Chichester, England; Hoboken, NJ: John Wiley & Sons; 2007:358.
8. Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–1088. doi:10.1016/S0140-6736(16)30579-7
9. Hajarizadeh B, Razavi-Shearer D, Merat S, Alavian SM, Malekzadeh R, Razavi H. Liver disease burden of hepatitis C virus infection in Iran and the potential impact of various treatment strategies on the disease burden. Hepat Mon. 2016;16(7):e15641. doi:10.5812/hepatmon.37234
10. Chhatwal J, Wang X, Ayer T, et al. Hepatitis C disease burden in the United States in the era of oral direct-acting antivirals. Hepatology. 2016;64(5):1442–1450. doi:10.1002/hep.28571
11. Wedemeyer H, Dore GJ, Ward JW. Estimates on HCV disease burden worldwide - filling the gaps. J Viral Hepat. 2015;22:1–5. doi:10.1111/jvh.12371
12. Younossi Z, Brown A, Buti M, et al. Impact of eradicating hepatitis C virus on the work productivity of chronic hepatitis C (CH-C) patients: an economic model from five European countries. J Viral Hepat. 2016;23(3):217–226. doi:10.1111/jvh.12483
13. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599–1608. doi:10.1053/j.gastro.2016.02.039
14. Organisation mondiale de la santé, editor. Global Tuberculosis Report 2017. Geneva: World health organization; 2017.
15. Silva DR, Muñoz-Torrico M, Duarte R, et al. Risk factors for tuberculosis: diabetes, smoking, alcohol use, and the use of other drugs. J Bras Pneumol. 2018;44(2):145–152. doi:10.1590/s1806-37562017000000443
16. Brooks GF. Jawetz, Melnick & Adelberg’s Medical Microbiology. New York; London: McGraw-Hill Medical; 2007.
17. Sharifzadeh M, Rasoulinejad M, Valipour F, Nouraie M, Vaziri S. Evaluation of patient-related factors associated with causality, preventability, predictability and severity of hepatotoxicity during antituberclosis treatment. Pharmacol Res. 2005;51(4):353–358. doi:10.1016/j.phrs.2004.10.009
18. Blumberg HM, Burman WJ, Chaisson RE, Daley CL. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–662.
19. Aires R, Matos M, Lopes C, et al. Prevalence of hepatitis B virus infection among tuberculosis patients with or without HIV in Goiânia City, Brazil. J Clin Virol. 2012;54(4):327–331. doi:10.1016/j.jcv.2012.04.006
20. Abdallah TM, Idriss MI, Ahmed AM, Ali -A-A-A, Saeed OK. Sero-prevalence of hepatitis B and hepatitis C viruses among tuberculosis patients in Kassala, Eastern Sudan. Glob J Infect Dis Clin Res. 2015;1(1):1–3. doi:10.17352/2455-5363.000001
21. Khalili H, Dashti KS, Rasoulinezhad M, Rezaei L, Etminani M. Anti-tuberculosis drugs related hepatotoxicity; incidence, risk factors, pattern of changes in liver enzymes and outcome; 2009.
22. Woldegiorgis AE, Erku W, Medhin G, Berhe N, Legesse M. Community-based sero-prevalence of hepatitis B and C infections in South Omo Zone, Southern Ethiopia. PLoS One. 2019;14(12):e0226890. doi:10.1371/journal.pone.0226890
23. Abera B, Adem Y, Yimer M, Mulu W, Zenebe Y, Mekonnen Z. Community seroprevalence of hepatitis B, C and human immunodeficiency virus in adult population in gojjam zones, northwest Ethiopia. Virol J. 2017;14(1):1–5. doi:10.1186/s12985-017-0696-6
24. Abebe A, Nokes D, Dejene A, Enquselassie F, Messele T, Cutts F. Seroepidemiology of hepatitis B virus in Addis Ababa, Ethiopia: transmission patterns and vaccine control. Epidemiol Infect. 2003;131(1):757–770. doi:10.1017/S0950268803008574
25. Kim WS, Lee SS, Lee CM, et al. Hepatitis C and not Hepatitis B virus is a risk factor for anti-tuberculosis drug induced liver injury. BMC Infect Dis. 2016;16(1):50. doi:10.1186/s12879-016-1344-2
26. Fernández-Villar A, Sopeña B, Fernández-Villar J, et al. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2004;8(12):1499–1505.
27. Chen L, Bao D, Gu L, et al. Co-infection with hepatitis B virus among tuberculosis patients is associated with poor outcomes during anti-tuberculosis treatment. BMC Infect Dis. 2018;18(1):1–10. doi:10.1186/s12879-018-3192-8
28. Wu P-H, Lin Y-T, Hsieh K-P, Chuang H-Y, Sheu -C-C. Hepatitis C virus infection is associated with an increased risk of active tuberculosis disease: a nationwide population-based study. Medicine. 2015;94(33):e1328. doi:10.1097/MD.0000000000001328
29. Population Census Commission. Summary and statistical report of the 2007 population and housing census. Population size by age and sex; 2008.
30. Nail AM, Ahmed NE, Gaddour MO. Seroprevalence of hepatitis B and C viruses among tuberculosis patients. Sudan J Med Sci. 2013;8(1):17–22.
31. Fissehatsion K, Ali I, Getachew A. Seroprevalence and risk factors of sexually transmitted infections (HIV, HBV and syphilis) among pregnant women provided health care services, Addis Ababa, Ethiopia. Am J Health Res. 2017;5(5):154–161.
32. Beijing Wantai Biological Pharmacy Enterprise Co. L. Diagnostic kit for hepatitis B virus Surface Antigen (ELISA); 2021. Available from: https://www.ystwt.cn/wp-content/uploads/2018/04/Wantai-HBsAg-ELISA.pdf.
33. Ethiopia Federal Minister of Health. Guidelines for Clinical and Programmatic Management of TB, Leprosy and TB/HIV in Ethiopia.
34. World Health Organization. Xpert MTB/RIF Implementation manual: technical and operational ‘How-To’; Practical considerations. World Health Organization; 2014. Report No.: 9241506709.
35. Federal Democratic Republic of Ethiopia Ministry of Health/ Ethiopian Public Heath Institute. Implementation Guideline for GeneXpert MTB/RIF Assay in Ethiopia. Federal Democratic Republic of Ethiopia Ministry of Health/ Ethiopian Public Heath Institute; 2014.
36. Federal Democratic Republic of Ethiopia Ministry of Health. National Guidelines for TB, Drug Resistant TB and Leprosy in Ethiopia.
37. Jasmer RM, Seaman CB, Gonzalez LC, Kawamura LM, Osmond DH, Daley CL. Tuberculosis treatment outcomes: directly observed therapy compared with self-administered therapy. Am J Respir Crit Care Med. 2004;170(5):561–566. doi:10.1164/rccm.200401-095OC
38. Lomtadze N, Kupreishvili L, Salakaia A, et al. Hepatitis C virus co-infection increases the risk of anti-tuberculosis drug-induced hepatotoxicity among patients with pulmonary tuberculosis. PLoS One. 2013;8(12):e83892. doi:10.1371/journal.pone.0083892
39. Agha MA, El-Mahalawy II, Seleem HM, Helwa MA. Prevalence of hepatitis C virus in patients with tuberculosis and its impact in the incidence of anti-tuberculosis drugs induced hepatotoxicity. Egypt J Chest Dis Tubercul. 2015;64(1):91–96. doi:10.1016/j.ejcdt.2014.09.009
40. Mo P, Zhu Q, Teter C, et al. Prevalence, drug-induced hepatotoxicity, and mortality among patients multi-infected with HIV, tuberculosis, and hepatitis virus. Int J Infect Dis. 2014;28:95–100. doi:10.1016/j.ijid.2014.06.020
41. Bushnell G, Stennis N, Drobnik A, et al. Characteristics and TB treatment outcomes in TB patients with viral hepatitis, New York City, 2000–2010. Epidemiol Infect. 2015;143(9):1972–1981. doi:10.1017/S0950268814002970
42. Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: epidemiology and prevention in developing countries. World J Hepatol. 2012;4(3):74. doi:10.4254/wjh.v4.i3.74
43. Nooredinvand HA, Connell DW, Asgheddi M, et al. Viral hepatitis prevalence in patients with active and latent tuberculosis. World J Gastroenterol. 2015;21(29):8920. doi:10.3748/wjg.v21.i29.8920
44. Akhtar JAJ, Qamar Mu Sa, Anwar J. Sero-prevalence of HBV and HCV in tuberculosis patients at Sheikh Zayed hospital Rahim Yar khan, Pakistan. Biomedica. 2013;29(2):69–72.
45. Merza MA, Haji SM, Alsharafani AMH, Muhammed SU. Low prevalence of hepatitis B and C among tuberculosis patients in Duhok Province, Kurdistan: are HBsAg and anti-HCV prerequisite screening parameters in tuberculosis control program? Int J Mycobacteriol. 2016;5(3):313–317. doi:10.1016/j.ijmyco.2016.06.019
46. Martin NK, Vickerman P, Khakoo S, et al. Chronic hepatitis B virus case-finding in UK populations born abroad in intermediate or high endemicity countries: an economic evaluation. BMJ Open. 2019;9(6):e030183. doi:10.1136/bmjopen-2019-030183
47. Health Protection Agency Centre for Infections. Tuberculosis in the UK: Annual Report on Tuberculosis Surveillance in the UK, 2010. London: Health Protection Agency Centre for Infections; 2010.
48. Ali SA, Donahue RM, Qureshi H, Vermund SH. Hepatitis B and hepatitis C in Pakistan: prevalence and risk factors. Int J Infect Dis. 2009;13(1):9–19. doi:10.1016/j.ijid.2008.06.019
49. Kasradze A, Kuchukhidze G, Baliashvili D, et al. Prevalence and risk factors for hepatitis B infection in the adult population of Georgia: a nationwide survey. J Hepatol. 2017;66(1):S468–S469. doi:10.1016/S0168-8278(17)31324-7
50. Imoru M, Bala A, Marafa A. Prevalence rates of HIV, HBsAg and HCV co-infections among tuberculosis patients in Sokoto Metropolis, Northwest Nigeria. Sri Lankan J Infect Dis. 2018;8(2):84. doi:10.4038/sljid.v8i2.8219
51. Ungo JR, Jones D, Ashkin D, et al. Antituberculosis drug–induced hepatotoxicity: the role of hepatitis C virus and the human immunodeficiency virus. Am J Respir Crit Care Med. 1998;157(6):1871–1876. doi:10.1164/ajrccm.157.6.9711039
52. Pedral-Sampaio DB, Alves CR, Netto EM, Brites C, Oliveira AS, Badaro R. Efficacy and safety of efavirenz in HIV patients on rifampin for tuberculosis. Braz J Infect Dis. 2004;8:211–216. doi:10.1590/S1413-86702004000300004
53. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167(11):1472–1477. doi:10.1164/rccm.200206-626OC
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.