Back to Journals » International Journal of General Medicine » Volume 16
Serine/Threonine Protein Kinase-3 Promotes Oral Squamous Cell Carcinoma by Activating Ras-MAPK Mediated Cell Cycle Progression
Received 28 March 2023
Accepted for publication 6 July 2023
Published 21 July 2023 Volume 2023:16 Pages 3115—3124
DOI https://doi.org/10.2147/IJGM.S412155
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Li Yue,* Yuedi Xu,* Ping Lu
Department of Stomatology, Liaocheng People’s Hospital, Liaocheng, Shandong, 252000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ping Lu, Department of Stomatology, Liaocheng People’s Hospital, Liaocheng, Shandong, 252000, People’s Republic of China, Tel +86 17763517077, Email [email protected]
Purpose: Serine/threonine protein kinase-3 (STK3) is a key molecule in the Hippo pathway, but its biological function in the development of oral squamous cell carcinoma (OSCC) remains unclear, we explored the roles of STK3 in OSCC.
Methods: In this study, GEPIA was used to analyse STK3 expression in different types of tumor patients. OSCC patients were then collected from Liaocheng People’s Hospital (Shandong, China), to further detect STK3 expression by qRT-PCR and Western blotting. To explore the function of STK3, overexpression and knockdown experiment were designed. Cell proliferation, migration and invasion were analyzed.
Results: First, STK3 is significantly up-regulated in OSCC patients, and high STK3 expression is associated with poor prognosis. Then, in vitro cell proliferation, migration, and invasion tests were used to determine the role of STK3. STK3 overexpression significantly promoted the proliferation, migration and invasion of OSCC cells. The downregulation of STK3 inhibited the proliferation, migration and invasion of OSCC cells. Finally, STK3 was demonstrated to promote oral squamous cell carcinoma by activating Ras-MAPK mediated cell cycle progression.
Conclusion: The results showed that STK3 was a potential cancer promoter for OSCC. It plays an important role in promoting the progression of oral squamous cell carcinoma. Inhibition of STK3 may prove beneficial as a therapeutic strategy for OSCC treatment.
Keywords: oral squamous cell carcinoma cancer, OSCC, serine/threonine protein kinase-3, STK3, proliferation, migration, invasion
Introduction
Oral squamous cell carcinoma (OSCC) is a very common tumor in the all of the world, with approximately 600,000 new cases diagnosed each year.1,2 This tumor is characterized by strong local aggressiveness, high incidence of tumor recurrence and metastasis (18–76% at 14 months), and low survival rate (42% at 3 years).3 OSCC is a complex and heterogeneous disease with multiple etiologies associated with tumor development.4–6 The poor survival of HNSC patients is because of failure in the early detection of this disease. The improvement of survival rates in HNSC patients largely depends on the early diagnosis of this disease.
Serine/threonine kinase 3 (STK3) is one of a key regulator in the Hippo pathway, controlling cell development, proliferation, apoptosis and various stress responses.7 The Hippo pathway was first discovered in fruit flies through a genetic screen designed to identify pathways that regulate tissue overgrowth.8–10 The typical core Hippo pathway consists of two histone kinases, two scaffold proteins, two coregulatory factors and a family of transcription factors. When the Hippo pathway is activated, these signaling elements act in tandem: MST1 and MST2 (also known as STK4 and STK3; When the Hippo pathway is inactivated, YAP1/TAZ is hypo-phosphorylated and can enter the nucleus, interact with TEAD (Sd) family transcription factors, and promote the expression of genes associated with cell survival and proliferation. Recently, the mammalian Hippo pathway has been shown to include several LatS1/2-activated kinases, such as MAP4Ks and TAOKs, which can act in parallel with MST1/2, implying added complexity in higher eukaryotes.11,12 The specific kinase that phosphorylates LATS1/2 depends on the nature of the stimulus and cell type.11,13 For example, in HEK293 cells, MST1/2 represents the major LATS1/2-activating kinase under osmotic stress and heat shock conditions.13,14 However, MAP4Ks and/or TAOKs appear to play an equal or greater role in serum starvation and contact suppression conditions.11,12 STK3 inhibits the progression of gastric cancer, hepatocellular carcinoma and breast cancer by activating Hippo signaling pathway.15–17 STK3 also modulates the immune system during infection.18 However, the role of STK3 in oral squamous cell carcinoma remains unclear.
In our research, we explored the role of STK3 in the progression of oral squamous cell carcinoma, and evaluated the role of STK3 as a potential new therapeutic target for oral squamous cell carcinoma.
Materials and Methods
OSCC Patient
The study was conducted according to the ethical guidelines of the Declaration of Helsinki. And patients in the current study were approved by the institutional ethics committee of the Liaocheng People’s Hospital (No. 2020(35), July 10, 2020). Informed consent was obtained from each patient.
Bioinformatic Analysis
Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia.cancer-pku.cn/index.html) was used to analyze STK3 expression in various tumor and normal samples. And the survivor ship curve was produced by Kaplan-Meier Plotter (KM plotter, http://kmplot.com/).
Cell Culture
Human squamous cell carcinoma (HSC-3) cells and mouse oral squamous cell carcinoma (MOC1) cells were derived from the cell bank of Shanghai Institute for Biological Studies, China. The cells were cultured in DMEM medium (True line, Kaukauna, WI, USA) with 10% FBS (Thermo Fisher Scientific), 1% penicillin/streptomycin (Solarbio, Beijing, P.R. China) and 2 mM L-glutamine. The cells were cultured in a 5% CO2, 37°C CO2 incubator.
RNA Isolation and qRT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Waltham, MA, USA). Reverse transcription was carried out using cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA) as instructed by the manufacturer. RT-PCR was conducted as follows: 95°C for 10 min then 40 cycles of 95°C for 15s, 60°C for 45s. The 2−ΔΔCt method was used to calculate relative gene expression, and GAPDH was used for normalization. Data represent average of three replicates. Primers used in this study were as follows: STK3 F 5’-ATGGCTCCTGAGGTGATT−3’, R 5-GTTGGTGGTGGATTTGTG−3; GAPDH F 5’-AAT CCCATCACCATCTTC-3’, R 5’-AGGCTGTTGTCATAC TTC-3’.
Western Blot
Full protein lysates were extracted cancer and paracancer tissue samples from OSCC patients using RIPA lysis buffer (JRDUN, Shanghai, China) and protease cocktail inhibitor (Roche, Heidelberg, Germany). Protein concentrations were tested with an enhanced BCA protein detection kit (Thermo Fisher Scientific). Twenty-five micrograms protein was isolated on 10% SDS-PAGE glue. It was transferred to nitrocellutrifiers membrane (Millipore, Billerica, MA, USA) and closed with 2% BSA for 2 h. It was then tested overnight at 4°C with primary antibody. PBST was washed three times, and then the second antibody was incubated (1:5000; Beyotime, Shanghai, China) at 37°C for 1 hour. Protein signal was detected by an enhanced chemiluminescence system (Tanon, Shanghai, P.R. China). The STK3 (ab52641) antibody was purchased from Abcam. GAPDH (5174) antibody was purchased from Cell Signaling Technology.
Immunofluorescence Histochemistry
A total of 5×104 cells were placed in a 24-well plate with pre-placed sliver, and transfected for 24 h. Cells were fixed in 4% (v/v) formaldehyde at 4°C for 30 min. And then it’s closed with 3% BSA. Rabbit primary antibody STK3 (Abcam, USA) was incubated overnight and then washed three times with PBST. The second antibody, Alexa Fluor®555 Donkey Anti-Rabbit IgG (H+L) (Invitrogen, Carlsbad, CA), was treated for 1h, and STK3 expression after transfection was detected. DAPI is used to stain the nucleus. The tablets were then sealed with an anti-fluorescence quencher (Beyotime, China). The sections were observed under fluorescence microscope (DMI4000B; Leica).
Knockdown and Overexpression
Short interfering RNAs targeting human STK3 (si STK3-1, 5’-ACTGTAATCAGAACATGCAT-3’; si STK3-2, 5’-ATGGCTCCTGAGGTGATT-3’; And si STK3-3, 5’-ACCCTTCCCTATGTCCAA-3’) and negative control siRNA (siNC, 5’-CGG AGTAATGATCAGGGCATGTGCT-3’) were synthesized by Shanghai Shenggong Biotech of China. For the overexpression study, the full-length CDS region of STK3 (NM_006281) was cloned into the overexpression plasmid pA4-EGFP, named pA4-STK3-EGFP. Blank plasmid was used as negative control (oeNC).
Cell Transfection
Lipofectamine 2000 was used to transfect plasmids into HSC-3 and MOC1 cells following the manufacturer’s instruction, and cells were cultured for 48 h prior to analysis.
Cell Proliferation
Cell proliferation was assessed using Cell Counting Kit-8 (CCK-8) assay (Signalway Antibody, Maryland, USA). Following 0, 12, 24, 48, and 72 h in culture, cells were incubated with CCK-8 solution (1:10) for 1 h. Quantification of cell proliferation was done on a microplate reader (Pulangxin, Beijing, P.R. China) and optical densities (ODs) at 450 nm wavelength were determined. Each experiment was conducted in triplicates.
Transwell Assay
Migration and invasion tests were conducted in the Transwell chamber (Corning, Inc. USA). The migration assay was performed with 1 × 104 cells per well. Cells were first starved serum-free for 24 h, then suspended in 200 μL serum-free RPMI-1640 medium and added to the upper cavity of transwell chamber. Five hundred microlitres RPMI-1640 medium containing 10% FBS was added into the inferior cavity. In the invasion test, the transwell chamber was first coated with a thin layer of matrix glue (cat. No. 356234; BD BioSciences). Then, 1 × 104 cells per well were added to the upper chamber and 500 μL of RPMI-1640 medium containing 10% FBS was added to the lower chamber.
The transwell chamber was incubated at 37°C for 48 h and fixed at room temperature with 4% formaldehyde for 15 min. After PBST cleaning, it was stained with 1% crystal purple solution for 5 min. After cleaning, drying, images were taken with an optical microscope (magnification, ×200).
Statistical Analysis
Statistical analysis was conducted using GraphPad Prism Software Version 7.0 (La Jolla, CA, USA). Data are shown as mean ± SD for at least three samples. Statistical analysis for multiple comparisons was done using one-way ANOVA. A p value of <0.05 was considered statistically significant.
Results
High Expression of STK3 in OSCC Clinical Samples Correlated with Poor Patient Prognosis
The expression level of STK3 in different tumors is different or even opposite. To investigate the expression level of STK3 in OSCC, we first analyzed STK3 protein expression in 31 kinds of tumor tissue expression data of clinical patients included in the GEPIA database. As shown in Figure 1A, the STK3 expression was significantly downregulated in Uterine Corpus Endometrial Carcinoma (UCEC), Pheochromocytoma and Paraganglioma (PCPG) and Testicular Germ Cell Tumors (TGCT) cancers. And the STK3 expression was significantly upregulated in six kinds of cancers, such as Cholangio carcinoma (CHOL), Pancreatic adenocarcinoma (PAAD), Stomach adenocarcinoma (STAD), Thymoma (THYM), Head and Neck squamous cell carcinoma (HNSC) and Glioblastoma multiforme (GBM) (Figure 1B and C). We also investigated whether the STK3 expression was correlated with prognosis in HNSC patients. In the GAPIA dataset, which included 499 samples of HNSC, the high STK3 expression was associated with poorer overall survival and progression-free survival prognosis (P value = 0.0022) (Figure 1D). As we known, HNSC is rather than a general cancer than OSCC. To confirm the result, then, we collected four OSCC patient in hospital to detect STK3 expression in OSCC. The qRT-PCR and Western blot result showed that STK3 is highly expression in OSCC cancer of patient rather than normal tissues (Figure 1E–G). These results showed that high expression of STK3 in OSCC cancer and correlated with poor patient prognosis.
Over Expression of STK3 Promotes the Proliferation of OSCC Cancer Cells
To further explore the role of STK3 in OSCC progression in vitro, we construction STK3 overexpression and knockdown system on the Human squamous cell carcinoma (HSC-3) and mouse oral squamous cell carcinoma (MOC1) cell line. Firstly, we detected STK3 mRNA transcript and protein expression by qRT-PCR and Western blot. The result showed that STK3 mRNA was downregulated by siRNA and overexpressed by specific overexpression vector in HSC-3 cell line (Figure 2A and B) and MOC1 cell line (Figure 2C and D). Then, we determined the effect of STK3 on the proliferation of OSCC in HSC-3 and MOC1 cell lines. The results showed that, compared with the control group, the proliferation of HSC-3 and MOC1 were significantly induced by overexpressing STK3, and reduced by knockdown of STK3 (Figure 2E and F).
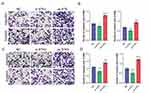
Over Expression of STK3 Improves the Migration and Invasion of OSCC Cancer Cells
Then, to analyze the function of STK3 in OSCC progression in in vitro, migration and invasion assay were carried out. As shown in the result, compared with the control group, the migration and the invasion of HSC-3 and MOC1 were significantly induced by overexpressing STK3, and reduced by knockdown of STK3 in HSC-3 cell line (Figure 3A and B) and in MOC1 cell line (Figure 3C and D). The result suggested that over expression of STK3 can induce OSCC migration and invasion. In contrast, downregulated of STK3 can inhibit OSCC migration and invasion.
STK3 Promoted Cells Proliferation and Invasion by Inducing RAS/MAPK Signal Pathway
It is reported that STK3 induce RAS/MAPK signal pathway to participate cell proliferation in gastric cancer, and colon Cancer.19,20 To identified the molecular mechanism of STK3 regulated OSCC cell proliferation, migration and invasion, we use qRT-PCR analysis relative genes transcript. AS shown in the result, RAS, RAF, ERK and MAPK genes transcript are all upregulated by STK3 overexpression. And these genes transcript are all downregulated by STK3 knockdown (Figure 4A–D). We also detected the biomarker gene of cell proliferation genes (Ki67 and PCNA) and cell migration/invasion biomarker genes (E-cadherin, Occludin and Vimentin) (Figure 4E–I). The data confirmed that STK3 can promote OSCC cell proliferation, migration and invasion, by inducing RAS/MAPK signal pathway.
Discussion
Recent studies have shown that key molecules of Hippo signal transduction pathway play an important role in inhibiting the occurrence and development of malignant tumors.21 Serine/threonine kinases (STKs) are key molecules in the Hippo pathway. STKs control organ growth and reduce tumor progression through their effect on Hippo pathway.22 Through bioinformatics analysis, this study found that STK3 expression level was high in oral squamous cell carcinoma tissues, and the high expression of STK3 was closely related to poor prognosis of ovarian cancer patients. As an important component of the Hippo pathway, STK3 is involved in the progression of many types of cancer. For example, the expression of STK3 is decreased in gastric cancer and lowest in tumors of patients with lymph node metastasis.23 Deletion of STK3 gene in hepatocytes also leads to the occurrence of hepatocellular carcinoma.24 However, STK3 is overexpressed in breast cancer tissues, promoting the proliferation of breast cancer cells and tumor progression.25 Increasing evidence suggests that STK3 expression may be regulated by genetic or epigenetic changes. For example, hypermethylation of the STK3 gene promoter region contributes to downregulation of STK3 expression in soft tissue sarcomas.26 However, this study found that in oral squamous cell carcinoma, the high expression of STK3 promoted the proliferation, migration and invasion of tumor cells. Therefore, we suggest that STK3 may be a potential target for oral squamous cell carcinoma.
The expression level of STK3 in different tumors is different or even opposite. Known reports have shown that STK3 expression is relatively low in glioblastoma, pancreatic cancer and non-small cell lung cancer tissues, and it can inhibit the progression of these tumors.27–29 However, it has been reported in liver cancer that the expression of STK3 in tumor tissues is higher than that in normal tissues, which can promote the occurrence of liver cancer.24 This implies that STK3 is expressed in different amounts in different types of tumors, and its role is different or even opposite. In this study, bioinformatics analysis was used to find that among the 31 kinds of tumor tissue expression data of clinical patients included in the GEPIA database, there were 6 kinds of tumors with significantly high expression of STK3 compared with normal tissues, 3 kinds with significantly low expression, and 22 kinds with no significant difference in expression. However, STK3 is significantly highly expressed in oral squamous cell carcinoma, and our study data further confirmed that the high expression of STK3 can promote the proliferation, migration and invasion of oral squamous cell carcinoma. This indicates that STK3 is an oncogenic gene in oral squamous cell carcinoma.
STK3 promotes oral squamous cell carcinoma proliferation and migration by RAS/RAF/MAPK signaling pathway. The Hippo pathway is highly conserved from Drosophila to mammals. Mammalian Hippo orthologs MST1/2 belong to the group II germinal center kinases. MST1/2 form heterodimers with SAV1 (Sav ortholog) through their C-terminal SARAH (sav/Rassf/Hpo) domains, and this interaction is required for MST1/2 to phosphorylate SAV1, MOB1 (Mats ortholog), and LATS1/2 kinase (Wts ortholog).30–32 LATS1/2 directly phosphorylate the Yki orthologs YAP (yes-associated protein) and TAZ (WW domain–containing transcription regulator protein 1) at multiple sites, thereby inhibiting their nuclear localization.33,34 Mechanistically, phosphorylated YAP/TAZ bind to 14–3–3 and are sequestered in the cytoplasm, resulting in YAP/TAZ inhibition. Further phosphorylation of YAP/TAZ by casein kinase 1 leads to β-TrCP–mediated ubiquitination and proteasomal degradation.33,35,36 However, the function and mechanism of MST3 is rarely little. It reports that STK3 expression is relatively low in glioblastoma, pancreatic cancer and non-small cell lung cancer tissues, and it can inhibit the progression of these tumors.27–29 In this study, we found that STK3, a novel oncogene in oral squamous cell carcinoma. It can promote cell proliferation through the MAPK signaling pathway mediated by RAS-RAF-MAPK axis. Our findings underscore the crucial roles of STK3 in oral squamous cell carcinoma growth and provide STK3 as a new target for developing targeting therapy of oral squamous cell carcinoma. The limitation of this study is that we have only 4 patient samples for analyze STK3 expression, which is a very small sample size. In our further study, we will collect more oral squamous cell carcinoma patient to confirm the results, and furtherly revealed the mechanism of STK3 in oral squamous cell carcinoma.
Conclusion
STK3 was upregulated in human oral squamous cell carcinoma and related to tumor stage. STK3 silencing could effectively slow down the growth rate of oral squamous cell carcinoma cells proliferation and accelerate cell apoptosis via activating MAPK signaling pathway mediated by RAS-RAF-MAPK axis. Overexpression of STK3 can promote cancer profession. This study provides evidence of a promising therapeutic target for patients with oral squamous cell carcinoma.
Acknowledgments
We thanked Dan Li, for her kindness guidance and for her very wonderful suggestions on the revision of the article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA. 2016;66(1):7–30. doi:10.3322/caac.21332
2. Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;2(26):123–141. doi:10.1016/j.coms.2014.01.001
3. Brands MT, Brennan PA, Verbeek ALM, Merkx MAW, Geurts SME. Follow-up after curative treatment for oral squamous cell carcinoma. A critical appraisal of the guidelines and a review of the literature. Eur J Surg Oncol. 2018;44(5):559–565. doi:10.1016/j.ejso.2018.01.004
4. Goldstein BY, Chang SC, Hashibe M, Vecchia CL, Zhang ZF. Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: an update. Eur J Cancer Prev. 2010;19(6):431–465. doi:10.1097/CEJ.0b013e32833d936d
5. Hashibe M. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. J Natl Cancer Inst. 2007;99(10):777–789. doi:10.1093/jnci/djk179
6. Brennan JA, Boyle JO, Koch WM, Steven K. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332(11):712–717. doi:10.1056/NEJM199503163321104
7. Thompson BJ, Sahai E. MST kinases in development and disease. J Cell Biol. 2015;210(6):871–882. doi:10.1083/jcb.201507005
8. Jia J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17(20):2514–2519. doi:10.1101/gad.1134003
9. Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121(4):1053–1063. doi:10.1242/dev.121.4.1053
10. Justice RW, Zilian O, Woods DF, Noll M, Bryant P. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9(5):534–546. doi:10.1101/gad.9.5.534
11. Steven W, Plouffe ZM, Kimberly C, Audrey W. Hong: characterization of hippo pathway components by gene inactivation - ScienceDirect. Mol Cell. 2016;64(5):993–1008. doi:10.1016/j.molcel.2016.10.034
12. Meng Z, Moroishi T, Mottier-Pavie V, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357–8366. doi:10.1038/ncomms9357
13. Hong AW, Meng Z, Plouffe SW, Lin Z, Zhang M, Guan KL. Critical roles of phosphoinositides and NF2 in Hippo pathway regulation. Genes Dev. 2020;1(34):511–525. doi:10.1101/gad.333435.119
14. Luo M, Meng Z, Moroishi T, et al. Heat stress activates YAP/TAZ to induce the heat shock transcriptome. Nat Cell Biol. 2020;22(12):1447–1459. doi:10.1038/s41556-020-00602-9
15. Zhang Y, Xin C, Qiu J, Wang Z. Essential oil from Pinus koraiensis pinecones inhibits gastric cancer cells via the HIPPO/YAP Signaling pathway. Molecules. 2019;24(21):3851–3857. doi:10.3390/molecules24213851
16. Park Y, Park J, Lee Y, et al. Lee YJJJoMM: mammalian MST2 kinase and human Salvador activate and reduce estrogen receptor alpha in the absence of ligand. J Mol Med. 2011;89(2):181–191. doi:10.1007/s00109-010-0698-y
17. Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16(5):425–438. doi:10.1016/j.ccr.2009.09.026
18. Shi H, Liu C, Tan H, et al. Hippo kinases Mst1 and Mst2 Sense and amplify IL-2R−STAT5 signaling in regulatory T cells to establish stable regulatory activity. Immunity. 2018;49(5):899–914. doi:10.1016/j.immuni.2018.10.010
19. Riffet M, Eid Y, Faisant M, et al. Deciphering promoter hypermethylation of genes encoding for RASSF/hippo pathway reveals the poor prognostic factor of RASSF2 gene silencing in colon cancers. Cancers. 2021;13(23):5975–5988. doi:10.3390/cancers13235957
20. Chen B, Chan W, Mui C, et al. STK3 promotes gastric carcinogenesis by activating Ras-MAPK mediated cell cycle progression and serves as an independent prognostic biomarker. Mol Cancer. 2021;20(1):147–155. doi:10.1186/s12943-021-01451-2
21. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–828. doi:10.1016/j.cell.2015.10.044
22. Rawat SJ, Chernoff J. Regulation of mammalian Ste20 (Mst) kinases. Trends Biochem Sci. 2015;40(3):149–156. doi:10.1016/j.tibs.2015.01.001
23. Yu FX, Meng Z, Plouffe SW, Guan KL. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol. 2014;77(1):201–227. doi:10.1146/annurev-physiol-021014-071733
24. Kim W, Khan SK, Gvozdenovic-Jeremic J, et al. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest. 2017;127(1):137–152. doi:10.1172/JCI88486
25. Zhou X, Wang S, Feng Z, Liu P, Lv X-B. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015;125(5):2123–2135. doi:10.1172/JCI79573
26. Seidel C, Schagdarsurengin U, Blümke K, Würl P, Dammann R. Frequent hypermethylation of MST1 and MST2 in soft tissue sarcoma. Mol Carcinog. 2010;46(10):865–871. doi:10.1002/mc.20317
27. Liu XL, Zuo R, Ou WB. The hippo pathway provides novel insights into lung cancer and mesothelioma treatment. J Cancer Res Clin Oncol. 2018;144(11):2097–2106. doi:10.1007/s00432-018-2727-0
28. Huang T, Kim CK, Alvarez AA, et al. MST4 phosphorylation of ATG4B regulates autophagic activity, tumorigenicity, and radioresistance in glioblastoma. Cancer Cell. 2017;32(6):840–855. doi:10.1016/j.ccell.2017.11.005
29. Erica JC, Evelina M, Daniele C, Bracci P. Steven gallinger ea: common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat Genet. 2015;47(8):911–916. doi:10.1038/ng.3341
30. Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18(5):311–321. doi:10.1016/j.cub.2008.02.006
31. Hergovich A, Schmitz D, Hemmings BA. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Biophys Res Commun. 2006;345(1):50–58. doi:10.1016/j.bbrc.2006.03.244
32. Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24(12):2076–2086. doi:10.1038/sj.onc.1208445
33. Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761. doi:10.1101/gad.1602907
34. Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421–434. doi:10.1016/j.cell.2005.06.007
35. Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF (beta-TRCP). Genes Dev. 2010;24(1):72–85. doi:10.1101/gad.1843810
36. Liu CY, Zha ZY, Zhou X, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFbeta-TrCP E3 ligase. J Biol Chem. 2010;285(48):37159–37169. doi:10.1074/jbc.M110.152942
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.