Back to Journals » Infection and Drug Resistance » Volume 15
Sequential Treatment by Antiviral Drugs Followed by Immunosuppressive Agents for COVID-19 Patients with Hematological Malignancy
Authors Seki M , Hashimoto K, Kondo N , Ohya Y, Kotajima F, Mitsutake K
Received 17 October 2022
Accepted for publication 29 November 2022
Published 5 December 2022 Volume 2022:15 Pages 7117—7124
DOI https://doi.org/10.2147/IDR.S393198
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Masafumi Seki,1,2 Kosuke Hashimoto,2 Nami Kondo,2 Yoshitaka Ohya,2 Futoshi Kotajima,2 Kotaro Mitsutake1,2
1Division of Infectious Diseases and Infection Control, International Medical Center, Saitama Medical University, Hidaka City, Saitama, Japan; 2COVID-19 Management Team, International Medical Center, Saitama Medical University, Hidaka City, Saitama, Japan
Correspondence: Masafumi Seki, Division of Infectious Diseases and Infection Control, International Medical Center, Saitama Medical University, Yamane 1397-1, Hidaka City, Saitama, 350-1298, Japan, Tel +81-42-984-4392, Fax +81-42-984-0280, Email [email protected]
Background: The detailed treatment regimen of COVID-19 patients with hematological malignancies has been unclear, and some fatalities have occurred, although combination therapy with antiviral agents and corticosteroids has been established for moderate to severe COVID-19 patients.
Case Series: Case 1 was a 57-year-old woman who had malignant lymphoma and received CHOP therapy with obinutuzumab, and case 2 was a 70-year-old-man who had myeloma and received molecular targeted therapy with weekly corticosteroid. In both cases, SARS-CoV-2 genes and antigens were detected from their nasal swabs, and treatment was started for moderate to severe COVID-19. Case 1 received antiviral agents with high doses of corticosteroids for a long term simultaneously, but the high titer of viral antigens in her nasal swabs persisted. Ground-glass opacities and interstitial shadows also worsened in both lungs, and she finally died on day 60. In contrast, in case 2, antiviral agents were started first, and restarted the immunosuppressive agents, such as gamma globulin and corticosteroids after no titer of SARS-CoV-2 antigens was confirmed. The patient survived, and his abnormal chest shadows showed gradual improvement. Both of the patients received two vaccinations, but showed the low antibody titers for SARS-CoV-2.
Conclusion: Administration of both antiviral agents and corticosteroids has been recommended for moderate to severe COVID-19 patients, but in patients with hematological malignancies, it might be better to use antiviral agents first to reduce the viral titers, and then add steroid and related immunosuppressive agents later appropriately to inhibit the excessive inflammatory state. The dose, timing, and order of the antivirals and immunosuppressive agents for COVID-19 should be considered carefully in the patients with hematological malignancies who showed low vaccine effectiveness.
Keywords: SARS-CoV-2, B cell depleting agents, corticosteroid, obinutuzumab, rituximab
Background
Coronavirus diseases 2019 (COVID-19) has been a significant issue, and treatment of SARS-CoV-2 infection, especially for moderate to severe immunosuppressed patients, may still be unclear.1,2 In the guideline for the management of COVID-19, simultaneous use of antiviral agents, such as remdesivir, and corticosteroids is recommended for moderate to severe hospitalized patients who require conventional oxygen and/or a high-flow nasal cannula (HFNC).3
However, two main processes may be thought to drive the pathogenesis of COVID-19. Early in the clinical course, the disease is primarily driven by the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and later in the clinical course, the disease appears to be driven by a dysregulated immune/inflammatory response to SARS-CoV-2 that leads to tissue damage.2,4 Based on this understanding, therapies that directly target SARS-CoV-2 are anticipated to have the greatest effect early in the course of the disease, whereas immunosuppressive/anti-inflammatory therapies are likely to be more beneficial in the later stages of COVID-19.4 Furthermore, when COVID-19 patients have received immunosuppressive drugs and/or are in an immunosuppressed state, clinicians should consider factors such as the underlying disease including hematological malignancies, the specific immunosuppressants being used, the potential for drug-drug interactions, and exacerbation of COVID-19.5
In hematological malignancies, the patients were considered at high risk of mortality from COVID-19, and large-scale surveillance has confirmed that COVID-19 patients with hematological malignancies were actually at high risk of lethal complications.6 However, improved COVID-19 prevention has reduced mortality despite an increase in the number of reported cases.7,8 Furthermore, it is reported that overall mortality rate by SARS-CoV-2 infection decreased according to vaccination status in the hematological patients those received targeted drugs, such as Bruton's kinase inhibitors, anti-CD20 other than rituximab, BCL2 inhibitors, and lenalidomide although detailed treatment regimens for COVID-19 were still unclear.9
In this report, two COVID-19 cases are presented. Both patients had hematological malignancies and a history of having recently received immunosuppressive therapy. They showed ground-glass opacities (GGOs) in both lung fields and needed supplemental oxygen on admission to our university hospital. The first case has received remdesivir and corticosteroids simultaneously for a long term, and the latter case received antiviral agents first, and then restarted corticosteroid therapy sequentially after confirmation of a viral antigen decrease. The latter case survived, whereas, unfortunately, the former case died.
These cases and the related study were approved as #2022-032 by the Institutional Review Board of Saitama Medical University International Medical Center on July 06, 2022 and registered as UMIN000047691, and the patients whose specimens were used provided written, informed consent to have their case details and any accompanying images published.
Case Report
Case 1
A 57-year-old woman with malignant lymphoma underwent chemotherapy, including CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisolone) with obinutuzumab as the B cell depleting agent in a tertiary hospital near our hospital for a year (Table 1). She had received two vaccinations for SARS-CoV-2 by BNT162b2 (Pfizer/BioNTech) seven and six months ago. She developed a sore throat on day −1 and fever on day 0. The polymerase chain reaction (PCR: by Cobas SARS-CoV-2, Roche, Basel, Switzerland) test for SARS-CoV-2 was positive on day 0. Two days later (day 2), her sore throat became much worse, and she was admitted to the tertiary hospital near our hospital. No shadows were found on chest computed tomography (CT) at day 2 in the tertiary hospital, and arterial oxygen saturation (SpO2) was 97% (normal), but the SARS-CoV-2 antigen (Ag) in the nasal swab showed a very high titer, >5000 IU (Cobas SARS-CoV-2 Ag, Roche, Basel, Switzerland). Therefore, she received neutral antibody therapy by sotrovimab (Glaxo-Smith Kline, London, UK) drop infusion. Her fever decreased, and she was discharged one week later, but she developed facial paralysis (Bell’s palsy) on day 12, and she had to start oral prednisolone therapy from 50 mg/day, with tapering by 5 mg every two days.
 |
Table 1 The Clinical Timeline of Case 1 and 2 |
On day 28, her fever increased again, and a dry cough appeared, although she had stayed at home. Her chest X-ray at a tertiary hospital near our hospital, showed interstitial shadows in both lung fields, and her SARS-CoV-2 Ag was positive again (3024 IU). She was diagnosed with moderate COVID-19 with pneumonia by reactivation of SARS-CoV-2, and she was readmitted to the tertiary hospital to receive antiviral therapy by remdesivir (Gilead, Foster City, CA, USA) drip infusion for five days. Four days later (day 32), dexamethasone 6 mg was started orally because her fever (>38°C) continued. Her fever decreased (<37°C), and her SARs-CoV-2 Ag was also decreased (126 IU) on day 34.
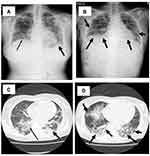
However, on day 40, her fever increased, and SpO2 decreased to less than 92% (room air), and supplemental oxygen (1 L by nasal cannula) was started. Her SARS-CoV-2 Ag also increased to 2237 IU again, and she was transferred to our university hospital. Laboratory data on admission at our university hospital were as follows: white blood cell (WBC) count, 1.49×103/μL, with 93.9% neutrophils, 0.7% lymphocytes, 5.4% monocytes, 0.0% eosinophils, and 0.0% basophils; platelet count, 72×103/μL; hemoglobin, 7.4 g/dL; blood urea nitrogen, 10.1 g/L; serum creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 23 U/L; alanine aminotransferase (ALT), 14 U/L; and C-reactive protein, 4.082 mg/dL. In addition, SARS-CoV-2 antibodies (Abs) were as follows: IgM, 0.1< (–) COI; IgG-spike protein, 49 IU/mL; and IgG-nucleocapsid protein 1.0<(–) (Table 2). Bone marrow suppression was suspected, and she was started on granulocyte-stimulating factor (G-CSF) therapy. Chest X-ray/CT showed massive GGOs in both lung fields (Figure 1A and C), although the SpO2 was 95% by nasal cannula 2 L, and SARS-CoV-2 Ag was increased to >5000 IU again at day 41.
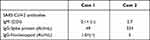 |
Table 2 The Antibody Titers for SARS-CoV-2 Nucleocapsid (N) and Spike (S) Proteins in Case 1 and 2 |
Molnupiravir (Merck, Darmstadt, Germany) 800 mg twice per day orally for five days, baricitinib 4 mg once per day orally, dexamethasone 6 mg per day intravenously, and gamma globulin 5 g per day intravenously were started. On day 45, the antigen level decreased to 2454 IU, but her chest X-ray worsened. Therefore, she was treated with methylprednisolone (mPSL) 500 mg per day intravenously for three days followed by mPSL 50 mg per day orally. One week later (day 52), SARS-CoV-2 Ag was increased to >5000 IU again, and she was treated with an HFNC because her chest X-ray/CT were also not improved (Figure 1B and D). She and her family refused mechanical ventilation, and she died on day 60 due to respiratory failure.
Case 2
A 74-year-old man with multiple myeloma underwent chemotherapy, including LD (lenalidomide and dexamethasone) therapy in the tertiary hospital (Table 1). He developed a fever and general malaise on day −1, and the PCR test for SARS-CoV-2 was positive. He had received two vaccinations for SARS-CoV-2 by BNT162b2 (Pfizer/BioNTech) six and five months ago.
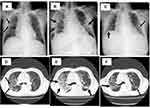
He stayed at home, but 10 days later (day 10), his condition did not improve, and cough and dyspnea had appeared. He was admitted to the tertiary hospital and transferred to our hospital. Laboratory data on admission at our hospital were as follows: white blood cell (WBC) count, 2.16×103/μL, with 68.9% neutrophils, 29.2% lymphocytes, 1.9% monocytes, 0.0% eosinophils, and 0.0% basophils; platelet count, 97×103/μL; hemoglobin, 8.8 g/dL; blood urea nitrogen, 17.4 g/L; serum creatinine, 0.80 mg/dL; aspartate aminotransferase (AST), 36 U/L; alanine aminotransferase (ALT), 59 U/L; and C-reactive protein, 17.35 mg/dL. In addition, SARS-CoV-2 Abs were as follows: IgM, 5.7 COI; IgG-spike protein, 334 IU/mL; and IgG-nucleocapsid protein 5 IU/mL (Table 1). Chest X-ray/CT showed massive GGOs in both lung fields (Figure 2A and D), although SpO2 was 94% by nasal cannula 3 L, and the SARS-CoV-2 Ag was increased to 217.0 IU.
Remdesivir 200 mg was started on day 10, followed by 100 mg per day intravenously for five days with dexamethasone 6 mg per day intravenously. However, on day 11, his respiratory status deteriorated, and HFNC had to be started at O2 60%, 40 L/h. It was suspected that the dexamethasone might have increased the viral titer rather than inhibited excessive inflammation, and then the dexamethasone was stopped. His condition did not change, but he continued the HFNC at the same setting. Gamma globulin 5 g per day intravenously was added for three days, with sotrovimab on day 12. His viral antigen titers decreased to 2.28 IU.
However, his chest X-ray/CT showed severe deterioration, and GGOs were increased on day 15 (Figure 2B and E). After confirmation that the Ag titer was almost negative, the dexamethasone was restarted at 6 mg intravenously. On day 20, because the chest X-ray/CT did not improve, the dexamethasone 6 mg was changed to mPSL 500 mg/day for three days. The SARS-CoV-2 Ag titer increased to 482 IU on day 23, and molnupiravir 800 mg twice per day orally was given for five days, along with sotrovimab again. The steroid was changed from mPSL 500 mg/day intravenously to prednisolone 30 mg/day orally and then gradually decreased by 5 mg/five days. On day 30, his chest X-ray and CT were improved (Figure 2C and F), and he could maintain SpO2 of 97% with nasal cannula O2 1 L. In addition, the SARS-CoV-2 Ag titer was only 1.36 IU on day 30, which suggested that negative viral status was maintained. Finally, he was discharged on day 40 without supplementary oxygen after receiving the tixagevimab 300 mg/ciligavimab 300 mg intramuscularly to prevent the reactivation of SARS-CoV-2.
Discussion
The patients with hematological malignancies have a high risk for infection due to immune compromise secondary to their underlying disease and subsequent therapy.5 The characteristics of SARS-CoV-2 infection and COVID-19 in the patients with hematological malignancies are not yet well known, but the underlying hematological disease could affect the inflammatory response and viral clearance and modify the manifestations and outcome of the disease, similar to viral infections such as RSV or influenza that are considered mild in immunocompetent hosts, but can become life-threatening in certain patients with hematological malignancies.10,11
In this case series, two COVID-19 patients with hematological malignancies who received systemic chemotherapy by specific regimens including obinutuzumab, such as a CD20+ B cell depleting agent, similar to rituximab, and corticosteroids were presented. Both cases became severe and needed supplementary oxygen because the COVID-19 progressed rapidly along with the increase of the viral antigen titers. These worse outcomes matched the previous reports that also suggested that COVID-19 patients with hematological diseases had a poor prognosis,5,12 and they also suggested that corticosteroids, tocilizumab, and anakinra treatment could be related to the higher mortality, compared with COVID-19 patients with hematological malignancies and non-hematological patients who survived.5 These results and reports suggested that these underlying diseases and anticancer therapies contribute to the worse outcomes of SARS-CoV-2 infection by lymphopenia and lymphocyte dysfunction, hypercoagulability, immunometabolic deregulation related to myeloid cell dysfunction, and converge in patients with hematological malignancies.5 Indeed, the two present cases showed worse clinical courses even though they received the appropriate regimen that combined antiviral drugs and anti-inflammatory drugs.
Lymphopenia and lymphocyte dysfunction were found, and the two patients might not have been able to clear the viral antigens and active SARS-CoV-2. In addition, we found much lower titers of IgG antibodies against SARS-CoV-2 nucleocapsid (N) and spike (S) proteins in case 1, compared with case 2 although both patients received two vaccinations (Table 2). Recent large-scale surveillance data suggested that the mortality of COVID-19 patients with hematological malignancies were not dependent on the targeted drugs use, but the vaccination status.6,7,9 These data might suggest that appropriate and correct use of immunosuppressive agents, rather than the avoid use of these agents, contributed to control the underlying diseases and COVID-19. Further study will be needed to clear the meaning of the immunosuppressive agents, including target drugs in the COVID-19 patients with hematological malignancies. The detailed regimen, such as dose, timing, and order of the agents, especially when combined with the antiviral agents and these immunosuppressive agents should be investigated in addition to the acquired higher antibody titers by booster vaccinations. The importance of the booster vaccinations, such as third and fourth vaccinations might become obviously clear.8
Furthermore, chemotherapy-induced neutropenia and monocytopenia might attenuate the hyperinflammatory response to the virus, whereas neutrophil recovery, treatment with G-CSF, or immunotherapy could enhance it.13 G-CSF was also used in case 1 to support the decline of viral activation, but administration of G-CSF might contribute to the reactivation and/or maintenance of a hyperinflammatory status. Fernandez-Cruz et al5 analyzed 71 COVID-19 cases with hematological diseases and showed that non-Hodgkin’s lymphoma was the most common (21 patients, 29.4%), followed by multiple myeloma (15 patients, 21.1%), similar to the present cases,14 and the careful use of G-CSF and other related immunological agents was suggested in these cancer patients and hematopoietic stem cell transplant recipients.13
Several series suggested that older age might contribute to the increased mortality of hematological patients.5,12,14 Age is an important prognostic factor in COVID-19, but in the present cases, case 1 was younger, but died, whereas in case 2, the patient who was over 70 years old survived. In addition, the majority of patients recovered from COVID-19, despite receiving recent systemic chemotherapy, and this suggests that systemic chemotherapy, where urgent, should be administered despite intercurrent COVID-19 infection, which should be managed according to standard pathways. However, delay or modification of therapy should be considered on an individual basis, but in the practical situation, physicians have hesitated and discontinued the systemic chemotherapy, similar to the present two cases. Age and continuous chemotherapy are important, but we should proceed with viral clearance first, and then try to control the excessive hyperimmunological reactions, inhibit the progression of the organized pneumonia and damage to the lungs, and maintain the patient's condition, including immunological status.
Of the two present cases, case 2 showed lower viral antigen titers, and treatment for viral suppression was given first, and then control of hyperimmune reactions and inhibition of lung damage progression was achieved by tapering corticosteroid therapy. Such sequential treatment should be considered for hematological patients with COVID-19. In contrast, case 1 showed higher viral antigen titers, and the viral antigen titers could not be controlled, although the hyperimmune responses decreased in the early stage. Antiviral drugs and corticosteroids were used simultaneously, but the simultaneous use of these two types of agents might have unfortunately been related to the poor outcome. In addition, the overdose and long-term use of the corticosteroids and vaccination without booster shots might be also affect the prognosis.
Conclusions
Two COVID-19 patients with hematological malignancies who progressed to severe disease were described. One case received simultaneous antiviral therapy and immunomodulatory therapy for a long term, but died, whereas the other patient received antiviral therapy followed by restart of the immunomodulatory therapy and survived. Sequential treatment aimed primarily to control viral antigen titers in the early stage followed by immunological control in the late stage should perhaps be considered for COVID-19 patients with hematological malignancies. The importance of the booster vaccinations was also suggested in these two patients. The regimens for COVID-19 have been established according to the severity and oxygenation levels, but the dose, timing, and order of the administration of recommended agents should be considered based on the host immunological condition and viral titers.
Acknowledgments
The authors would like to thank to all medical staff, including physicians, pharmacists, nurses, and medical clinical microbiological technicians for their kind support with COVID-19 management in Saitama Medical University International Medical Center.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi:10.1001/jama.2020.1585
2. Seki M. Trends in the management of infectious disease under SARS-CoV-2 era: from pathophysiological comparison of COVID-19 and influenza. World J Virol. 2021;10:62–68. doi:10.5501/wjv.v10.i2.62
3. Bhimraj A, Morgan R, Shumaker AH, et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa478
4. Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi:10.1136/bmj.m3379
5. Fernández-Cruz A, Puyuelo A, Núñez Martín-Buitrago L, et al. Higher mortality of hospitalized haematologic patients with COVID-19 compared to non-haematologic is driven by thrombotic complications and development of ARDS: an age-matched cohorts study. Clin Infect Pract. 2022;13:100137. doi:10.1016/j.clinpr.2022.100137
6. Pagano L, Salmanton-García J, Marchesi F, et al. COVID-19 infection in adult patients with hematological malignancies: a European hematology association survey (EPICOVIDEHA). J Hematol Oncol. 2021;14:168. doi:10.1186/s13045-021-01177-0
7. Pagano L, Salmanton-García J, Marchesi F, et al. COVID-19 in vaccinated adult patients with hematological malignancies: preliminary results from EPICOVIDEHA. Blood. 2022;139:1588–1592. doi:10.1182/blood.2021014124
8. Salmanton-García J, Marchesi F, Glenthøj A, et al. Improved clinical outcome of COVID-19 in hematologic malignancy patients receiving a fourth dose of Anti-SARS-CoV-2 vaccine: an EPICOVIDEHA report. Hemasphere. 2022;6:e789. doi:10.1097/HS9.0000000000000789
9. Infante MS, Salmanton-García J, Fernández-Cruz A, et al. B-cell malignancies treated with targeted drugs and SARS-CoV-2 infection: a European hematology association survey (EPICOVIDEHA). Front Oncol. 2022;12:992137. doi:10.3389/fonc.2022.992137
10. Kmeid J, Vanichanan J, Shah DP, et al. Outcomes of influenza infections in hematopoietic cell transplant recipients: application of an immunodeficiency scoring index. Biol Blood Marrow Transplant. 2016;22:542–548. doi:10.1016/j.bbmt.2015.11.015
11. Sheshadri A, Chemaly R, Alousi AM, et al. Pulmonary impairment after respiratory viral infections is associated with high mortality in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2019;25:800–809. doi:10.1016/j.bbmt.2018.11.022
12. Fox TA, Troy-Barnes E, Kirkwood AA, et al. Clinical outcomes and risk factors for severe COVID-19 in patients with haematological disorders receiving chemo- or immunotherapy. Br J Haematol. 2020;191:194–206. doi:10.1111/bjh.17027
13. Chamilos G, Lionakis M, Kontoyiannis DP. Are all patients with cancer at heightened risk for severe coronavirus disease 2019 (COVID-19)? Clin Infect Dis. 2021;72:351–356. doi:10.1093/cid/ciaa1079
14. García-Suárez J, de la Cruz J, Cedillo Á, et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;13:133. doi:10.1186/s13045-020-00970-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.