Back to Journals » Infection and Drug Resistance » Volume 16
Sequential Therapy of Linezolid and Contezolid to Treat Vancomycin-Resistant Enterococcus faecium Pneumonia in a Centenarian Patient: Case Report
Authors Chen P , An L, Zhang Z
Received 29 December 2022
Accepted for publication 6 March 2023
Published 18 March 2023 Volume 2023:16 Pages 1573—1578
DOI https://doi.org/10.2147/IDR.S401533
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Pengzhi Chen, Li An, Zhijian Zhang
Department of Respiratory and Critical Care Medicine, The Second Medical Center & National Clinical Research Center for Geriatric Disease, Chinese PLA General Hospital, Beijing, People’s Republic of China
Correspondence: Zhijian Zhang, Department of Respiratory and Critical Care Medicine, The Second Medical Center & National Clinical Research Center for Geriatric Disease, Chinese PLA General Hospital, Beijing, People’s Republic of China, Tel +86 17701090515, Email [email protected]
Abstract: Enterococcus faecium (E. faecium) is one of the core components of enterococci and causes serious illnesses in the elderly and immunocompromised patients. Due to its adaptive traits and antibiotic resistance, E. faecium has evolved as a worldwide hospital-associated pathogen, especially vancomsycin-resistant Enterococcus faecium (VREfm). Pneumonia caused by VREfm is quite rare in clinical settings, and optimal treatment has not yet been determined. Here, we present a case of nosocomial VREfm pneumonia with lung cavitation following adenovirus infection, which was successfully treated with linezolid and contezolid.
Keywords: vancomycin-resistant enterococcus, Enterococcus faecium, pneumonia, contezolid
Corrigendum for this paper has been published.
Introduction
Enterococci are hardy, facultatively anaerobic Gram-positive cocci and found in human gastrointestinal tract. In special conditions, they cause infections such as urinary tract infection, bacteremia, infective endocarditis, surgical wound infections,1 and rarely cause pneumonia.2–4 Among the Enterococcus species, Enterococcus faecium (E. faecium) has emerged as one of the most significant causes of hospital-acquired infections by multiple adaptive mechanisms that increase virulence and resistance in nosocomial setting.5 Thus, the treatment of multi-drug resistant E. faecium has presented a challenge in clinical practice.
Linezolid has long been the first choice oxazolidinone antibiotic against vancomycin-resistant enterococci (VRE). Nevertheless, the serious adverse effects of linezolid, especially thrombocytopenia, limit its application in elderly patients with multiple underlying diseases as well as severely ill patients.6,7 Contezolid is a novel oxazolidinone antibiotic designed for multidrug-resistant Gram-positive bacterial infections, including VRE.8 Furthermore, in a Phase III clinical trial, contezolid showed a lower tendency to alter platelet counts than linezolid, suggesting that it might be an appropriate alternative treatment for patients who are unable to tolerate linezolid.9 Here, we report a case of nosocomial vancomycin-resistant Enterococcus faecium (VREfm) pneumonia in a centenarian patient who was successfully treated by the sequential therapy of linezolid and contezolid.
Case Presentation
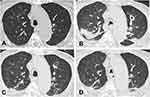
A 101-year-old male veteran was admitted to our hospital with a chief complaint of a 1-day history of cough, productive sputum, and wheezing without febrile. His medical history is significant for hypertension, coronary artery disease, old cerebral infarction, and diabetes mellitus. He had no history of recent travel to populated areas and had stayed at home for months. Initial laboratory studies revealed that WBC 9.28×109/L (neutrophils 80.4%, lymphocytes 11.3%); C-reactive protein (CRP) 2.67 mg/dl; Procalcitonin (PCT) <0.05 ng/mL. CT scan of the lung showed new patchy ground glass opacities in the right upper lung and right middle lobe (Figure 1A). The patient was diagnosed with community acquired pneumonia at admission and received regular infection treatment with flomoxef and levofloxacin (Figure 2).
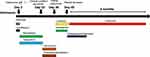 |
Figure 2 Antibiotics used in the patient and correlated changes with the progress of the clinical condition. Abbreviation: VREfm, Vancomycin-resistant Enterococcus faecium. |
On day 2 after admission, he developed a fever of 38.3°C. The serological tests showed adenovirus IgM positive twice, suggesting adenovirus as a probable pathogen for the patient’s right lung pneumonia. Considering that bacterial infections might also exist along with the viral infection, meropenem and tigecycline were prescribed for the patient (Figure 2). However, after 3 weeks of antibiotic treatment, there was no significant improvement in his body temperature and his clinical condition began to deteriorate with persistent fever, lethargy, thrombocytopenia, and elevated creatinine and transaminase levels, which was accompanied by a remarkable increase in PCT (peak at 1.78 ng/mL). As a result, antibiotic treatment was switched to vancomycin and piperacillin/tazobactam for 13 days, but the patient’s condition had not changed (Figure 2).
On day 36 after admission, the patient developed respiratory failure, and CT scan of the chest revealed new bilateral patchy infiltrates and a notable lung cavity (7 mm × 8 mm) in the anterior segment of the left upper lobe, though the primary ground glass opacity lesions in the right lung were resolved (Figure 1B). A bronchoscopy was then performed, during which the yellowish-white secretion almost blocked the lumen of the left main bronchus, and the mucosa was edematous and congested. Samples were collected from the anterior segment of the left upper lobe by bronchoalveolar lavage. Metagenomic next-generation sequencing (mNGS) was applied to identify pathogens in bronchoalveolar lavage fluid (BALF). Within 24 hours, mNGS analysis of the BALF revealed the presence of E. faecium with 10, 376 sequences (Table 1). The result of pathogen identification was subsequently confirmed by BALF culture. By use of the Kirby-Bauer disk method, the isolate was found to be resistant to vancomycin, which indicated VREfm, and sensitive to linezolid. Based on the drug susceptibility results, the patient began to receive linezolid (600 mg IV q12d). Following a 13-day course of linezolid, the patient’s clinical condition improved, but his platelets dropped sharply (from 425 ×109/L to 44 × 109/L). For this reason, linezolid was discontinued, and contezolid (400 mg PO q12d) was used as an alternative treatment for VREfm. Thrombopoietin was administered at the same time to elevate the level of platelets. After 10 days of taking contezolid, clinical improvement was noted in the patient’s condition with platelet counts returning back to normal. In addition, the cavitary lesion was also resolved, with decreased patchy infiltrations in the left upper lobe (Figure 1C). Following this, the patient received a nearly 4-month course of contezolid therapy during which contezolid was well tolerated and his platelet levels remained relatively stable. A follow-up CT scan performed after contezolid was discontinued revealed that the lung cavity lesion and patchy infiltrations on both sides of the lung had completely disappeared (Figure 1D).
 |
Table 1 The Microorganisms Detected by mNGS in the BALF from the Lung Cavity Lesion (The Complete Sequencing Results are Available in the Supplementary Material). |
Discussion
Enterococci are common flora of the gastrointestinal tract of humans.10 Some of the species, specifically E. faecalis and E. faecium, can cause a variety of life-threatening infections in humans, including endocarditis and bacteremia.1 Moreover, E. faecium has emerged as a worldwide healthcare-associated pathogen by adapting to hospital environments and building resistance to antibiotics.5,11 In numerous studies, VRE has been linked to high mortality rates and poses a serious threat to current health-care practices all over the world.5,12 Various factors contribute to VRE emergence, such as increased medical device use, prolonged hospitalization, and improper antibiotic treatment.12
These pathogenic Enterococcus species are thought to rarely cause respiratory illnesses.10,13,14 A study of the US military health system revealed that 577 patients were identified with VRE infections from 2009 to 2015. Among them, only 2.9% of VRE infections were located in the respiratory tract.15 A similar study in Osogbo, Nigeria revealed that during a 6-month period in 2009, only 9 out of 118 cases of hospital acquired enterococci infection were associated with respiratory tract infection.16 According to the current findings, Enterococcus species have been reported to cause sinusitis, infections of the trachea and bronchi, and pneumonia, especially in patients with ventilation, immune system impairment, cancer, old age, and patients receiving broad-spectrum antibiotics.2–4,17 Notably, there is a risk that Enterococcus pneumonia can progress to lung necrosis, abscess formation, and parenchymal cavitation, which can complicate the clinical condition and lead to sepsis and multiple organ failure.18–20 Our patient was also found to have a lung cavity lesion when his condition became severe and mNGS BALF from the lesion revealed the causative agent as E. faecium. Additionally, a subsequent drug resistance test indicated VREfm.
The emergence of vancomycin resistance isolates in our case may be related to the use of combined broad-spectrum antibiotics and the patient’s advanced age.12 The empirical use of broad-spectrum antibiotics is a common practice for elderly patients with multiple underlying diseases in hospitals, as it provides coverage for a wide range of potential pathogens. However, it also carries the risk of promoting the emergence of antibiotic-resistant bacteria, such as VRE.21,22 The isolation of VRE from the patient’s BALF indicates that the empirical use of different antibiotics did not effectively treat the underlying infection and may have contributed to the development of antibiotic resistance. Thus, health-care providers must balance the need to effectively treat infections with the potential risks of promoting antibiotic resistance.
As a classic oxazolidinone drug, linezolid exerts its antibiotic effect by inhibiting the initiation process of protein synthesis and plays a crucial role in the therapy of multidrug-resistant Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and VRE. Despite its high efficacy against Gram-positive infections, linezolid has been proven to have certain safety limitations, such as hematological effects.7 Thrombocytopenia is one of the most frequent hematological changes during linezolid treatment, the mechanism of which is uncertain.7 Previous studies reported that linezolid-induced platelet decrease was associated with bone marrow suppression. However, recent evidence has suggested the involvement of immune-mediated platelet destruction23 and abnormal cellular pathway regulation of megakaryocytes24 in this process.
Contezolid is a novel oxazolidinone antibiotic that replaces the morpholine in linezolid with a piperidinone.25 This drug has a significantly reduced risk of myelosuppression and has been proven to be less likely to cause abnormalities in platelet counts as compared to linezolid based on a phase III clinical trial26. It was approved by the National Medical Products Administration of China (NMPAC) in June 2021 to treat complicated skin and soft tissue infections (cSSTI), including, but not limited to, methicillin-susceptible S. aureus, MRSA, Streptococcus pyogenes and Streptococcus agalactiae.8 According to a recent study, contezolid exhibited considerable activity in vitro against VRE isolates.27 In addition to cSSTI, contezolid has also been found to be effective in lung infections, such as pulmonary tuberculosis.28,29 Based on our knowledge, this is the first published report of a patient with VREfm pneumonia who was treated successfully with contezolid.
Though the treatment of VREfm pneumonia has not been established, several reports suggested the necessity of prolonged antibiotic treatment, ranging from 3 to 5 weeks based on the radiological evaluation.18–20 In our case, the patient received contezolid 400 mg q12d for nearly 4 consecutive months. During this time period, we evaluated the patient’s CT scan every month and stopped the drug as soon as the lesions completely disappeared. The long-term treatment might result from the patient’s old age and degenerative conditions. It is also worth noting that the dose of contezolid we used on this patient is lower than the recommended dose of 800 mg q12d8 considering the patient’s advanced age and potential liver and renal impairments.
In conclusion, contezolid exhibited good tolerance and effective antibacterial activity in the treatment of VREfm pneumonia. Though linezolid has been considered as a standard-of-care treatment for VRE infections, contezolid can be utilized as an alternative when linezolid-induced thrombocytopenia occurs. However, randomized controlled trials still need to be conducted to determine the efficacy and safety of contezolid in treating VREfm-related lung infections.
Ethical Approval Statement
The studies involving human participants were reviewed and approved by The Second Medical Center, Chinese PLA general hospital. The patient’s spouse has provided written informed consent for the case details to be published.
Acknowledgments
The authors express their sincere gratitude to all the staff of the Department of Respiratory and Critical Care Medicine, The Second Medical Center of Chinese PLA General Hospital in Beijing, China.
Disclosure
The authors declare no conflict of interest. The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fiore E, Van Tyne D, Gilmore MS. Pathogenicity of enterococci. Microbiol Spectr. 2019;7(4):1. doi:10.1128/microbiolspec.GPP3-0053-2018
2. Li F, Wang Y, Sun L, Wang X. Vancomycin-resistant Enterococcus faecium pneumonia in a uremic patient on hemodialysis: a case report and review of the literature. BMC Infect Dis. 2020;20(1):167. doi:10.1186/s12879-020-4892-4
3. Grupper M, Kravtsov A, Potasman I. Enterococcal-associated lower respiratory tract infections: a case report and literature review. Infection. 2009;37(1):60–64. doi:10.1007/s15010-007-7123-7
4. Kimura Y, Kobayashi I. 溺水後にEnterococcus faeciumによる肺炎をきたした1例[A case of pneumonia due to Enterococcus faecium after near drowing]. Kansenshogaku Zasshi. 2011;85(4):380–383. Japanese. doi:10.11150/kansenshogakuzasshi.85.380
5. Zhou X, Willems RJL, Friedrich AW, Rossen JWA, Bathoorn E. Enterococcus faecium: from microbiological insights to practical recommendations for infection control and diagnostics. Antimicrob Resist Infect Control. 2020;9(1):130. doi:10.1186/s13756-020-00770-1
6. Bi LQ, Zhou J, Huang M, Zhou SM. Efficacy of linezolid on gram-positive bacterial infection in elderly patients and the risk factors associated with thrombocytopenia. Pak J Med Sci. 2013;29(3):837–842. doi:10.12669/pjms.293.2925
7. Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. J Infect. 2009;59(Suppl 1):S59–74. doi:10.1016/s0163-4453(09)60009-8
8. Hoy SM. Contezolid: first approval. Drugs. 2021;81(13):1587–1591. doi:10.1007/s40265-021-01576-0
9. MicuRx Pharmaceuticals, Inc. MicuRx reports positive top-line results of a China Phase 3 clinical trial for novel antibiotic contezolid in complicated skin and soft tissue infections. MicuRx Pharmaceuticals; 2019.
10. Agudelo Higuita NI, Huycke MM. Enterococcal disease, epidemiology, and implications for treatment. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Massachusetts Eye and Ear Infirmary; 2014.
11. Alduhaidhawi AHM, AlHuchaimi SN, Al-Mayah TA, et al. Prevalence of CRISPR-cas systems and their possible association with antibiotic resistance in Enterococcus faecalis and Enterococcus faecium collected from hospital wastewater. Infect Drug Resist. 2022;15:1143–1154. doi:10.2147/idr.S358248
12. Raza T, Ullah SR, Mehmood K, Andleeb S. Vancomycin resistant Enterococci: a brief review. J Pak Med Assoc. 2018;68(5):768–772.
13. Patterson JE, Sweeney AH, Simms M, et al. An analysis of 110 serious enterococcal infections epidemiology, antibiotic susceptibility, and outcome. Medicine. 1995;74(4):191–200.
14. Levitus M, Rewane A, Perera TB. Vancomycin-resistant enterococci. In: StatPearls. StatPearls Publishing; 2022.
15. Stagliano DR, Susi A, Adams DJ, Nylund CM; Lt Col. Epidemiology and outcomes of vancomycin-resistant enterococcus infections in the U.S. military health system. Mil Med. 2021;186(Supplement_1):100–107. doi:10.1093/milmed/usaa229
16. Olawale KO, Fadiora SO, Taiwo SS. Prevalence of hospital-acquired enterococci infections in two primary-care hospitals in Osogbo, southwestern Nigeria. Afr J Infect Dis. 2011;5(2):40–46. doi:10.4314/ajid.v5i2.66513
17. Savini V, Gherardi G, Astolfi D, et al. Insights into airway infections by enterococci: a review. Recent Pat Antiinfect Drug Discov. 2012;7(1):36–44. doi:10.2174/157489112799829774
18. Morris JF, Okies JE. Enterococcal lung abscess: medical and surgical therapy. Chest. 1974;65(6):688–691. doi:10.1378/chest.65.6.688
19. Vanschooneveld T, Mindru C, Madariaga MG, Kalil AC, Florescu DF. Enterococcus pneumonia complicated with empyema and lung abscess in an HIV-positive patient. Case report and review of the literature. Int J STD AIDS. 2009;20(9):659–661. doi:10.1258/ijsa.2008.008456
20. Levora J, Teplan V, Viklický O. Enterococcus faecium as a cause of pulmonary abscesses in kidney transplant recipient. Transpl Int. 2007;20(3):297–298. doi:10.1111/j.1432-2277.2006.00403.x
21. Giarratano A, Green SE, Nicolau DP. Review of antimicrobial use and considerations in the elderly population. Clin Interv Aging. 2018;13:657–667. doi:10.2147/cia.S133640
22. Beckett CL, Harbarth S, Huttner B. Special considerations of antibiotic prescription in the geriatric population. Clin Microbiol Infect. 2015;21(1):3–9. doi:10.1016/j.cmi.2014.08.018
23. Nishijo N, Tsuji Y, Matsunaga K, et al. Mechanism underlying linezolid-induced thrombocytopenia in a chronic kidney failure mouse model. J Pharmacol Pharmacother. 2017;8(1):8–13. doi:10.4103/jpp.JPP_167_16
24. Tajima M, Kato Y, Matsumoto J, et al. Linezolid-induced thrombocytopenia is caused by suppression of platelet production via phosphorylation of myosin light chain 2. Biol Pharm Bull. 2016;39(11):1846–1851. doi:10.1248/bpb.b16-00427
25. Meng J, Zhong D, Li L, et al. Metabolism of MRX-I, a novel antibacterial oxazolidinone, in humans: the oxidative ring opening of 2,3-Dihydropyridin-4-one catalyzed by non-P450 enzymes. Drug Metab Dispos. 2015;43(5):646–659. doi:10.1124/dmd.114.061747
26. Zhao X, Huang H, Yuan H, Yuan Z, Zhang Y. A Phase III multicentre, randomized, double-blind trial to evaluate the efficacy and safety of oral contezolid versus linezolid in adults with complicated skin and soft tissue infections. J Antimicrob Chemother. 2022;77(6):1762–1769. doi:10.1093/jac/dkac073
27. Wang S, Cai C, Shen Y, et al. In vitro activity of contezolid against methicillin-resistant staphylococcus aureus, vancomycin-resistant enterococcus, and strains with linezolid resistance genes from China. Front Microbiol. 2021;12:729900. doi:10.3389/fmicb.2021.729900
28. Kang Y, Ge C, Zhang H, Liu S, Guo H, Cui J. Compassionate use of contezolid for the treatment of tuberculous pleurisy in a patient with a leadless pacemaker. Infect Drug Resist. 2022;15:4467–4470. doi:10.2147/idr.S373082
29. Shoen C, DeStefano M, Hafkin B, Cynamon M. In vitro and in vivo activities of contezolid (MRX-I) against mycobacterium tuberculosis. Antimicrob Agents Chemother. 2018;62(8):1. doi:10.1128/aac.00493-18
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.