Back to Journals » International Journal of Nanomedicine » Volume 18
Sensitization Strategies of Lateral Flow Immunochromatography for Gold Modified Nanomaterials in Biosensor Development
Authors He X, Hao T, Geng H, Li S, Ran C, Huo M, Shen Y
Received 15 September 2023
Accepted for publication 28 November 2023
Published 21 December 2023 Volume 2023:18 Pages 7847—7863
DOI https://doi.org/10.2147/IJN.S436379
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Xingyue He,1 Tianjiao Hao,1 Hongxu Geng,2 Shengzhou Li,1 Chuanjiang Ran,1 Meirong Huo,1 Yan Shen1
1State Key Laboratory of Nature Medicines, China Pharmaceutical University, Nanjing, 210009, People’s Republic of China; 2School of Pharmacy, Yantai University, Yantai, 264005, People’s Republic of China
Correspondence: Yan Shen; Meirong Huo, Email [email protected]; [email protected]
Abstract: Gold nanomaterials have become very attractive nanomaterials for biomedical research due to their unique physical and chemical properties, including size dependent optical, magnetic and catalytic properties, surface plasmon resonance (SPR), biological affinity and structural suitability. The performance of biosensing and biodiagnosis can be significantly improved in sensitivity, specificity, speed, contrast, resolution and so on by utilizing multiple optical properties of different gold nanostructures. Lateral flow immunochromatographic assay (LFIA) based on gold nanoparticles (GNPs) has the advantages of simple, fast operation, stable technology, and low cost, making it one of the most widely used in vitro diagnostics (IVDs). However, the traditional colloidal gold (CG)-based LFIA can only achieve qualitative or semi-quantitative detection, and its low detection sensitivity cannot meet the current detection needs. Due to the strong dependence of the optical properties of gold nanomaterials on their shape and surface properties, gold-based nanomaterial modification has brought new possibilities to the IVDs: people have attempted to change the morphology and size of gold nanomaterials themselves or hybrid with other elements for application in LFIA. In this paper, many well-designed plasmonic gold nanostructures for further improving the sensitivity and signal output stability of LFIA have been summarized. In addition, some opportunities and challenges that gold-based LFIA may encounter at present or in the future are also mentioned in this paper. In summary, this paper will demonstrate some feasible strategies for the manufacture of potential gold-based nanobiosensors of post of care testing (POCT) for faster detection and more accurate disease diagnosis.
Keywords: gold nanomaterials, LFIA, sensitivity, POCT
Introduction
Post of care testing (POCT) is crucial for providing rapid diagnostic results and timely on-site treatment. Over the past two decades, lateral flow immunochromatographic assay (LFIA) has received increasing attention and has become the most prominent POCT.1,2 It has been widely used in clinical diagnosis, animal and plant disease monitoring, and other fields. The results of LFIA can be easily read with the naked eye or quantified using portable devices.2,3 The immunochromatographic test strip mainly consists of sample pad, conjugation pad, nitrocellulose (NC) membrane, absorbent pad, and plastic backing. As shown in Figure 1, the plastic backing serves as the support body, and other parts are stacked and adhered to it in sequence, antibodies (Abs) labeled with colored nanomaterials (such as gold nanoparticles (GNPs)) are fixed on the binding pad, two pre fixed immune reagent detection lines: test line (T line) and control line (C line) are marked on the NC membrane. The T line is used to determine the test results, and the C line is used to determine the effectiveness of the test strip. The sample to be tested moves forward from the sample pad due to chromatography. According to the different number of binding epitopes of the detected target, LFIA can be divided into two categories: double antibody sandwich method and competitive method.4 The double antibody sandwich method generally detects macromolecular targets containing multiple antigenic epitopes, such as proteins, pathogens, viruses, etc. Competitive method generally detects small molecule antigen (Ag) containing only a single antigenic epitope, such as agricultural and veterinary drug residues, hormones, toxins, etc.
 |
Figure 1 The structure of immunochromatographic test strip. Abbreviations: Ag, antigen; Ab, antibody. |
LFIA has been widely used in rapid detection due to its unique advantages, but its detection sensitivity still lags far behind other instrument measurement methods,5,6 such as mass spectrometry (MS), high-performance liquid chromatography (HPLC), enzyme-linked immunosorbent assay (ELISA) and so on. The improvement of sensitivity will undoubtedly further expand its application fields. In recent years, the main strategies to improve the sensitivity of LFIA include the use of highly sensitive nano labeling materials7 and signal amplification strategies.
As far as we know, gold nanomaterials are the most commonly used nano labeling materials in LFIA because of their unique physical and chemical properties,8–11 such as optical absorption of light at specific wavelengths, high conductivity with abundant surface electrons, ease of surface modification with thiol groups, and gold nanomaterials can have different shapes and compositions at the nanoscale.12 Their nanoscale size falls within the typical size range of biomolecules and organelles.13,14
Traditional Colloid gold immunochromatographic assay (GICA) typically uses 30–40 nm GNPs as labeling materials,15–20 but the optical signals intensity of GNPs within this particle size range is not sufficient, and the capture rate of antibodies is not high (<5%), ultimately resulting in the detection sensitivity is not high.21–23
In order to make GICA more sensitive, more accurate, have quantitative reading ability, and better adapt to today’s POCT demand, researchers have made a lot of efforts to introduce some new gold-based nanomaterials with stronger optical and color signals as labelling nanomaterials, such as different forms of gold nanomaterials (gold nanoflowers, gold nanorods and so on) and hybrid gold nanomaterials (gold magnetic nanomaterials and so on) based on adjustable size, shape, and easy surface modification characteristics of gold nanomaterials. These new gold nanomaterials provide ideal characteristics for chemical and biological detection, such as significant body surface area ratio, optical properties endowed by strong surface plasmon resonance (SPR) signals,24 wide absorption in the visible light region of the electromagnetic spectrum,25 fine tunable surface chemistry, the wide compatibility of the structure and higher colloidal stability.26,27 LFIA biosensors based on these well-designed gold nanostructures have higher detection sensitivity and various signal output functions. So far, various LFIA biosensors based on gold nanomaterials have been developed and reported. We anticipate that further development of POCT techniques based on gold nanomaterials will provide unique analytical methods with higher sensitivity and multiplexing capabilities for modern clinical laboratories.
In this paper, we will provide a detailed introduction to the principles, methods, and results of improving LFIA detection sensitivity based on these well-designed different forms of gold nanostructures and hybrid gold nanostructures, as well as different LFIA signal output methods and amplification strategies based on different gold nanostructures. Additionally, we will introduce some synthesis methods that have been reported in recent years to improve the stability of these gold nanomaterials.
Carefully Designed Gold Modified Nanomaterials for LFIA Sensitization
Multimodal Gold Nanostructures
Multimodal gold nanostructures in this paper refers to adjusting the size, morphology, and aggregation state of gold nanomaterials. At the nanoscale, gold can have different shapes and compositions,12 and their very small size endows them with unique physical and chemical properties,13,14 such as significant specific surface area and optical performance endowed by strong SPR signals.24,28 In addition to the widely researched and applied spherical gold nanomaterials, some different forms of gold nanostructures are also worth paying attention to, such as29,30 gold nanoflowers (GNFs),31,32 gold nanorods (GNRs), etc. These structures tend to have complex three-dimensional structures, rough irregular surfaces and larger specific surface area,22,33,34 which can further improve the binding efficiency of biological molecules on their surfaces. At the same time, some irregular protrusions on the surface of nanostructures can bring more SPR effects, higher molar extinction coefficient and stronger optical signal.30,35 These well-designed plasma nanostructures have generally improved the sensitivity and colloidal stability of gold nanomaterials in IVD technology.8
Gold Nanoflowers
GNFs are a kind of multi-branched nanostructure. Compared with the traditional GNPs, the multi-branched GNFs has higher extinction performance because the interaction between the tip and the core tip acts as an antenna, resulting in enhanced electromagnetic field and SPR, resulting in strong light extinction at visible wavelengths.29,36,37 In addition, because of its complex three-dimensional structure, GNFs have higher colloidal stability and larger total surface area than GNPs of the same size, and because the spatial resistance of protein to the surface of GNFs is reduced, the large specific surface area of GNFs is beneficial to improve the immobilization rate of Abs.38 In recent years, the use of GNFs as a Surface Enhanced Raman Scattering (SERS) probe and colorimetric method in chemical and biological analysis has been reported.39,40 Because of its high signal-to-noise ratio (S/N) (blue-black/white), it has more obvious color signal under the aggregation of the same number of NPs. Ji et al41 reported a technique for improving the sensitivity of immunochromatographic strip (ICS) using large multi-branched GNFs. Blue GNFs with an average diameter of 75 ± 5 nm were synthesized and used as a signal amplification probe for ultra-sensitive quantitative detection of aflatoxin B1 (AFB1) in rice. Ren et al42 used GNFs of about 60 nm as detection markers for sensitive detection of fumonisin B1 (FB1) with ICS. The sensitivity of this method is 5.0 ng/mL, which is four times higher than that of conventional test strip using CG as Ab labelled probe. Based on the fact that large GNFs show higher light absorbance than small GNFs, Zhang et al43 synthesized five different sizes of GNFs: 33,47,79,152 and 195 nm and used them as report markers of ICS to detect human chorionic gonadotropin (HCG) in order to clarify the effect of GNF size on the sensitivity of sandwich LFIA (Figure 2). The results showed that the sensitivity of medium GNFs with a diameter of 47–79 nm was the highest, which was 9 mIU/mL. These results suggest that medium-sized GNFs can be used as the best LFIA marker for highly sensitive detection of disease-related protein biomarkers.
However, the performance of traditional GNFs is not ideal because of its complex synthesis, lack of uniformity and general optical properties. Zhang et al44 provided a new scheme for the synthesis of GNFs. A novel chrysanthemum-like gold@polydopamine (GNC@PDA) prepared by one-pot method was compared with GNF@polydopamine (GNF@PDA) prepared by traditional three-step method. These GNFs synthesized by one-pot method have more branching structure, more uniform petal shape and narrower particle size distribution. The gray value of GNC@PDA in aqueous solution and NC membrane is about three times higher than that of GNF@PDA. And because of the polydopamine (PDA) coating, the stability and coupling efficiency of GNC@PDA are much higher than those of traditional nanoprobes. These results show that GNC@PDA has excellent morphological and optical properties. When GNC@PDA was applied to the detection of HCG by LFIA, compared with LFIA based on GNPs and GNF@PDA, the limit of detection (LOD) of LFIA based on GNC@PDA increased by 6.0 times and 3.3 times, respectively. These results can provide a basis for future researchers to develop new probes in practical LFIA applications.
Gold Nanorods
GNRs are also one of the most studied precious metal nanomaterials in recent years. The different aspect ratio of GNRs enables it to display different absorption spectra or different colors, which is very suitable for rapid screening and visualization, and has been widely used in a variety of biological detection applications.31,45 GNRs has two plasma peaks, one around 520 nm (transverse SPR) and the other at 800 nm (longitudinal SPR). In addition, GNRs also have excellent photothermal conversion effect, which can support their thermal comparison to obtain higher detection sensitivity. Pang et al46 developed a LFIA based on GNRs to quickly detect C-reactive protein (CRP) by simultaneously monitoring colorimetric and temperature signals. The detection signal can be directly observed by the naked eye or read directly by the thermal imager.
Pan et al47 introduced the progress in the determination of zearalenone (ZEN) in cereals by LFIAs with GNRs as signal. The developed LFIAs can detect the analyte ZEN in a short time (10 min) and reach LOD. Yu et al48 developed a transversely mobile nucleic acid biosensor using AuNRs of about 60 nm as a color tag to detect DNA probe. It has the characteristics of short detection time and high sensitivity for visual detection of DNA. The optimized ICS can detect 10 pM DNA without using the instrument. Quantitative analysis can be established by using portable strip meter to measure the strength of testing strip. The LOD of this method is 250 times lower than that of GNPs. This work not only provides a sensitive, rapid and economical strategy for nucleic acid detection, but also opens up new prospects for disease diagnosis and clinical application.
In the traditional synthesis, cetyltrimethylammonium bromide (CTAB) is used to prepare GNRs, which results in poor biocompatibility and cytotoxicology, so it is difficult to further functionalize. However, traditional methods usually require multiple centrifugal cycles to completely remove CTAB. This can lead to unexpected GNR aggregations. Tao et al49 describe a dialysis assistance scheme for ligand exchange on GNR. CTAB was separated from aqueous solution by concentration gradient driven dialysis. The concentration gradient of CTAB in the dialysis bag could avoid GNR aggregation caused by drastic environmental changes. It also supports the ligand exchange rate on the surface of GNR. The modified GNR was used for LFIA of food pathogen Escherichia coli (E. coli) O157:H7. Compared with the GNR prepared by ligand exchange through multiple centrifugal cycles, the GNR prepared by dialysis-assisted ligand exchange showed better binding to Abs and enhanced the visual signal of the test line in ICS. Its detection performance is better than that of conventional test strips using centrifugally modified GNR. Tao et al50 also skillfully took advantage of the different photothermal capabilities of GNRs with different aspect ratios to develop a unique thermal sensor. In the presence of glucose oxidase (GOX), glucose can react with dissolved oxygen to form hydrogen peroxide (H2O2). With the help of Fe2+, H2O2 can etch GNRs into different aspect ratios. With the decrease of the aspect ratio of GNR, the LSPR peak blue shifts, resulting in the weakening of NIR photothermal effect and the decrease of system temperature. A simple thermometer is used as a reading to detect glucose.
Aggregation of Gold Nanostructures
A transformative idea for improving the sensitivity of LFIA detection is to amplify GNP’s plasmons. The most commonly used strategy for plasmon amplification is to assemble a large number of GNPs on larger carrier particles. The first method is to gather a large number of gold nanomaterials on the carrier or be wrapped in the carrier with the help of an intermediate carrier to form a new signal labeling material, which is used in the test strip. The aggregation of gold nanomaterials makes the label have stronger luminous intensity, so a darker strip color can be obtained on the test line during the detection to realize signal amplification51,52. Table 1 lists the ICS which is the first method to aggregate gold nanomaterials to achieve signal amplification, and summarizes the intermediate carriers, performance improvement multiples (vs ICS based on spherical gold nanomaterials), detection targets and detection limits.
 |
Table 1 ICS of Signal Amplification Realized by Aggregation of Gold Nanomaterials |
The second method of aggregating nanomaterials is bilayer method, that is, a pair of specifically binding substances are used to bind nanomaterials respectively. At present, these substances are mainly Abs-anti-Abs, biotin and streptavidin (SA) and bovine serum albumin (BSA)-anti-BSA Abs. The interaction between these substances is used to realize the aggregation of gold nanomaterials and enhance the luminous intensity of the detection signal. Chen et al59 labeled gold nanomaterials with Abs and anti-Abs respectively, and immunochromatography formed nanogold-Ab-anti-Ab-nanogold complex on the test strip, thus realizing the aggregation of gold nanomaterials. This method was used to detect E. coli O157:H7. The LOD is 1.14 × 103 CFU/mL. Yan et al60 realized the aggregation of magnetic nanomaterials by using the interaction between Abs and anti-Abs, and LOD for furazolidone metabolites was 0.044 ng/mL, which was 10 times higher than that of traditional methods. Fang et al61 labeled gold nanomaterials with SA and biotinylated monoclonal antibodies (mAbs) respectively to form nanogold-SA- biotin-Ab-nanogold complex. The sensitivity of this method for the detection of imidacloprid is 160 times higher than that of the traditional method. Although the bilayer method improves the detection sensitivity of ICS, the tedious double labeling process increases the preparation time of the test strip and affects the large-scale production of the test strip. At the same time, there is a certain steric hindrance effect that affects the binding efficiency between the target and Ab to be tested, and affects the repeatability of the test results.
Hybrid Gold Nanocomposites
Nanoparticles (NPs) based on single element do not have perfect detection function to a certain extent, so many researchers focus on multi-element NPs in order to obtain immune signal carriers with better properties.62 Researchers have found that multi-component nanomaterials can obtain better properties than single-component nanomaterials, and the diversity of their composition and structure can greatly enrich their applications in various fields. Recently, there are also a large number of reports about the attempt of hybrid gold nanomaterials as biosensors for LFIA. Some inorganic non-metallic nanomaterials, such as carbon and silicon, have high physical stability, loading capacity, and are easy to modify on the surface. They can be hybridized with gold nanomaterials to amplify signals while improving probe stability and biocompatibility.55,63–65 The deposition of inorganic metal nanomaterials such as silver66,67 and copper68 on the surface of gold nanomaterials or their hybridization with gold nanomaterials can amplify the size and enhance the SPR effect, further enhancing molar extinction and amplifying optical signals;69 the hybridization of iron NPs with gold not only amplifies the SPR effect, but also serves to concentrate and enrich samples, making it possible to achieve hypersensitive disease detection;70,71 in addition, some macromolecular organic structures such as metal frameworks72 and bacterial fungi, as carriers of GNPs, can also significantly improve the optical performance of gold nanomaterial probes and enhance the sensitivity of LFIA.
Inorganically Modified Gold Nanostructures
Carbon nanomaterials show great potential in biomedical applications, mainly due to their unique chemical and physical properties.73 Carbon nanotubes are one of the most widely used carbon nanomaterials because of their physical and chemical stability and their high surface area-to-weight ratio. Song et al74 coupled single-walled carbon nanotubes (SWCNT) with GICA. It was used for identification of mycoplasma pneumoniae in children. The LOD was 1 × 102 copies/mL. Jia et al75 established a low-cost, sensitive, intuitive and rapid immunochromatographic method for the detection of carcinoembryonic antigen (CEA) on cotton thread using a novel carbon nanotube/gold nanoparticles (CNT/GNPs) nanocomposite reporter probe. The principle of cotton thread biosensor immunochromatographic detection was demonstrated by using CEA, a biomarker of lung cancer protein, as the analyte. Compared with traditional GNPs or carbon nanotubes (CNTs) as reporter probes, the sensitivity of this method is improved by 2–3 orders of magnitude. Under the optimum conditions, the biosensor can detect 2.32 ng/mL CEA (S/N≥3) and has sufficient sensitivity for clinical diagnosis.
Thanks to its inertia, high load capacity, biocompatibility and good surface modification, silicon is often used to modify gold to develop highly sensitive biosensors and biometrics. Xu et al76 constructed a fast and highly sensitive visual detection method for protein by using silica nanorods to modify GNPs (GNP-SiNRs) tags. Compared with the traditional GICA, LOD is reduced by 50 times.
Silver-enhanced labeling technique is developed from immunogold silver staining technique. Silver staining technique was first put forward by Holgate et al in 1983. They applied it to Ag display in tissue sections under light microscope, resulting in a great increase in sensitivity (up to 200 times). Although silver staining has been successfully applied in immunohistochemistry, it has not been widely used in the field of in vitro diagnostics (IVDs) until recent years. The specific step is to increase the size of GNPs by depositing layer by layer of silver around GNPs. In addition, visually, under the background of white NC membrane, the black that generates from metallic silver is stronger than the original red of GNPs. Yu et al77 reported a silver staining LFIA for simultaneous detection of FB1 and deoxynivalenol (DON) in corn samples. The test is based on competition between the target mycotoxin and the corresponding coating Ag fixed on the test line to bind to a limited number of GNPs labeled Abs. The detection signal was further amplified by silver staining of GNP. In this process, silver ions are catalyzed by GNP to form metallic silver, which is deposited on the surface of GNP, which can not only expand the particle size of GNP, but also form a clearer black in the test area. Under the optimized conditions, the cut-off value of silver staining LFIA for FB1 is 2.0 ng/mL, and for DON is 40 ng/mL. For DON in the buffer, it is at least 2 times higher than the GNPs-based method. This method is further used to detect FB1 and DON in naturally contaminated corn samples, which is consistent with the data obtained by HPLC-MS/MS. Liao et al78 developed an LFIA for rapid screening of AFB1 in food using corresponding mAbs immobilized on nanoparticles with silver nuclei and gold shells (Ag@Au) as detection reagents. The membrane-based test strip consists of a test line containing AFB1 conjugated to BSA and a control line containing goat anti-mouse immunoglobulin G (IgG). By using red Ag@Au NPs coated with anti-AFB1 mAb as signal reagents, one or two color lines were formed on the membrane. Under the best conditions, the LOD of AFB1 is lower, which is 0.1 ng/mL. Compared with GNPs, Ag@Au NPs greatly improve the sensitivity, reproducibility and stability of detection. The natural pollution samples including rice, wheat, sunflower, cotton, pepper and almond were evaluated and found to have a good correlation with the data obtained by ELISA. The simple and non-instrumental test strip method can be further extended to the screening of other mycotoxins in food. Silver can effectively increase the extinction coefficient of gold nanomaterials. Deng et al79 reported an LFIA method based on SERS for the determination of Sudan I in food samples. Au-silver core-shell bimetallic nanorods (Au@AgNRs) were synthesized and characterized and used as substrates for the preparation of LFIA (Figure 3). Sudan I polyclonal antibody was immobilized on the surface of Au@AgNRs carrying Raman reporter gene 5-dithiobenzene (2-nitrobenzoic acid). Sudan red I was quantified by Raman scattering intensity on the detection line. The determination was done in 15 min. The half maximal inhibitory concentration (IC50) and LOD were 30 pg/mL and 0.2 pg/mL, respectively. There is no large cross reaction (CR) with sunset yellow, lemon yellow, bright blue, but only 3.53–9.74% CR with Sultan II, III and IV. The detection recovery of Sudan I in food samples ranged from 88.9% to 107.6%, with a relative standard deviation of - 8.7% to 3.7% (n = 3).
Kim et al80 established a semi-quantitative LFIA platform for prostate specific antigen (PSA) detection in blood using Au-Ag NPs assembled on silica (SiO2@Au-AgNPs) as a signal reporting factor. The synthesized SiO2@Au-AgNPs has high absorbance in a wide wavelength range (400~800 nm), and the color intensity of the test line depends on the PSA concentration (0.3–10.00 ng/mL).
Magnetic NPs (MNPs), such as Fe3 O4 NPs, are particularly useful for imaging and separation techniques. It has the ability to extract analytes by magnetic pre-concentration, but compared with GNPs, it is not feasible to attach biometric elements to MNPs by passive adsorption. A simple strategy is to coat MNPs with GNPs. This gold magnetic nanoparticles (GMNs) can bind with biomolecules due to hydrophobic attraction and ion interaction.81 They can cooperatively combine the advantages of two kinds of nanoparticles,82–84 which can be used as an optical reporter for visual detection while realizing the pre-concentration of analytes. It has aroused great interest in diagnostic applications. For example, Tang et al85 developed GMNs consisting of MNPs as the core, GNPs as the shell, and biological functionalization with monoclonal anti-AFB2 Abs for the detection of aflatoxin B2 (AFB2). The experimental results show that the visual LOD (vLOD) of GMNs-based LFIA is about three times lower than that of conventional immunoassay using GNPs as detection reagent (Figure 4). Qualitative results (yes/no) can be obtained within 15 minutes without expensive equipment. GMNs usually need to be modified because of their low surface binding activity. For example, GMNs are coupled with anti-human immunoglobulin M (IgM, mucosa specificity) after being modified by polymethacrylic acid (PMAA)86 to construct a lateral flow detection device. This device is used to detect IgM of four types of pathogens. Compared with commercial GICA, this method has higher sensitivity. Yang et al87 developed a simple coating program for GMNs and polyacrylic acid (PAA). GMNs coated PAA (PGMNs) is stable and monodisperse. Based on this, a new LFIA system was established. The recombinant Treponema pallidum antigen (r-Tp) was combined with PGMNs to construct a probe for the detection of anti-Tp Ab. The LOD was as low as 1 national clinical unit/mL (NCU/mL).
 |
Figure 4 The fabrication process of the synthesized GMNs and principle of the detection method. |
Copper modified GNPs also have application cases in LFIA. Shao et al88 proposed a new GNPs-LFIA system to detect E. coli O157:H7 by metal growth mediated by polyallylamine hydrochloride (PAH). The developed method relies on the height-controlled growth of the copper shell on the GNP core and allows a highly enhanced chromaticity signal to be achieved by controlling PAH as a growth framework. The introduction of polycyclic aromatic hydrocarbons eliminates the non-specific adsorption of copper ions on NC membrane to provide the maximum S/N, so that the LOD of E. coli O157:H7 is 9.8 CFU/mL. In addition, the newly developed detection method has good repeatability (coefficient of variation <13%), remarkable stability and practicability. PAH-mediated signal enhancement system paves the way for stable metal growth methods based on gold, silver and other metals, and is very suitable for rapid, stable and sensitive detection of foodborne pathogens using GNPs-LFIA platform.
Inorganic Nanocatalytic Materials Modified Gold Nanostructures
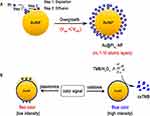
Although many efforts have been made to improve the extinction coefficient and optical properties of gold nanomaterials as much as possible, these strategies are simple and practical, it is still a challenge to greatly improve the sensitivity of GNP-based colorimetric LFIA without increasing complexity. Because the detectable color signal in GNP-based LFIA comes from the plasma polaritons of GNP, the detection sensitivity is basically limited by the inherent plasma activity. In order to avoid the limitation of the inherent plasma characteristics of gold, it is considered that some inorganic nanocatalytic materials, also known as metal NPs with enzyme mimic activity (inorganic nanoenzymes). Their catalytic performance is less affected by external environmental conditions, their chemical properties are stable, they are easy to surface modify, and their production cost is lower than that of proteases.89 The signal amplification of LFIA can also be achieved by utilizing the color generated by the substrate catalyzed by these inorganic nanoenzymes to enhance the signal of the test line. Among them, platinum-containing nanocatalytic materials showed high peroxidase and catalase mimic activity, and had significant stability90,91 in the extreme range of pH, temperature and inhibitor concentration. Gao et al92 first reported a unique bifunctional Au nanoparticles (AuNPs) to greatly improve the detection sensitivity of GICA. This bifunctional AuNPs is based on a 10-atomic layer of ultra-thin platinum coating on traditional AuNP (Au@PtNPs) (Figure 5A). This Au@PtNPs retains the plasma activity of the initial AuNP and has the ultra-high catalytic activity achieved by the platinum atomic layer. The dual functions of plasmon and catalysis provide two different detection options: one is generated only by the color generated by plasmon (low-sensitivity mode), and the other more sensitive color is catalyzed by chromogenic substrate (high-sensitivity mode) (Figure 5B). The detection performance can be adjusted “on demand”. Compared with conventional AuNP, Au@PtNPs can increase the sensitivity of PSA by two orders of magnitude.
Considering that platinum is mostly produced on gold seeds by seed-mediated method, the process is tedious and difficult to control. Panferov et al93 proposed a new method for the synthesis of inorganic nanoenzymes, which can effectively disperse platinum atoms on the surface of the GNPs. The synthesis of inorganic nanoenzyme includes three steps: the synthesis of GNPs, the overgrowth of silver layer on GNPs (Au@AgNPs, 6 kinds of silver shell with different thickness), and the replacement of silver with PtCl6 couple, resulting in the formation of trimetal. Au@Ag-Pt nanoparticles. The Au@Ag-Pt NPs have uniform deposition catalytic center and high platinum utilization efficiency. Au@Ag-Pt NPs combined with mAb were used as a colorimetric and catalytic marker in LFIA of CRP, a biomarker of inflammation. The LOD of CRP in serum with Au@Ag-Pt NPs-based LFIA (15 pg/mL) is 65 times higher than that of GNPs-based LFIA.
Bai et al94 developed citrate capped tricrystal Au@Ag-Pt NPs by using seed-mediated growth and electrical substitution reactions under convenient conditions, which can be directly converted into industrial production. Human cardiac troponin I (cTnI), one of the most specific markers of heart injury, was selected as a detection model. The hypersensitive colorimetric detection of human cTnI has been successfully achieved, and the LOD of cTnI is as low as 20 pg/mL. This strategy is robust, can ensure the stability and repeatability of peroxidase activity without precise control, and can be directly docked with the industrial production of traditional ICS, and is easy to adapt to on-demand clinical diagnosis.
However, considering that this detection model may not be accurate when detected in high endogenous peroxidase activity (EPA) samples. Panferov et al90 reported the detection of samples with high EPA using Au@Pt core@shell NPs. Unlike the inhibition of EPA in plant extracts by high concentration of H2O2 (>20 mM), the EPA of nanoenzyme remained stable at high concentration of H2O2 (up to 4 M), and the stability of Au@Pt NPs to substrate can eliminate the interference of EPA. This method was used to detect virus X (PVX) in the extracts from leaves and tubers of potato in order to reach the lower LOD and eliminate the interference of LFIA background. The established peroxidase inhibition method is a general method for bioanalysis, and its applicability is confirmed by eliminating EPA in three substrates (serum, potato leaf and tuber extract).
These examples show that LFIA enhanced by inorganic nanoenzymes show a good prospect. During the epidemic of coronavirus disease 2019 (COVID-19), Fu et al95 used Au@Pt NPs with excellent peroxidase-mimic catalysis (PMA) to detect severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) spike protein (S1) by colorimetry. This method was developed for rapid and accurate diagnosis of COVID-19 caused by SARS-CoV-2. The linear range of protein concentration was 10~100 ng/mL and the LOD was 11 ng/mL.
This method is a new type of sensitization method in immunochromatography and has been widely studied, and research has shown that it can significantly improve detection sensitivity. However, this method requires a secondary operation after conventional immunochromatography, which will increase analysis time and reduce efficiency. It cannot be denied that this is also a worthwhile research direction for LFIA. The future focus is to find a balance between sensitization and acceleration of detection, as well as achieve stable output of detection results.
Organically Modified Gold Nanostructures
Some researchers have begun to attempt to apply some new organic polymeric materials or organic compounds to the sensitization experiments of LFIA. Organic polymeric materials include novel biomimetic adhesive polymers such as polydopamine (PDA) and metal organic frameworks with large specific surface area and high loading capacity. At the same time, organic compounds such as bacteria also exhibit strong flexibility, high loading capacity, and biocompatibility. The excellent properties of these materials applied to gold nanostructures can also increase the sensitivity of LFIA to varying degrees.
Studies have shown that after coating the surface of NPs (such as silica NPs (SiNPs) and MNPs) with PDA, significantly higher UV Vis absorption levels can be observed.96,97 Therefore, some researchers have applied this characteristic of PDA to GNPs to enhance their color intensity and facilitate the detection of test strips. In Xu’s work,98 PDA-coated GNPs (Au@PDAs) were synthesized by the oxidative self-polymerization of dopamine (DA) on the surface of GNPs and applied for the first time as a signal-amplification label in LFIAs for the sensitive detection of ZEN in maize. The PDA layer functioned as a linker between GNPs and anti-ZEN mAbs to form a probe (Au@PDA-mAb). Compared with GNPs, Au@PDA had excellent color intensity, colloidal stability, and mAb coupling efficiency. The LOD of the Au@PDA-based LFIA was 7.4 pg/mL, which was 10-fold lower than that of the traditional GNP-based LFIA (76.1 pg/mL).
Metal organic frameworks (MOFs), a new class of crystalline porous material with flexible structures,99 are usually formed on the basis of the connection between organic linkers and metal nodes.100 Featured with extremely high surface area and tunable pore size,100 MOFs have exhibited significant application potentials in various fields, such as separation, catalysis, sensing, imaging, and drug deliver.101 Yuan et al54 synthesized MOF@GNPs with significantly enhanced color signal intensity by in situ growth of GNPs on MOFs skeleton. Taking advantage of high surface area of MOFs, numerous densely packed GNPs were embedded in the surface and interior of MOFs, showing ultrahigh loading capacity and uniform distribution of GNPs on the MOF@GNPs. Accordingly, compared with the traditional 40 nm GNPs (GNP40), the absorbance of MOF@GNP is 16.7 times higher. As a signal amplification tag of HCG, the LOD of enhanced LFIA is 1.69 mIU/mL, which is about 10.6 times higher than that of traditional GNP40-LFIA.
As novel signal carriers, the bacteria are not only the correct size (1–3 μm) and uniformity, are stable and can be dispersed, but they are also naturally occurring and biocompatible, and exhibit a better protection for the antibody. Additionally, the bacteria have an ultrahigh capacity for nanomaterials, owing to the abundant functional groups distributed on their surface, eg polysaccharides and proteins, which can be easily modified and activated. Huang et al53 constructed a new type of probe by loading a large amount of GNPs on the surface of bacteria which used as signal carriers. Bacteria can load large amounts of GNPs on their surfaces, which means that much fewer Abs are needed to produce visible results (Figure 6). After optimizing the analytical conditions such as bacterial species, coupling method and concentration, the vLOD for clenbuterol is 0.1 ng/mL, which is 20 times higher than that of traditional GICA. This work opens a new way for signal amplification and improving the performance of LFIA and proves that inactivated bacteria can also be environmentally friendly and powerful signal carriers for other biosensors.
 |
Figure 6 Illustration of the improved LFIA: comparison of the traditional GICA and novel bacteria@Au based LFIA for the detection of weakly positive sample. |
Different Signal Output Strategies Used in LFIA
After introducing the different gold nanomaterials applied to LFIA, we need to further introduce the different signal output strategies for these well-designed gold-based nanoprobes.
GICA has some disadvantages, such as low sensitivity and uncertainty in quantitative analysis, and it is difficult to explain the visual reading.102–104 At present, the quantitative strategy of LFIA is to record the optical density of the signal by testing the strip reader.105 The basis of detection using the reader signal is to convert the color density of the T line or line into optical density (OD), which can be used to accurately quantify the signal. In early studies, most quantitative test strip analysis was achieved by recording the OD of the corresponding test line (ODT). However, the ODs of the test and control line depend not only on the analyte concentration, but also on the immune reaction time, operating temperature, immune reaction time, signal changes between bands, and the influence of the sample matrix.106 In addition, the pH value, ionic strength and matrix of the sample also affect the efficiency of immune reaction between Ab and Ag. Therefore, the test results determined only by the OD of the test line are not very accurate with low reproducibility, which usually used as semi-quantitative test. The researchers found that the ratio of ODT to the OD of the control line (ODC) can effectively offset the effects of these parameters. A research team has previously reported quantitative detection of clenbuterol using LFIA through ODT/ODC ratios and portable note readers. LOD in standard pig urine samples reached 220 pg/mL in 10 minutes.
It has been reported that by correlating the temperature change with the target concentration, many biosensors using thermometers as readout devices have been developed. The heat sources of these reports, such as iron oxide NPs,107,108 new infrared (NIR) sensitive dye109 and aggregating GNPs,110,111 all have significant photothermal effects. All these studies show that the thermometer can be used as an accurate reading device for POCT. The thermal signal produced by antibody-labeled GNPs as a contrast agent can be amplified and detected by a thermal detector112 (Figure 7). The laser wavelength can be matched with SPR peak of GNPs, and the temperature rise can be detected by NIR detector, and finally the quantitative detection of GNPs signal can be realized. In addition, many nanomaterials with excellent photothermal properties also provide more options for the development of in vitro detection technology based on thermal signals.50
The excellent SERS signal sensing ability of gold nanomaterials cannot be ignored. Some studies have shown that using the thermal and SERS signals of nanomaterials to analyze target substances can improve the sensitivity of ICS.113–115 SERS is an ultra-sensitive vibration spectrum technology, which has the advantages of anti-photobleaching, short response time, high band resolution, rich information and so on. The immunochromatographic method based on SERS not only has the high sensitivity and spectral resolution of SERS, but also has the specificity and convenience of immunochromatography. Lin et al116 adsorbed 4-aminophenylthiophenol molecules on the surface of gold nanostars, using them as signal labels for the test strip. By collecting raman signals from the test strip, the LOD for bisphenol A (BPA) was 0.073 ng/mL, and the sensitivity was 20 times higher than that based on colorimetric methods.
In recent years, LFIA based on fluorescent signal has also been one of the research hotspots. Fluorescent nanomaterials, including gold nanoclusters (AuNCs), could significantly enhance the sensitivity of test strips owing to their excellent photoluminescent characteristic, good photostability, easy synthesis, and good biocompatibility.117–119
In addition, quantitative detection of magnetic flux through the magnetic properties of GMNs is also a very sensitive strategy. GMNs is a promising composite material in LFIA,71 not only because of its excellent colorimetric sensitivity, but also because of its magnetic sensitivity brought by iron oxide NPs.70 MNPs is a key factor in the development of a new generation of biosensors based on LFIA. They can be coupled to an external reader to monitor changes in the magnetic flux in order to sensitively detect analytes for quantitative measurement.120,121
Conclusion
LFIA is one of the most widely used real-time detection reagents. Generally, an ideal POCT detection reagent should have the following characteristics: fast diagnosis, easy to use, low cost, and reliability. The size dependent optical properties, diversity of surface functionalization, and high optical extinction coefficient of GNPs have driven the development of various sensitization and signal amplification strategies, making highly sensitive real-time detection possible. These detection methods are expected to be used for early diagnosis and monitoring of some diseases. However, there are still many barriers that need to be overcome to achieve the clinical transformation of gold nanomaterial diagnostic technology in the field of POCT. The main problems at present and the development direction in the future are briefly described as follows:
a. GNPs as a biosensor structure played a crucial influence on detection performance, and with the deepening of the research, a variety of composite materials have been applied due to their excellent properties, but these nanomaterials mostly have the disadvantages of complex synthesis process and poor reproducibility, which violates the original intention of IVDs technology development. In the main text, we also introduced different synthesis methods for some types of gold nanomaterials to improve stability and achieve mass production. Developing more stable, green, and batch synthesized synthesis methods and surface modification methods for gold nanomaterials remain a key focus at present and in the future; b. at the same time, we do not yet have a mature theory on the intrinsic mechanism of the binding of gold nanomaterials to biomolecules, often relying on past experience. Generally, covalent binding and electrostatic adsorption are commonly used. What we currently know is that the successful binding of traditional GNPs (30–40 nm) with some proteins depends on pH value. Before labeling, the pH value of the gold dispersion must be adjusted to a slightly alkaline PI of the protein to be labeled. Finally, the degree of binding can be determined by the depth of color development. However, the binding between gold nanomaterials and proteins is not only influenced by electrostatic forces, but also by hydrophobic forces, van der Waals forces, etc. In addition, their binding is also influenced by protein spatial conformation and amino acid sequence. Therefore, this method does not have universality, and cannot guarantee the stability of multiple experimental results; at present, there are also some methods that can accurately measure the binding efficiency of proteins with gold, such as Bicinchoninic Acid Assay (BCA), sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and other methods; however, the interaction between gold and proteins cannot be explored through simple binding or not. We need to study and discuss the forms in which proteins bind to gold nanomaterials, the most suitable forms, and the factors that affect the binding between gold nanomaterials and proteins to better control the process of binding; many methods are now used to study protein–nanoparticle interactions, such as radio-labeled proteins, spectrophotometry to measure shifts in plasmon resonance and dynamic light scattering (DLS) to measure hydrodynamic diameter, require separation of unbound protein from the conjugate prior to analysis and are therefore not truly a measure of the system at equilibrium. Alternatively, fluorescence correlation spectroscopy (FCS) and differential centrifugal sedimentation (DCS) have been introduced for the in situ analysis of protein adsorption on NPs. In recent years, nanoparticle tracking analysis (NTA) has also been used to quantitatively evaluate the adsorption of Abs on GNP as a function of pH; c. LFIA based on photothermal and raman signals require corresponding sensors, which increases the cost of LFIA. Developing portable and inexpensive sensors is the trend of future development.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81972892). We thank Mr. Changyou Luo for his contribution to the construction of the review framework and literature research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu D, Wang J, Wu L, et al. Trends in miniaturized biosensors for point-of-care testing. Trends Anal Chem. 2020;2020:122.
2. Shrivastava S, Trung TQ, Lee NE. Recent progress, challenges, and prospects of fully integrated mobile and wearable point-of-care testing systems for self-testing. Chem Soc Rev. 2020;49(6):1812–1866. doi:10.1039/c9cs00319c
3. Liu D, Zhang Y, Zhu M, et al. Microfluidic-integrated multicolor immunosensor for visual detection of HIV-1 p24 antigen with the naked eye. Anal Chem. 2020;92(17):11826–11833. doi:10.1021/acs.analchem.0c02091
4. Li F, You M, Li S, et al. Paper-based point-of-care immunoassays: recent advances and emerging trends. Biotechnol Adv. 2020;39:107442. doi:10.1016/j.biotechadv.2019.107442
5. Vidotti M, Carvalhal RF, Mendes RK, Ferreirab DCM, Kubota LT. Biosensors based on gold nanostructures. I Brazil Chem Soc. 2011;22(1):3–20. doi:10.1590/S0103-50532011000100002
6. Li Y, Zhou Y, Chen X, Huang X, Xiong Y. Comparison of three sample addition methods in competitive and sandwich colloidal gold immunochromatographic assay. Anal Chim Acta. 2020;1094:90–98. doi:10.1016/j.aca.2019.09.079
7. Mirica AC, Stan D, Chelcea IC, Mihailescu CM, Ofiteru A, Bocancia-Mateescu LA. Latest trends in lateral flow immunoassay (LFIA) detection labels and conjugation process. Front Bioeng Biotechnol. 2022;10:922772. doi:10.3389/fbioe.2022.922772
8. Huang X, Aguilar ZP, Xu H, Lai W, Xiong Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens Bioelectron. 2016;75:166–180. doi:10.1016/j.bios.2015.08.032
9. Tanaka R, Yuhi T, Nagatani N, et al. A novel enhancement assay for immunochromatographic test strips using gold nanoparticles. Anal Bioanal Chem. 2006;385(8):1414–1420. doi:10.1007/s00216-006-0549-4
10. Huang SH. Gold nanoparticle-based immunochromatographic test for identification of Staphylococcus aureus from clinical specimens. Clin Chim Acta. 2006;373(1–2):139–143. doi:10.1016/j.cca.2006.05.026
11. Guo YR, Liu SY, Gui WJ, Zhu GN. Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Anal Biochem. 2009;389(1):32–39. doi:10.1016/j.ab.2009.03.020
12. Cortie MB. The weird world of nanoscale gold. Gold Bulletin. 2004;37(1–2):12–19. doi:10.1007/BF03215512
13. Navya PN, Daima HK. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016;3(1):1. doi:10.1186/s40580-016-0064-z
14. Kamat PV. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J Phys Chem B. 2002;106:7729–7744. doi:10.1021/jp0209289
15. Preechakasedkit P, Pinwattana K, Dungchai W, et al. Development of a one-step immunochromatographic strip test using gold nanoparticles for the rapid detection of Salmonella typhi in human serum. Biosens Bioelectron. 2012;31(1):562–566. doi:10.1016/j.bios.2011.10.031
16. Zhao Y, Zhang G, Liu Q, Teng M, Yang J, Wang J. Development of a lateral flow colloidal gold immunoassay strip for the rapid detection of enrofloxacin residues. J Agric Food Chem. 2008;56(24):12138–12142. doi:10.1021/jf802648z
17. Frohnmeyer E, Tuschel N, Sitz T, et al. Aptamer lateral flow assays for rapid and sensitive detection of cholera toxin. Analyst. 2019;144(5):1840–1849. doi:10.1039/C8AN01616J
18. Fu X, Xie R, Wang J, et al. Development of colloidal gold-based lateral flow immunoassay for rapid qualitative and semi quantitative analysis of ustiloxins A and B in rice samples. Toxins. 2017;9(3):79. doi:10.3390/toxins9030079
19. Wu S, Liu L, Duan N, Li Q, Zhou Y, Wang Z. Aptamer-based lateral flow test strip for rapid detection of zearalenone in corn samples. J Agric Food Chem. 2018;66(8):1949–1954. doi:10.1021/acs.jafc.7b05326
20. Singh J, Sharma S, Nara S. Evaluation of gold nanoparticle based lateral flow assays for diagnosis of enterobacteriaceae members in food and water. Food Chem. 2015;170:470–483. doi:10.1016/j.foodchem.2014.08.092
21. Choi DH, Lee SK, Oh YK, et al. A dual gold nanoparticle conjugate-based lateral flow assay (LFA) method for the analysis of troponin I. Biosens Bioelectron. 2010;25(8):1999–2002. doi:10.1016/j.bios.2010.01.019
22. Guo J, Chen S, Guo J, Ma X. Nanomaterial labels in lateral flow immunoassays for point-of-care-testing. J Mater Sci Technol. 2021;60:90–104. doi:10.1016/j.jmst.2020.06.003
23. Jacobs JM, Adkins JN, Qian WJ, et al. Utilizing human blood plasma for proteomic biomarker discovery. J Proteome Res. 2005;4(4):1073–1085. doi:10.1021/pr0500657
24. Zhang D, Wang Z, Wang L, et al. High-performance identification of human bladder cancer using a signal self-amplifiable photoacoustic nanoprobe. ACS Appl Mater Interfaces. 2018;10(34):28331–28339. doi:10.1021/acsami.8b08357
25. Link S, Wang ZL, El-Sayed MA. Alloy formation of gold-silver nanoparticles and the dependence of the plasmon absorption on their composition. J Phys Chem B. 1999;103:3529–3533. doi:10.1021/jp990387w
26. Kim JE, Choi JH, Colas M, Kim DH, Lee H. Gold-based hybrid nanomaterials for biosensing and molecular diagnostic applications. Biosens Bioelectron. 2016;80:543–559. doi:10.1016/j.bios.2016.02.015
27. X-M M, Sun M, Lin Y, et al. Progress of visual biosensor based on gold nanoparticles. Chin J Anal Chem. 2018;46(1):1–10. doi:10.1016/S1872-2040(17)61061-2
28. Liu P, Han L, Wang F, Petrenko VA, Liu A. Gold nanoprobe functionalized with specific fusion protein selection from phage display and its application in rapid, selective and sensitive colorimetric biosensing of Staphylococcus aureus. Biosens Bioelectron. 2016;82:195–203. doi:10.1016/j.bios.2016.03.075
29. Nehl CL, Liao H, Hafner JH. Optical properties of star-shaped gold nanoparticles. Nano Lett. 2006;6(4):683–688. doi:10.1021/nl052409y
30. Guerrero-Martínez A, Barbosa S, Pastoriza-Santos I, Liz-Marzán LM. Nanostars shine bright for you. Curr Opin Colloid Interface Sci. 2011;16(2):118–127. doi:10.1016/j.cocis.2010.12.007
31. Pérez-Juste J, Pastoriza-Santos I, Liz-Marzán LM, Mulvaney P. Gold nanorods: synthesis, characterization and applications. Coord Chem Rev. 2005;249(17–18):1870–1901. doi:10.1016/j.ccr.2005.01.030
32. Bai Y, Gao C, Yin Y. Fully alloyed Ag/Au nanorods with tunable surface plasmon resonance and high chemical stability. Nanoscale. 2017;9(39):14875–14880. doi:10.1039/C7NR06002E
33. Xu S, Ouyang W, Xie P, et al. Highly uniform gold nanobipyramids for ultrasensitive colorimetric detection of influenza virus. Anal Chem. 2017;89(3):1617–1623. doi:10.1021/acs.analchem.6b03711
34. Wu M, Wu Y, Liu C, et al. Development and comparison of immunochromatographic strips with four nanomaterial labels: colloidal gold, new colloidal gold, multi-branched gold nanoflowers and Luminol-reduced Au nanoparticles for visual detection of Vibrio parahaemolyticus in seafood. Aquaculture. 2021;2021:539.
35. Li N, Zhao P, Astruc D. Anisotropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angew Chem Int Ed Engl. 2014;53(7):1756–1789. doi:10.1002/anie.201300441
36. Dondapati SK, Sau TK, Hrelescu C, Klar TA, Stefani FD, Feldmann J. Label-free biosensing based on single gold nanostars as plasmonic transducers. ACS Nano. 2010;4(11):6318–6322. doi:10.1021/nn100760f
37. Hao F, Nehl CL, Hafner JH, Nordlander P. Plasmon resonances of a gold nanostar. Nano Lett. 2007;7(3):729–732. doi:10.1021/nl062969c
38. Truong PL, Kim BW, Sim SJ. Rational aspect ratio and suitable antibody coverage of gold nanorod for ultra-sensitive detection of a cancer biomarker. Lab Chip. 2012;12(6):1102–1109. doi:10.1039/c2lc20588b
39. Xie JP, Zhang QB, Lee JY, Wang DI. The synthesis of SERS-active gold nanoflower tags for in vivo applications. ACS nano. 2008;2(12):2473–2480. doi:10.1021/nn800442q
40. Li J, Wu J, Zhang X, et al. Controllable synthesis of stable urchin-like gold nanoparticles using hydroquinone to tune the reactivity of gold chloride. J Phy Chem C. 2011;115(9):3630–3637. doi:10.1021/jp1119074
41. Ji Y, Ren M, Li Y, et al. Detection of aflatoxin B(1) with immunochromatographic test strips: enhanced signal sensitivity using gold nanoflowers. Talanta. 2015;142:206–212. doi:10.1016/j.talanta.2015.04.048
42. Ren W, Huang Z, Xu Y, Li Y, Ji Y, Su B. Urchin-like gold nanoparticle-based immunochromatographic strip test for rapid detection of fumonisin B1 in grains. Anal Bioanal Chem. 2015;407(24):7341–7348. doi:10.1007/s00216-015-8896-7
43. Zhang W, Duan H, Chen R, et al. Effect of different-sized gold nanoflowers on the detection performance of immunochromatographic assay for human chorionic gonadotropin detection. Talanta. 2019;194:604–610. doi:10.1016/j.talanta.2018.10.080
44. Zhou Y, Chen Y, Liu W, et al. Development of a rapid and sensitive quantum dot nanobead-based double-antigen sandwich lateral flow immunoassay and its clinical performance for the detection of SARS-CoV-2 total antibodies. Sens Actuators B Chem. 2021;343:130139. doi:10.1016/j.snb.2021.130139
45. Chen H, Shao L, Li Q, Wang J. Gold nanorods and their plasmonic properties. Chem Soc Rev. 2013;42(7):2679–2724. doi:10.1039/c2cs35367a
46. Pang R, Zhu Q, Wei J, et al. Development of a gold-nanorod-based lateral flow immunoassay for a fast and dual-modal detection of C-reactive protein in clinical plasma samples. RSC Adv. 2021;11(45):28388–28394. doi:10.1039/D1RA04404D
47. Pan M, Ma T, Yang J, Li S, Liu S, Wang S. Development of lateral flow immunochromatographic assays using colloidal au sphere and nanorods as signal marker for the determination of zearalenone in cereals. Foods. 2020;9(3). doi:10.3390/foods9030281
48. Yu Q, Zhang J, Qiu W, et al. Gold nanorods-based lateral flow biosensors for sensitive detection of nucleic acids. Mikrochim Acta. 2021;188(4):133. doi:10.1007/s00604-021-04788-z
49. Tao Y, Yang J, Chen L, et al. Dialysis assisted ligand exchange on gold nanorods: amplification of the performance of a lateral flow immunoassay for E. coli O157:H7. Mikrochim Acta. 2018;185(7):350. doi:10.1007/s00604-018-2897-0
50. Tao Y, Luo F, Lin Y, Dong N, Li C, Lin Z. Quantitative gold nanorods based photothermal biosensor for glucose using a thermometer as readout. Talanta. 2021;230:122364. doi:10.1016/j.talanta.2021.122364
51. Bu T, Jia P, Sun X, Liu Y, Wang Q, Wang L. Hierarchical molybdenum disulfide nanosheets based lateral flow immunoassay for highly sensitive detection of tetracycline in food samples. Sensors and Actuat B Chem. 2020;2020:320.
52. Su L, Wang L, Yao X, et al. Small size nanoparticles-Co(3)O(4) based lateral flow immunoassay biosensor for highly sensitive and rapid detection of furazolidone. Talanta. 2020;211:120729. doi:10.1016/j.talanta.2020.120729
53. Huang Q, Bu T, Zhang W, et al. An improved clenbuterol detection by immunochromatographic assay with bacteria@Au composite as signal amplifier. Food Chem. 2018;262:48–55. doi:10.1016/j.foodchem.2018.04.085
54. Yuan J, Chen X, Duan H, et al. Gold nanoparticle-decorated metal organic frameworks on immunochromatographic assay for human chorionic gonadotropin detection. Mikrochim Acta. 2020;187(12):640. doi:10.1007/s00604-020-04617-9
55. Yao X, Wang Z, Zhao M, et al. Graphite-like carbon nitride-laden gold nanoparticles as signal amplification label for highly sensitive lateral flow immunoassay of 17beta-estradiol. Food Chem. 2021;347:129001. doi:10.1016/j.foodchem.2021.129001
56. Hao L, Chen J, Chen X, et al. A novel magneto-gold nanohybrid-enhanced lateral flow immunoassay for ultrasensitive and rapid detection of ochratoxin A in grape juice. Food Chem. 2021;336:127710. doi:10.1016/j.foodchem.2020.127710
57. Zhang M, Bu T, Bai F, et al. Gold nanoparticles-functionalized three-dimensional flower-like manganese dioxide: a high-sensitivity thermal analysis immunochromatographic sensor. Food Chem. 2021;341(Pt 1):128231. doi:10.1016/j.foodchem.2020.128231
58. Liu X, Yang J, Li Q, et al. A strip test for the optical determination of influenza virus H3 subtype using gold nanoparticle coated polystyrene latex microspheres. Mikrochim Acta. 2020;187(5):306. doi:10.1007/s00604-020-04255-1
59. Chen M, Yu Z, Liu D, et al. Dual gold nanoparticle lateflow immunoassay for sensitive detection of Escherichia coli O157:H7. Anal Chim Acta. 2015;876:71–76. doi:10.1016/j.aca.2015.03.023
60. Yan L, Dou L, Bu T, et al. Highly sensitive furazolidone monitoring in milk by a signal amplified lateral flow assay based on magnetite nanoparticles labeled dual-probe. Food Chem. 2018;261:131–138. doi:10.1016/j.foodchem.2018.04.016
61. Fang Q, Wang L, Cheng Q, et al. A bare-eye based one-step signal amplified semiquantitative immunochromatographic assay for the detection of imidacloprid in Chinese cabbage samples. Anal Chim Acta. 2015;881:82–89. doi:10.1016/j.aca.2015.04.047
62. Prodan E, Radloff C, Halas NJ, Nordlander P. A hybridization model for the plasmon response of complex nanostructures. Science. 2003;302(5644):419–422. doi:10.1126/science.1089171
63. Su L, Wang L, Xu J, et al. Competitive lateral flow immunoassay relying on Au-SiO(2) janus nanoparticles with an asymmetric structure and function for furazolidone residue monitoring. J Agric Food Chem. 2021;69(1):511–519. doi:10.1021/acs.jafc.0c06016
64. Preechakasedkit P, Osada K, Katayama Y, et al. Gold nanoparticle core-europium(iii) chelate fluorophore-doped silica shell hybrid nanocomposites for the lateral flow immunoassay of human thyroid stimulating hormone with a dual signal readout. Analyst. 2018;143(2):564–570. doi:10.1039/C7AN01799E
65. Jia X, Wang K, Li X, et al. Highly sensitive detection of three protein toxins via SERS-lateral flow immunoassay based on SiO(2)@Au nanoparticles. Nanomedicine. 2022;41:102522. doi:10.1016/j.nano.2022.102522
66. Yang H, He Q, Lin M, et al. Multifunctional Au@Pt@Ag NPs with color-photothermal-Raman properties for multimodal lateral flow immunoassay. J Hazard Mater. 2022;435:129082. doi:10.1016/j.jhazmat.2022.129082
67. Huang X, Chen L, Zhi W, et al. Urchin-Shaped Au-Ag@Pt sensor integrated lateral flow immunoassay for multimodal detection and specific discrimination of clinical multiple bacterial infections. Anal Chem. 2023;95:13101–13112. doi:10.1021/acs.analchem.3c01631
68. Peng T, Jiao X, Liang Z, et al. Lateral flow immunoassay coupled with copper enhancement for rapid and sensitive SARS-CoV-2 nucleocapsid protein detection. Biosensors. 2021;12(1). doi:10.3390/bios12010013
69. Gupta Y, Ghrera AS. Recent advances in gold nanoparticle-based lateral flow immunoassay for the detection of bacterial infection. Arch Microbiol. 2021;203(7):3767–3784. doi:10.1007/s00203-021-02357-9
70. Moyano A, Serrano-Pertierra E, Salvador M, Martinez-Garcia JC, Rivas M, Blanco-Lopez MC. Magnetic lateral flow immunoassays. Diagnostics. 2020;10(5). doi:10.3390/diagnostics10050288
71. Razo SC, Panferov VG, Safenkova IV, Varitsev YA, Zherdev AV, Dzantiev BB. Double-enhanced lateral flow immunoassay for potato virus X based on a combination of magnetic and gold nanoparticles. Anal Chim Acta. 2018;1007:50–60. doi:10.1016/j.aca.2017.12.023
72. Zhang G, Deng S, Fang B, et al. Lateral flow immunoassay based on polydopamine-coated metal-organic framework for the visual detection of enrofloxacin in milk. Anal Bioanal Chem. 2022;414(24):7315–7323. doi:10.1007/s00216-022-04283-1
73. Ojeda I, Barrejon M, Arellano LM, et al. Grafted-double walled carbon nanotubes as electrochemical platforms for immobilization of antibodies using a metallic-complex chelating polymer: application to the determination of adiponectin cytokine in serum. Biosens Bioelectron. 2015;74:24–29. doi:10.1016/j.bios.2015.06.001
74. Song M, Zhang Y, Li S, et al. A sensitive and rapid immunoassay for Mycoplasma pneumoniae in children with pneumonia based on single-walled carbon nanotubes. Sci Rep. 2017;7(1):16442. doi:10.1038/s41598-017-16652-3
75. Jia X, Song T, Liu Y, Meng L, Mao X. An immunochromatographic assay for carcinoembryonic antigen on cotton thread using a composite of carbon nanotubes and gold nanoparticles as reporters. Anal Chim Acta. 2017;969:57–62. doi:10.1016/j.aca.2017.02.040
76. Xu H, Chen J, Birrenkott J, et al. Gold-nanoparticle-decorated silica nanorods for sensitive visual detection of proteins. Anal Chem. 2014;86(15):7351–7359. doi:10.1021/ac502249f
77. Yu Q, Li H, Li C, Zhang S, Shen J, Wang Z. Gold nanoparticles-based lateral flow immunoassay with silver staining for simultaneous detection of fumonisin B1 and deoxynivalenol. Food Control. 2015;54:347–352. doi:10.1016/j.foodcont.2015.02.019
78. Liao J-Y, Li H. Lateral flow immunodipstick for visual detection of aflatoxin B1 in food using immuno-nanoparticles composed of a silver core and a gold shell. Microchimica Acta. 2010;1713–4.
79. Deng D, Yang H, Liu C, Zhao K, Li J, Deng A. Ultrasensitive detection of Sudan I in food samples by a quantitative immunochromatographic assay. Food Chem. 2019;277:595–603. doi:10.1016/j.foodchem.2018.10.129
80. Kim HM, Kim J, An J, et al. Au-Ag assembled on silica nanoprobes for visual semiquantitative detection of prostate-specific antigen. J Nanobiotechnology. 2021;19(1):73. doi:10.1186/s12951-021-00817-4
81. Hwang J, Kwon D, Lee S, Jeon S. Detection of Salmonella bacteria in milk using gold-coated magnetic nanoparticle clusters and lateral flow filters. RSC Advances. 2016;6(54):48445–48448. doi:10.1039/C6RA05446C
82. Bao J, Chen W, Liu T, et al. Bifunctional Au-Fe3O4 nanoparticles for protein separation. ACS Nano. 2007;1(4):293–298. doi:10.1021/nn700189h
83. Huang J, Xie Z, Xie L, et al. Au/Fe(3)O(4) core-shell nanoparticles are an efficient immunochromatography test strip performance enhancer-a comparative study with Au and Fe(3)O(4) nanoparticles. RSC Adv. 2018;8(25):14064–14071. doi:10.1039/C8RA00185E
84. Karamipour S, Sadjadi MS, Farhadyar N. Fabrication and spectroscopic studies of folic acid-conjugated Fe3O4@Au core-shell for targeted drug delivery application. Spectrochim Acta A Mol Biomol Spectrosc. 2015;148:146–155. doi:10.1016/j.saa.2015.03.078
85. Tang D, Sauceda JC, Lin Z, et al. Magnetic nanogold microspheres-based lateral-flow immunodipstick for rapid detection of aflatoxin B2 in food. Biosens Bioelectron. 2009;25(2):514–518. doi:10.1016/j.bios.2009.07.030
86. Li X, Zhang Q, Hou P, et al. Gold magnetic nanoparticle conjugate-based lateral flow assay for the detection of IgM class antibodies related to TORCH infections. Int J Mol Med. 2015;36(5):1319–1326. doi:10.3892/ijmm.2015.2333
87. Yang D, Ma J, Zhang Q, et al. Polyelectrolyte-coated gold magnetic nanoparticles for immunoassay development: toward point of care diagnostics for syphilis screening. Anal Chem. 2013;85(14):6688–6695. doi:10.1021/ac400517e
88. Shao Y, Xu W, Zheng Y, et al. Controlled PAH-mediated method with enhanced optical properties for simple, stable immunochromatographic assays. Biosens Bioelectron. 2022;206:114150. doi:10.1016/j.bios.2022.114150
89. Jiang D, Ni D, Rosenkrans ZT, Huang P, Yan X, Cai W. Nanozyme: new horizons for responsive biomedical applications. Chem Soc Rev. 2019;48(14):3683–3704. doi:10.1039/C8CS00718G
90. Panferov VG, Safenkova IV, Zherdev AV, Dzantiev BB. The steadfast Au@Pt soldier: peroxide-tolerant nanozyme for signal enhancement in lateral flow immunoassay of peroxidase-containing samples. Talanta. 2021;225:121961. doi:10.1016/j.talanta.2020.121961
91. Yang H, He Q, Chen Y, et al. Platinum nanoflowers with peroxidase-like property in a dual immunoassay for dehydroepiandrosterone. Mikrochim Acta. 2020;187(11):592. doi:10.1007/s00604-020-04528-9
92. Gao Z, Ye H, Tang D, et al. Platinum-decorated gold nanoparticles with dual functionalities for ultrasensitive colorimetric in vitro diagnostics. Nano Lett. 2017;17(9):5572–5579. doi:10.1021/acs.nanolett.7b02385
93. Panferov VG, Byzova NA, Zherdev AV, Dzantiev BB. Peroxidase-mimicking nanozyme with surface-dispersed Pt atoms for the colorimetric lateral flow immunoassay of C-reactive protein. Mikrochim Acta. 2021;188(9):309. doi:10.1007/s00604-021-04968-x
94. Bai T, Wang L, Wang M, et al. Strategic synthesis of trimetallic Au@Ag-Pt nanorattles for ultrasensitive colorimetric detection in lateral flow immunoassay. Biosens Bioelectron. 2022;208:114218. doi:10.1016/j.bios.2022.114218
95. Fu Z, Zeng W, Cai S, et al. Porous Au@Pt nanoparticles with superior peroxidase-like activity for colorimetric detection of spike protein of SARS-CoV-2. J Colloid Interface Sci. 2021;604:113–121. doi:10.1016/j.jcis.2021.06.170
96. Lin LS, Cong ZX, Cao JB, et al. Multifunctional Fe3O4@Polydopamine core–shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano. 2014;8:3876–3883.
97. Liu M, Jiang W, Chen Q, et al. A facile one-step method to synthesize SiO2@polydopamine core–shell nanospheres for shear thickening fluid. RSC Advances. 2016;6(35):29279–29287. doi:10.1039/C5RA25759J
98. Xu S, Zhang G, Fang B, Xiong Q, Duan H, Lai W. Lateral flow immunoassay based on polydopamine-coated gold nanoparticles for the sensitive detection of zearalenone in maize. ACS Appl Mater Interfaces. 2019;11(34):31283–31290. doi:10.1021/acsami.9b08789
99. Guo H, Zheng Z, Zhang Y, Lin H, Xu Q. Highly selective detection of Pb2+ by a nanoscale Ni-based metal–organic framework fabricated through one-pot hydrothermal reaction. Sensors and Actuat B Chem. 2017;248:430–436. doi:10.1016/j.snb.2017.03.147
100. Elsaidi SK, Mohamed MH, Banerjee D, Thallapally PK. Flexibility in Metal–Organic Frameworks: a fundamental understanding. Coord Chem Rev. 2018;358:125–152. doi:10.1016/j.ccr.2017.11.022
101. Huang X, He Z, Guo D, et al. “Three-in-one” nanohybrids as synergistic nanoquenchers to enhance no-wash fluorescence biosensors for ratiometric detection of cancer biomarkers. Theranostics. 2018;8(13):3461–3473. doi:10.7150/thno.25179
102. Choi JR, Tang R, Wang S, Wan Abas WA, Pingguan-Murphy B, Xu F. Paper-based sample-to-answer molecular diagnostic platform for point-of-care diagnostics. Biosens Bioelectron. 2015;74:427–439. doi:10.1016/j.bios.2015.06.065
103. Ge X, Asiri AM, Du D, Wen W, Wang S, Lin Y. Nanomaterial-enhanced paper-based biosensors. TrAC Trends Anal Chem. 2014;58:31–39. doi:10.1016/j.trac.2014.03.008
104. Parolo C, Merkoci A. Paper-based nanobiosensors for diagnostics. Chem Soc Rev. 2013;42(2):450–457. doi:10.1039/C2CS35255A
105. Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM. Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem. 2008;80(10):3699–3707. doi:10.1021/ac800112r
106. Yang Q, Gong X, Song T, et al. Quantum dot-based immunochromatography test strip for rapid, quantitative and sensitive detection of alpha fetoprotein. Biosens Bioelectron. 2011;30(1):145–150. doi:10.1016/j.bios.2011.09.002
107. Fu G, Sanjay ST, Dou M, Li X. Nanoparticle-mediated photothermal effect enables a new method for quantitative biochemical analysis using a thermometer. Nanoscale. 2016;8(10):5422–5427. doi:10.1039/C5NR09051B
108. Fu G, Sanjay ST, Zhou W, Brekken RA, Kirken RA, Li X. Exploration of nanoparticle-mediated photothermal effect of TMB-H(2)O(2) colorimetric system and its application in a visual quantitative photothermal immunoassay. Anal Chem. 2018;90(9):5930–5937. doi:10.1021/acs.analchem.8b00842
109. Zhang JJ, Duan XH, Wu Y, Yang JC, Guo LN. Transition-metal free C-C bond cleavage/borylation of cycloketone oxime esters. Chem Sci. 2019;10(1):161–166. doi:10.1039/C8SC03315C
110. Tao Y, Luo F, Guo L, Qiu B, Lin Z. Target-triggered aggregation of gold nanoparticles for photothermal quantitative detection of adenosine using a thermometer as readout. Anal. Chim. Acta. 2020;1110:151–157. doi:10.1016/j.aca.2020.03.023
111. Zhou W, Hu K, Kwee S, et al. Gold nanoparticle aggregation-induced quantitative photothermal biosensing using a thermometer: a simple and universal biosensing platform. Anal Chem. 2020;92(3):2739–2747. doi:10.1021/acs.analchem.9b04996
112. Su LH, Chen YQ, Wang LL, et al. Dual-signal based immunoassay for colorimetric and photothermal detection of furazolidone. Sens Actuators B Chem. 2021;2021:331.
113. Wang R, Kim K, Choi N, et al. Highly sensitive detection of high-risk bacterial pathogens using SERS-based lateral flow assay strips. Sensors and Actuat B Chem. 2018;270:72–79. doi:10.1016/j.snb.2018.04.162
114. Sheng E, Lu Y, Xiao Y, Li Z, Wang H, Dai Z. Simultaneous and ultrasensitive detection of three pesticides using a surface-enhanced Raman scattering-based lateral flow assay test strip. Biosens Bioelectron. 2021;181:113149. doi:10.1016/j.bios.2021.113149
115. Li Y, Tang S, Zhang W, et al. A surface-enhanced Raman scattering-based lateral flow immunosensor for colistin in raw milk. Sensors and Actuat B Chem. 2019;282:703–711. doi:10.1016/j.snb.2018.11.050
116. Lin LK, Stanciu LA. Bisphenol A detection using gold nanostars in a SERS improved lateral flow immunochromatographic assay. Sens Actuators B Chem. 2018;276:222–229. doi:10.1016/j.snb.2018.08.068
117. Peng T, Wang J, Zhao S, et al. Highly luminescent green-emitting Au nanocluster-based multiplex lateral flow immunoassay for ultrasensitive detection of clenbuterol and ractopamine. Anal Chim Acta. 2018;1040:143–149. doi:10.1016/j.aca.2018.08.014
118. Zheng P, Peng T, Wang J, et al. Fluorescent lateral flow immunoassay based on gold nanocluster for detection of pyrrolizidine alkaloids. Mikrochim Acta. 2021;188(1):11. doi:10.1007/s00604-020-04672-2
119. Deng -H-H, Shi X-Q, Wang -F-F, et al. Fabrication of water-soluble, green-emitting gold nanoclusters with a 65% photoluminescence quantum yield via host–guest recognition. Chem Mater. 2017;29(3):1362–1369. doi:10.1021/acs.chemmater.6b05141
120. Liu Y, Zhang Z, Wang Y, et al. A highly sensitive and flexible magnetic nanoprobe labeled immunochromatographic assay platform for pathogen Vibrio parahaemolyticus. Int J Food Microbiol. 2015;211:109–116. doi:10.1016/j.ijfoodmicro.2015.07.005
121. Wang Y, Xu H, Wei M, Gu H, Xu Q, Zhu W. Study of superparamagnetic nanoparticles as labels in the quantitative lateral flow immunoassay. Mater Sci Eng C. 2009;29(3):714–718. doi:10.1016/j.msec.2009.01.011
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.