Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Senegenin Inhibits Aβ1-42-Induced PC12 Cells Apoptosis and Oxidative Stress via Activation of the PI3K/Akt Signaling Pathway
Authors Ren X , Zhang J, Zhao Y, Sun L
Received 8 November 2021
Accepted for publication 10 February 2022
Published 4 March 2022 Volume 2022:18 Pages 513—524
DOI https://doi.org/10.2147/NDT.S346238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Xing Ren,1 Jiwei Zhang,2 Yunnan Zhao,3 Lingzhi Sun4
1College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, 250014, People’s Republic of China; 2College of Acupuncture and Massage, Shandong University of Traditional Chinese Medicine, Jinan, 250014, People’s Republic of China; 3Editorial Office of Journal of Shandong University of Traditional Chinese Medicine, Jinan, 250014, People’s Republic of China; 4Department of Neurology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, 250011, People’s Republic of China
Correspondence: Lingzhi Sun, Department of Neurology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, 42 Wenhua West Road, Jinan, 250011, People’s Republic of China, Tel +86 53 168 616 039, Fax +86 53 182 950 062, Email [email protected]
Background/Aim: Apoptosis and oxidative stress have been considered as key events in the pathogenesis of Alzheimer’s disease (AD). Senegenin (Sen), the major and most effective ingredient of Radix Polygalae, which has anti-apoptotic and anti-oxidative effects. The aim of this study was to investigate the anti-apoptotic and anti-oxidant effects of Sen on Aβ1-42-induced PC12 cells apoptosis and oxidative stress as well as its possible signaling pathway.
Methods: Rat pheochromocytoma (PC12) cells were treated by 20 μM Aβ1-42 and then divided into 5 different treatment groups (Control; Aβ1-42 20 μM; Aβ1-42 20 μM + Sen 10 μM; Aβ1-42 20 μM + Sen 30 μM; Aβ1-42 20μM + Sen 60 μM). PC12 cells activity was detected by MTT assay. Colony formation assay was performed to assess the clonogenic ability of cells. The cell apoptosis was detected by Annexin-V/PI staining. The pro-apoptotic protein (Bax), anti-apoptotic protein (Bcl-2), anti-oxidative stress factor (HO-1, Nuclear Nrf2, Total Nrf2) and pathway-related protein (Akt, P-Akt, PI3K, P-PI3K) were tested by Western blot. The reactive oxygen species (ROS) level was assessed with a DCFH-DA probe.
Results: The results indicated that Sen dose-dependently increased cell viability and reduced the number of apoptotic cells. The ratio of P-PI3K/PI3K and P-Akt/Akt increased in a dose-dependent manner under the treatment of Sen, suggesting that Sen might activate the PI3K/Akt signaling pathway. Moreover, Sen upregulates the ratio of Bcl-2/Bax. Further study revealed that Sen can play an antioxidant role in enhancing HO-1, promoting Nrf2 nuclear translocation and reducing ROS accumulation to reduce oxidative stress.
Conclusion: Sen is effective in inhibiting apoptosis and oxidative stress in Aβ1-42-induced PC12 cells, which likely contribute to the development of novel therapies for AD.
Keywords: Alzheimer’s disease, senegenin, apoptosis, Aβ, oxidative stress, PC12 cells
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia, accounting for approximately 60% to 80% of cases.1 Although the pathogenesis of AD is still unclear, β-amyloid (Aβ) deposition, neurofibrillary tangles and neuronal loss have been found to be closely related to cognitive dysfunction in patients.2–4 Numerous studies have demonstrated the toxicity of Aβ in vitro and in vivo, causing neuronal loss, tau phosphorylation and microglial proliferation5–9 and can lead to neuronal cell death.10 Two major forms of Aβ, such Aβ1-42 and Aβ1-40, considered as a disease marker in AD. As Aβ1–42 is more prone to aggregation than Aβ1–40 and the accumulation and precipitation of Aβ1–42 was considered a neuropathological event s associated with the progression and development of AD.11,12 Notably, the mechanism of Aβ induced cell death is still controversial. Some studies believe that Aβ inhibit cell viability and increase the percentage of DNA damage through necrosis.13–15 However, other studies suggest that apoptosis is the main mechanism of Aβ-induced neuronal death.16–18 In our study, we are more support the mechanism of apoptosis, but the pathway and mechanism of Aβ-induced apoptosis still need to be further studied.
The phosphatidylinositol 3-kinases (PI3Ks), a family of intracellular lipid kinases, are crucial roles in the upstream of the PI3K/Akt signaling pathway.19 The PI3K/Akt signaling pathway is directly regulated by Aβ exposure, and many downstream factors have an important impact on AD.20–22 Furthermore, the PI3K/Akt signaling pathway, as a key regulator of apoptosis, is believed to play a vital role in AD.23–25 In the apoptotic pathway, Bax and Bcl-2 are two critical downstream target proteins.26 PI3K/Akt signaling pathway promotes or inhibits Bcl-2 and Bax by phosphorylation.27 Therefore, it was important to study the expression of Bcl-2 and Bax to explore apoptosis. In addition, there is substantial evidence supporting that oxidative stress is a common pathogenesis of AD.28,29 Oxidative stress can induce excessive production of reactive oxygen species (ROS) and excessive ROS also promote oxidative stress, ultimately, cell death.30,31 ROS is induced by PI3K/Akt pathway, which triggers various intracellular reactions.32,33 Moreover, PI3K/AKT pathway may be an important way to activate/synthesize various antioxidant factors, such as Nuclear factor E2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1).34–36 Therefore, drugs that block apoptosis and oxidative stress may be useful in preventing neuronal cell death and treating AD.17,37–39
Senegenin (Sen) is the active component of Polygala tenuifolia root, a traditional herbal medicine that has been widely used in China (Figure 1). Biochemical analysis has proved that the active component of P. tenuifolia are mainly saponins derived from presenegenin. Current research shows that P. tenuifolia has neuroprotective and intellectual promoting activities, which can safely and effectively reduce memory damage40,41 and reduce Aβ secretion.42,43 Moreover, a previous study has showed that Sen has anti-apoptosis and anti-oxidation effects in hypoxia or reoxygenation injury.44
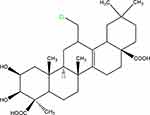 |
Figure 1 Chemical structure of senegenin. |
In the present study, we focused on the effects of Sen on apoptosis and oxidative stress in PC12 cells, as well as its possible pathway and the underlying mechanism. This may provide new evidence for a better understanding of the neuroprotective mechanism of Sen.
Materials and Methods
Materials
Aβ1-42, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Senegenin (purity>98%) was purchased from Mansite Pharmaceutical Co., LTD. (Chengdu, China). Dulbecco’s modified Eagle medium (DMEM), penicillin, fetal bovine serum (FBS) and streptomycin were purchased from Solarbio (Beijing, China). The Annexin-V/PI staining kit was purchased from KeyGEN (Nanjing, China). 2′, 7′-dichlorofluorescin diacetate (DCFH-DA) probe and Nuclear and Cytoplasmic Protein Extraction Kit were purchased from Beyotime Biotechnology (Shanghai, China). Antibodies against Akt (cat.no.ab18785), P-Akt (cat.no. ab38449), PI3K (cat.no. ab139307), P-PI3K (cat.no. ab182651), Bax (cat.no. ab232479), Bcl-2 (cat.no. ab59348), Nrf2 (cat.no. ab62352) and β-actin (cat.no. ab179467) were from Abcam (Cambridge, United Kingdom). HO-1 (cat.no. GTX101147) was from Gene Tex (Irvine, CA, USA) and Lamin A (cat.no. 201015-3D6) was from Zen-Bio (Chengdu, China).
Cell Culture and Drug Treatment
Rat pheochromocytoma (PC12) cells were obtained from the Chinese Academy of Sciences. The cells were maintained in DMEM containing 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C in a 5% CO2 incubator, the medium was changed every other day. Aβ1–42 was dissolved in PBS and incubated at 37°C for seven days to induce aggregation.45,46 Next, the aggregated Aβ1–42was diluted to the desired concentration. A 2mM stock solution of Sen was prepared by dissolving 20mg Sen powder in 15mL DMEM and placed at −20°C for long-term storage. Working solutions were diluted from the stock solutions at an appropriate concentration for further use.
Cell Viability Assay
PC12 cells were seeded into 96-well plates (0.5 × 104cells/well) and assessed for response to Aβ1-42 and Sen using the MTT assay. Briefly, the cells were pretreated with Sen (0, 10, 30, 60 or 80 μM) only for 1 h and then exposed to Aβ1-42 for 24 h in the continued presence of Sen (0, 10, 30, 60 or 80 μM). After incubations, PC12 cells were processed with 5 mg/mL MTT for 4 h at 37°C and the medium was carefully removed. Formazan crystals were dissolved in 150 μL DMSO. The absorbance at 570 nm was read using Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, USA) which correlated to the viability of cells.
Colony Formation Assay
To assess the clonogenic ability of cells, a colony formation assay was performed. The cultured PC12 cells were plated before the treatment with Aβ1-42. Cells were harvested from a stock culture and plated at proper dilutions into dishes. After the attachment of the cells to the dishes, which generally takes 2 h or more, the cells were treated with 20µM of Aβ1-42 and co-treated with different concentrations of Sen (0, 10, 30 or 60 μM). After treatment, place the dishes in the incubator for at least 2–3 weeks. The colonies were stained with Giemsa stain solution. Clones containing more than fifty cells were counted under an inverted microscope (Nikon, Tokyo, Japan).
Apoptosis Assays
Apoptosis was tested using the Annexin V-FITC/PI double staining Kit (Keygen Biotech Co., Nanjing, China). In the end of incubation, PC12 cells were washed in cold PBS then centrifuged twice at 2000 rpm for five minutes and resuspended in 500 μL of binding buffer. Then FITC labeled Annexin V (5μL) and propidium iodide (PI, 5μL) were added to the cells, and then incubated for 20 minutes at room temperature in the dark according to the manufacturer’s instructions. The cells were analyzed by flow cytometry (Becton Dickinson, USA).
Western Blot Analysis
Firstly, PC12 cells were dissolved with RIPA buffer. Nuclear and cytoplasmic proteins were extracted using Nuclear and Cytoplasmic Protein Extraction Kit. Protein concentrations were measured by the bicinchoninic acid protein assay kit according to the protocol provided by the manufacturer (CoWin Biosciences Co., Ltd., China), and 100μg of total protein was separated by 10% SDS-PAGE electrophoresis. Then the proteins were transferred to a PVDF membrane. The membrane was blocked with 5% milk for 1 h at room temperature, and the primary antibodies (P-Akt, Akt, PI3K, P-PI3K, Bax, Bcl-2, HO-1, Nuclear Nrf2, Total Nrf2, LaminA and β-actin) were incubated at 4°C overnight. Chemiluminescence was used for color development, and image analysis was calculation with Quantity One software version 4.4.6 (Media Cybernetics, Inc., Rockville, MD, USA).
Determination of Intracellular ROS
To measure intracellular ROS level in PC12 cells after exposure to the oxidation-sensitive fluorescent dye DCFH-DA. The fluorescence was detected by the microplate reader, the excitation of which was set as 488 nm and the emission as 525 nm. 1×104 PC12 cells per well were seeded in 96-well culture plates. Cells were pretreated with Sen (0, 10, 30 or 60μM) for 1 h then exposed to Aβ1-42 (20μM) for 24 h. Subsequently, DCFH-DA (10 μM) treated cells for 30 min at 37°C in the dark and cells were washed with PBS. The fluorescence was detected by the fluorescence microplate reader.
Statistical Analysis
All data are expressed as mean ± standard deviation (SD) and analyzed by SPSS 26.0 statistical software (SPSS Inc., Chicago, IL, USA). Each procedure was performed in duplicate in three independent experiments. Statistical analyses were performed using analysis of variance (one-way ANOVA) and Student’s t test was used when two groups were compared. Data were considered significant at P <0.05.
Results
Sen Reduced Aβ1-42-Induced Toxicity of PC12 Cells
To assess the impact of Sen on Aβ-induced apoptosis, we first to assess the dose response to Aβ1-42 of PC12 cell viability by MTT assay. Cells were treated with 0, 10, 20, 30, or 40 μM Aβ1-42 as shown in Figure 2A, 20 μM Aβ1-42 significantly decreased PC12 cell viability and 40 μM Aβ1-42 resulted in the minimal cell viability of 53.5%, indicating a dose-dependent effect.
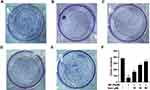
Next we assessed the effect of Sen alone on cell viability, which showed no statistical difference at Sen concentrations ranging from 0 to 60 μM (Figure 2B). Previous studies have shown that cells with activity of about 70% will be selected for the next experiment, which with 20 μM Aβ.25,47–49 For these studies, we explored the effect of Sen on cell viability in PC12 cells treated with 20 μM Aβ1-42. To determine the optimal dose of Sen, we first incubated PC12 cells with 10, 30, 60μM of Sen for 1 h, respectively, before adding 20 μM Aβ1-42. We found that Sen induced an increase in PC12 cell viability in a dose-dependent manner (Figure 2C). Subsequently, the proliferation of PC12 cells was analyzed by colony formation assay. Aβ1-42 has a significant inhibitory effect on cell proliferation and Sen significantly restored the proliferation of Aβ1-42-treated PC12 cells. Colony formation assay further confirmed the neuroprotective effect of Sen (Figure 3).
Sen Decreased Aβ1-42-Induced Apoptosis Rate in PC12 Cells
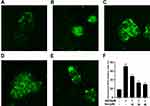
Apoptosis rate was detected with Annexin V/PI double staining. Our data indicated that 20 μM Aβ1-42 was sufficient to induce PC12 cell apoptosis with a rate of 64.27% by 24h. However, pretreatment with Sen (10, 30, 60 μM) for 1 h prior to Aβ1-42 exposure decreased the apoptotic rate to 35.65%, 26.25% and 15.74% in a dose-dependent manner, respectively (Figure 4). The results imply that Sen is anti-apoptotic in PC12 cells.
Sen Increase the Protein Expression of Nrf2 and HO-1
In order to examine the effect of Sen on oxidative stress, we analyzed HO-1 and Nrf2 by Western blot analysis. Compared with control group, the protein levels of HO-1, Nuclear Nrf2, and Total Nrf2 were significantly decreased in Aβ1-42 treatment group, while in Sen treatment groups, the protein expression of HO-1, Nuclear Nrf2, and Total Nrf2 was significantly increased in a dose-dependent manner (Figure 5). The result exhibited Sen is resistant to oxidation.
Sen Increased the Bcl-2/Bax Ratio
As shown in Figure 5, we detected the expression of anti-apoptosis protein Bcl-2 and pro-apoptosis protein Bax in different treatment groups. Bcl-2/Bax ratio in the Aβ1-42 treatment group was decreased compared with the control group, but after Sen treatment, this ratio was increased compared with the Aβ1-42 treatment group in a dose-dependent manner (Figure 6). The results indicated that Sen confers an anti-apoptotic effect under Aβ1-42.
Sen Attenuated Aβ1-42‑Induced Accumulation of Intracellular ROS Production
We investigated the effect of Sen on Aβ1-42-induced ROS by DCFH-DA staining. The results demonstrated that intracellular ROS levels significantly increased by Aβ1-42 stimulation, and significantly decreased after treated with Sen in a dose-dependent manner (P<0.01). These results indicate that Sen may has anti-oxidative effect by attenuating intracellular ROS levels in PC12 cells (Figure 7).
Sen Activated the PI3K/Akt Pathway
To examine the effects of Sen on PI3K phosphorylation (P-PI3K) and Akt phosphorylation (P-Akt), we analyzed P-PI3K and P-Akt by Western blot analysis. As shown in Figure 8, the ratio of P-PI3K/PI3K and P-Akt/Akt in the Aβ1-42 treatment group was decreased compared with the control group. Sen increased in the ratio of P-PI3K/PI3K and P-Akt/Akt compared to the Aβ1-42 group in a dose-dependent manner (p < 0.01). These results demonstrated that Sen may activate the PI3K/Akt signaling pathway, providing evidence that PI3K/Akt pathway may play an important role in the protective effects of Sen.
Discussion
Extensive neuronal cell death is an important hallmark of AD.50–52 Although the pathological mechanism is not clear, preventing nerve cell death is a promising method for the treatment of AD.53 The PC12 cell line has been widely used as an in vitro model to explore mechanisms in AD. In addition, the cells are particularly sensitive to Aβ-induced injury.54,55 In the present study, we established a PC12 cell model induced by Aβ1–42 to explore the neuroprotective effects and potential mechanisms of Sen.
Apoptosis is a common physiological phenomenon that leads to cell death.56,57 The PI3K/Akt signaling pathway, as a key regulator of apoptosis, is believed to play a vital role in AD.23–25 Increasing the phosphorylation of PI3K and Akt can inhibit the apoptosis, on the contrary, promote the apoptosis.58,59 The Bcl-2 family proteins are classified into anti-apoptotic proteins, including Bcl-2, and pro-apoptotic proteins, such as Bax.60,61 Bcl-2 and Bax are two key downstream target proteins in the PI3K/Akt signaling pathway.26 The ratio of Bcl-2/Bax determines whether or not a cell could undergo apoptosis.62,63 In this study, we found that Aβ1–42 treatment decreased the ratio of P-PI3K/PI3K, P-Akt/Akt and Bcl-2/Bax. Notably, Sen could increasing the phosphorylation of PI3K and Akt, which was consistent with the previous study.64 Therefore, Sen may reduce apoptosis by regulating the expression of Bcl-2 family proteins.
Oxidative stress is designated as an imbalance between the production of ROS and antioxidant defenses, which may be induced by exposure to Aβ and thought to contribute to memory decline.65,66 ROS may be induced by PI3K/Akt pathway32,33 and mediates Aβ-induced neurotoxicity in the neurodegenerative process of AD.67,68 Considering the important role of ROS in Aβ-induced neurotoxicity, compounds with antioxidant effects may have a potential neuroprotective effect on AD. Consistent with a previous research study, our results also showed that PC12 cells treated with Sen have been found to inhibit the intracellular production of ROS.44 In addition, Sen activated PI3K/Akt pathway, so we speculate that the antioxidant effect of Sen may related with PI3K/Akt pathway.
Nrf2 is a transcription factor and it has been shown to produce obvious antioxidant effects.69 When stimulated by external oxidative stress, Nrf2 will transfer to the nucleus and activate various protective molecules, thereby enhancing the cell’s resistance to external stimulation.70 In addition, Nrf2 can regulate the coordinated expression of cytoprotective genes, including enzymes such as HO-1.71 Pharmacological induction of HO-1 or overexpression of HO-1 in neuronal cells may have a protective effect on oxidative stress.72,73 Previous studies demonstrated that the PI3K/Akt signaling pathway has been demonstrate to be part of a central pathway involved in the activation and translocation of Nrf2 for highly specialized protein synthesis, including HO-1.34–36 In this study, the results showed that Sen that may be achieved by upregulating the levels of PI3K/Akt phosphorylation increased the expressions of HO-1 and promoted Nrf2 translocation into the nucleus in a dose-dependent manner.
Conclusion
In summary, this study provides evidence that Sen may be effective in inhibiting apoptosis and oxidative stress in Aβ1-42-induced PC12 cells, which might occurs by activating the PI3K/Akt signaling pathway. We describe in Figure 9 the possible molecular mechanism of Sen in exerting its neuroprotective effects in PC12 cells. Further researches are needed to better reveal the neuroprotective mechanism of Sen, which likely contribute to the development of novel therapies for AD.
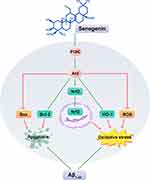 |
Figure 9 Schematic diagram of the possible mechanism underlying the neuroprotective effects of Sen on PC12 cells. |
Acknowledgments
This work was supported by Natural Science Foundation of Shandong Province (ZR2021MH180).
Author Contributions
All authors made a significant contribution to the conception, study design, execution, and acquisition of data, analysis and interpretation. They participated in drafting, revising or critically reviewing the article. They gave final approval of the version to be published and agreed upon the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17:327–406. doi:10.1002/alz.12328
2. Adachi N, Numakawa T, Richards M, Nakajima S, Kunugi H. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: implications in brain-related diseases. World J Biol Chem. 2014;5(4):409–428. doi:10.4331/wjbc.v5.i4.409
3. Imbrici P, Camerino DC, Tricarico D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front Genet. 2013;4:76. doi:10.3389/fgene.2013.00076
4. Um JW, Kaufman AC, Kostylev M, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron. 2013;79(5):887–902. doi:10.1016/j.neuron.2013.06.036
5. Troy CM, Rabacchi SA, Friedman WJ, Frappier TF, Brown K, Shelanski ML. Caspase-2 mediates neuronal cell death induced by beta-amyloid. J Neurosci. 2000;20(4):1386–1392. doi:10.1523/jneurosci.20-04-01386.2000
6. Cleary JP, Walsh DM, Hofmeister JJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8(1):79–84. doi:10.1038/nn1372
7. Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging. 2005;26(9):1235–1244. doi:10.1016/j.neurobiolaging.2005.05.022
8. Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 2006;281(29):20315–20325. doi:10.1074/jbc.M601016200
9. Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA. Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat Med. 1998;4(7):827–831. doi:10.1038/nm0798-827
10. Mao P, Reddy PH. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: implications for early intervention and therapeutics. Biochim Biophys Acta. 2011;1812(11):1359–1370. doi:10.1016/j.bbadis.2011.08.005
11. Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi:10.1126/science.1566067
12. Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi:10.1152/physrev.2001.81.2.741
13. Behl C, Davis JB, Klier FG, Schubert D. Amyloid beta peptide induces necrosis rather than apoptosis. Brain Res. 1994;645(1–2):253–264. doi:10.1016/0006-8993(94)91659-4
14. Castillo WO, Aristizabal-Pachon AF, Sakamoto-Hojo E, Gasca CA, Cabezas-Fajardo FA, Takahashi C. Caliphruria subedentata (Amaryllidaceae) decreases genotoxicity and cell death induced by β-amyloid peptide in SH-SY5Y cell line. Mutat Res Genet Toxicol Environ Mutagen. 2018;836:54–61. doi:10.1016/j.mrgentox.2018.06.010
15. Castillo WO, Aristizabal-Pachon AF, de Lima Montaldi AP, Sakamoto-Hojo ET, Takahashi CS. Galanthamine decreases genotoxicity and cell death induced by β-amyloid peptide in SH-SY5Y cell line. Neurotoxicology. 2016;57:291–297. doi:10.1016/j.neuro.2016.10.013
16. Behl C. Apoptosis and Alzheimer’s disease. J Neural Transm. 2000;107(11):1325–1344. doi:10.1007/s007020070021
17. Calissano P, Matrone C, Amadoro G. Apoptosis and in vitro Alzheimer disease neuronal models. Commun Integr Biol. 2009;2(2):163–169. doi:10.4161/cib.7704
18. Wang X, Xu W, Chen H, Li W, Li W, Zhu G. Astragaloside IV prevents Aβ(1-42) oligomers-induced memory impairment and hippocampal cell apoptosis by promoting PPARγ/BDNF signaling pathway. Brain Res. 2020;1747:147041. doi:10.1016/j.brainres.2020.147041
19. Long HZ, Cheng Y, Zhou ZW, Luo HY, Wen DD, Gao LC. PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front Pharmacol. 2021;12:648636. doi:10.3389/fphar.2021.648636
20. Zhao WQ, De Felice FG, Fernandez S, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22(1):246–260. doi:10.1096/fj.06-7703com
21. Griffin RJ, Moloney A, Kelliher M, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93(1):105–117. doi:10.1111/j.1471-4159.2004.02949.x
22. Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. FEBS J. 2005;272(16):4211–4220. doi:10.1111/j.1742-4658.2005.04833.x
23. Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9(1):59–71. doi:10.1111/j.1582-4934.2005.tb00337.x
24. Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407(6805):802–809. doi:10.1038/35037739
25. Zhang D, Wang Z, Sheng C, et al. Icariin prevents amyloid beta-induced apoptosis via the PI3K/Akt pathway in PC-12 cells. Evid Based Complement Alternat Med. 2015;2015:235265. doi:10.1155/2015/235265
26. Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170(6):2163–2172. doi:10.1097/01.ju.0000096060.92397.ed
27. Chu D, Zhang Z. Trichosanthis pericarpium aqueous extract protects H9c2 cardiomyocytes from hypoxia/reoxygenation injury by regulating PI3K/Akt/NO pathway. Molecules. 2018;23(10):2409. doi:10.3390/molecules23102409
28. Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23(1):134–147. doi:10.1016/s0891-5849(96)00629-6
29. Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem. 2006;96(1):1–13. doi:10.1111/j.1471-4159.2005.03530.x
30. Raza MH, Siraj S, Arshad A, et al. ROS-modulated therapeutic approaches in cancer treatment. J Cancer Res Clin Oncol. 2017;143(9):1789–1809. doi:10.1007/s00432-017-2464-9
31. Malik B, Pirzadah TB, Tahir I, Rehman RU, Hakeem KR, Abdin MZ. Plant signaling: response to reactive oxygen species. In: Plant Signaling: Understanding the Molecular Crosstalk. 2014:1–38.
32. Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthr Cartil. 2020;28(4):400–409. doi:10.1016/j.joca.2020.02.027
33. Spangle JM, Roberts TM, Zhao JJ. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer. 2017;1868(1):123–131. doi:10.1016/j.bbcan.2017.03.002
34. Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69(3):1033–1040. doi:10.1124/mol.105.018374
35. Calabrese V, Ravagna A, Colombrita C, et al. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J Neurosci Res. 2005;79(4):509–521. doi:10.1002/jnr.20386
36. Martin D, Rojo AI, Salinas M, et al. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279(10):8919–8929. doi:10.1074/jbc.M309660200
37. Ankarcrona M, Winblad B. Biomarkers for apoptosis in Alzheimer’s disease. Int J Geriatr Psychiatry. 2005;20(2):101–105. doi:10.1002/gps.1260
38. Camins A, Pallas M, Silvestre JS. Apoptotic mechanisms involved in neurodegenerative diseases: experimental and therapeutic approaches. Methods Find Exp Clin Pharmacol. 2008;30(1):43–65. doi:10.1358/mf.2008.30.1.1090962
39. Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287(24):3223–3229. doi:10.1001/jama.287.24.3223
40. Park CH, Choi SH, Koo JW, et al. Novel cognitive improving and neuroprotective activities of Polygala tenuifolia Willdenow extract, BT-11. J Neurosci Res. 2002;70(3):484–492. doi:10.1002/jnr.10429
41. Karakida F, Ikeya Y, Tsunakawa M, et al. Cerebral protective and cognition-improving effects of sinapic acid in rodents. Biol Pharm Bull. 2007;30(3):514–519. doi:10.1248/bpb.30.514
42. Jia H, Jiang Y, Ruan Y, et al. Tenuigenin treatment decreases secretion of the Alzheimer’s disease amyloid beta-protein in cultured cells. Neurosci Lett. 2004;367(1):123–128. doi:10.1016/j.neulet.2004.05.093
43. Lv J, Jia H, Jiang Y, et al. Tenuifolin, an extract derived from tenuigenin, inhibits amyloid-beta secretion in vitro. Acta Physiol. 2009;196:419–425. doi:10.1111/j.1748-1716.2009.01961.x
44. Zhu XQ, Li XM, Zhao YD, et al. Effects of Senegenin against hypoxia/reoxygenation-induced injury in PC12 cells. Chin J Integr Med. 2016;22(5):353–361. doi:10.1007/s11655-015-2091-8
45. Tong Y, Bai L, Gong R, Chuan J, Duan X, Zhu Y. Shikonin protects PC12 cells against β-amyloid peptide-induced cell injury through antioxidant and antiapoptotic activities. Sci Rep. 2018;8(1):26. doi:10.1038/s41598-017-18058-7
46. Zhu Y, Shi Y, Cao C, et al. Jia-Wei-Kai-Xin-San, an herbal medicine formula, ameliorates cognitive deficits via modulating metabolism of beta amyloid protein and neurotrophic factors in hippocampus of Aβ(1-42) induced cognitive deficit mice. Front Pharmacol. 2019;10:258. doi:10.3389/fphar.2019.00258
47. Yu X, Li Y, Mu X. Effect of quercetin on PC12 Alzheimer’s disease cell model induced by Aβ (25-35) and its mechanism based on sirtuin1/Nrf2/HO-1 pathway. Biomed Res Int. 2020;2020:8210578. doi:10.1155/2020/8210578
48. Cheng W, Chen W, Wang P, Chu J. Asiatic acid protects differentiated PC12 cells from Aβ(25-35)-induced apoptosis and tau hyperphosphorylation via regulating PI3K/Akt/GSK-3β signaling. Life Sci. 2018;208:96–101. doi:10.1016/j.lfs.2018.07.016
49. Lou H, Fan P, Perez RG, Lou H. Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-β-induced neuronal cell death. Bioorg Med Chem. 2011;19(13):4021–4027. doi:10.1016/j.bmc.2011.05.021
50. Yu W, Mechawar N, Krantic S, Quirion R. Evidence for the involvement of apoptosis-inducing factor-mediated caspase-independent neuronal death in Alzheimer disease. Am J Pathol. 2010;176(5):2209–2218. doi:10.2353/ajpath.2010.090496
51. Eckert A, Marques CA, Keil U, Schüssel K, Müller WE. Increased apoptotic cell death in sporadic and genetic Alzheimer’s disease. Ann N Y Acad Sci. 2003;1010(1):604–609. doi:10.1196/annals.1299.113
52. Smale G, Nichols NR, Brady DR, Finch CE, Horton WE
53. Donev R, Kolev M, Millet B, Thome J. Neuronal death in Alzheimer’s disease and therapeutic opportunities. J Cell Mol Med. 2009;13(11–12):4329–4348. doi:10.1111/j.1582-4934.2009.00889.x
54. Das KP, Freudenrich TM, Mundy WR. Assessment of PC12 cell differentiation and neurite growth: a comparison of morphological and neurochemical measures. Neurotoxicol Teratol. 2004;26(3):397–406. doi:10.1016/j.ntt.2004.02.006
55. Westerink RH, Ewing AG. The PC12 cell as model for neurosecretion. Acta Physiol. 2008;192(2):273–285. doi:10.1111/j.1748-1716.2007.01805.x
56. González-Mariscal L, Miranda J, Raya-Sandino A, Domínguez-Calderón A, Cuellar-Perez F. ZO-2, a tight junction protein involved in gene expression, proliferation, apoptosis, and cell size regulation. Ann N Y Acad Sci. 2017;1397(1):35–53. doi:10.1111/nyas.13334
57. Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J Biol Chem. 2007;282(25):18357–18364. doi:10.1074/jbc.M700202200
58. Chandra V, Fatima I, Manohar M, et al. Inhibitory effect of 2-(piperidinoethoxyphenyl)-3-(4-hydroxyphenyl)-2H-benzo(b)pyran (K-1) on human primary endometrial hyperplasial cells mediated via combined suppression of Wnt/β-catenin signaling and PI3K/Akt survival pathway. Cell Death Dis. 2014;5(8):e1380. doi:10.1038/cddis.2014.334
59. Ma D, Li S, Cui Y, et al. Paclitaxel increases the sensitivity of lung cancer cells to lobaplatin via PI3K/Akt pathway. Oncol Lett. 2021;21(3):219. doi:10.3892/ol.2021.12480
60. Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi:10.1038/nrm2308
61. Hata AN, Engelman JA, Faber AC. The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015;5(5):475–487. doi:10.1158/2159-8290.Cd-15-0011
62. Karmakar S, Banik NL, Ray SK. Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells. Neurochem Res. 2007;32(12):2103–2113. doi:10.1007/s11064-007-9376-z
63. Christodoulou MI, Kontos CK, Halabalaki M, Skaltsounis AL, Scorilas A. Nature promises new anticancer agents: interplay with the apoptosis-related BCL2 gene family. Anticancer Agents Med Chem. 2014;14(3):375–399. doi:10.2174/18715206113139990089
64. Pi T, Zhou XW, Cai L, et al. PI3K/Akt signaling pathway is involved in the neurotrophic effect of senegenin. Mol Med Rep. 2016;13(2):1257–1262. doi:10.3892/mmr.2015.4652
65. Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77(6):817–827. doi:10.1016/0092-8674(94)90131-7
66. Perrig WJ, Perrig P, Stähelin HB. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc. 1997;45(6):718–724. doi:10.1111/j.1532-5415.1997.tb01476.x
67. Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi:10.2174/157015909787602823
68. Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013;62:90–101. doi:10.1016/j.freeradbiomed.2012.11.014
69. Yu H, Chen B, Ren Q. Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1α/BNIP3 pathway. Artif Cells, Nanomed Biotechnol. 2019;47(1):3657–3663. doi:10.1080/21691401.2019.1657879
70. Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004;37(4):433–441. doi:10.1016/j.freeradbiomed.2004.04.033
71. Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91(21):9926–9930. doi:10.1073/pnas.91.21.9926
72. Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75(1):304–313. doi:10.1046/j.1471-4159.2000.0750304.x
73. Zhang F, Wang S, Zhang M, et al. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke. 2012;43(5):1390–1397. doi:10.1161/strokeaha.111.647420
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.