Back to Journals » Journal of Pain Research » Volume 13
Selective Ablation of Descending Serotonin from the Rostral Ventromedial Medulla Unmasks Its Pro-Nociceptive Role in Chemotherapy-Induced Painful Neuropathy
Authors Liu X , Wang G, Ai G, Xu X, Niu X , Zhang M
Received 3 August 2020
Accepted for publication 22 October 2020
Published 24 November 2020 Volume 2020:13 Pages 3081—3094
DOI https://doi.org/10.2147/JPR.S275254
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Robert B. Raffa
Xijiang Liu,1 Gongming Wang,1 Geyi Ai,1 Xiqiang Xu,2 Xinhuan Niu,1 Mengyuan Zhang1
1Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, People’s Republic of China; 2Department of Orthopedics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, People’s Republic of China
Correspondence: Xinhuan Niu; Mengyuan Zhang
Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, People’s Republic of China
Tel +86-53168776471
; +86-53168776472
Email [email protected]; [email protected]
Purpose: Chemotherapy-induced painful neuropathy (CIPN) is a severe adverse effect of many anti-neoplastic drugs that is difficult to manage. Serotonin (5-hydroxytryptamine, 5-HT) is an important neurotransmitter in the rostral ventromedial medulla (RVM), which modulates descending spinal nociceptive transmission. However, the influence of the descending 5-HT from the RVM on CIPN is poorly understood. We investigated the role of 5-HT released from descending RVM neurons in a paclitaxel-induced CIPN rat model.
Methods: CIPN rat model was produced by intraperitoneally injecting of paclitaxel. Pain behavioral assessments included mechanical allodynia and heat hyperalgesia. 5-HT content was analyzed by high-performance liquid chromatography (HPLC). Western blot and immunohistochemistry were used to determine tryptophan hydroxylase (Tph) and c-Fos expression. The inhibitors p-chlorophenylalanine (PCPA) and SB203580 were administrated by stereotaxical RVM microinjection. Ondansetron was injected through intrathecal catheterization.
Results: The results demonstrated that Tph, the rate-limiting enzyme in 5-HT synthesis, was significantly upregulated in the RVM, and that spinal 5-HT release was increased in CIPN rats. Intra-RVM microinjection of Tph inhibitor PCPA significantly attenuated mechanical and thermal pain behavior through Tph downregulation and decreased spinal 5-HT. Intra-RVM administration of p38 mitogen-activated protein kinase (p38 MAPK) inhibitor SB203580 alleviated paclitaxel-induced pain in a similar manner to PCPA. Intrathecal injection of ondansetron, a 5-HT3 receptor antagonist, partially reversed paclitaxel-induced pain, indicating that 5-HT3 receptors were involved in descending serotoninergic modulation of spinal pain processing.
Conclusion: The results suggest that activation of the p38 MAPK pathway in the RVM leads to increased RVM Tph expression and descending serotoninergic projection to the spinal dorsal horn and contributes to the persistence of CIPN via spinal 5-HT3 receptors.
Keywords: chemotherapy-induced painful neuropathy, paclitaxel, rostral ventromedial medulla, descending facilitation, 5-hydroxytryptamine
Introduction
Chemotherapy is part of the standard of care for cancer. Widely used chemotherapeutic drugs in clinical practice include taxanes, vinca alkaloids, and platinum-based drugs.1 These are used against the most common types of cancer, but may cause chemotherapy-induced painful neuropathy (CIPN). CIPN is an important clinical problem that impedes cancer treatment by limiting therapeutic options (eg, reducing doses, switching to less effective treatments, and discontinuing treatment).2,3 Paclitaxel, a member of the taxane class, is among the most effective and commonly used anti-cancer drugs. The neurotoxicity caused by paclitaxel usually manifests as sensory neuropathy. It is reported that neuropathic symptoms occur in 20–80% of all patients treated with paclitaxel depending on the dosage used.4,5 The most common symptoms are spontaneous pain, mechanical allodynia, and numbness in the hands and feet. These sensory symptoms usually start symmetrically from the feet, sometimes appearing on both hands and feet simultaneously, in a “stocking and glove” distribution.2,4 Although most patients’ symptoms disappear within months after paclitaxel therapy discontinuation, paresthesia and pain sometimes become persistent. This persistent pain affects the patient’s quality of life.4,6
In recent years, important progress has been made on the neural mechanisms underlying CIPN, particularly the functions of spinal dorsal root ganglia and spinal sensory neurons.1,7 However, the mechanism of CIPN is still not fully understood. Descending modulation of nociceptive transmission from the supraspinal center plays an important role in the maintenance of persistent pain.8 The rostral ventromedial medulla (RVM) is a critical component of the descending pain modulatory system that constitutes a major mechanism of spinal nociceptive transmission regulation during different pain states.9,10 The RVM consists of the nucleus raphe magnus (NRM), nucleus reticularis gigantocellularis pars alpha (GiA), and the ventral nucleus reticularis gigantocellularis (vGi). The RVM is a major source of serotonergic projections into the spinal dorsal horn.11 The RVM receives neuronal inputs from supraspinal sites, and is likely the final common relay in descending modulation of nociception from the brain.9,11,12
There is an anatomically discrete group of serotonin (5-hydroxytryptamine, 5-HT)-containing neurons in the RVM. 5-HT is an important neurotransmitter involved in the descending pain modulatory pathway from the RVM. The spinal projection of RVM 5-HT-containing neuronal axons constitutes the descending serotoninergic pathway to the spinal cord.11,13 5-HT plays a crucial role in the development of central sensitization and amplification of pain responses in the spinal cord.12 Some studies have shown that activation of mitogen-activated protein kinases (MAPKs) in some brain regions plays an important role in the descending nociception modulation after peripheral tissue injury.14–17 Tryptophan hydroxylase (Tph) is the rate-limiting enzyme in 5-HT biosynthesis.18 It has been reported that the transcription of Tph could be regulated by phosphorylation of p38 MAPK.19 Recent studies have indicated that selectively depleting 5-HT in RVM neurons with regional ablation of Tph inhibited inflammatory and neuropathic pain.13 Despite these results, the role of 5-HT in the pathogenesis of CIPN is unclear. The aim of the present study was to investigate the role of 5-HT released from RVM neurons in descending pain modulation during CIPN development in a paclitaxel-induced CIPN rat model.
Materials and Methods
Animals
Adult male Sprague Dawley rats weighing 250–300 g were housed in a temperature-controlled room (22°C–24°C) with free access to food and water under a natural light/dark cycle. Animals were placed in the testing equipment to habituate to the environment before experiments. All experimental protocols were approved by the Experimental Animal Care and Use Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University (NO:2019–134) and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the International Association for the Study of Pain guidelines for pain research. All rats were randomly divided into groups in each experiment.
Modeling of CIPN in Rats
We produced the paclitaxel-induced pain model by intraperitoneally injecting 1 mg/kg paclitaxel (Taxol®, Bristol–Myers–Squibb, New York City, NY, USA) on four alternating days (days 1, 3, 5, and 7).20 Sham rats were intraperitoneally injected with the vehicle (a 2:1 mixture of saline/Cremophor EL). Naïve rats did not undergo any treatment. None of the rats showed signs of ill health such as diarrhea, alopecia, or weight loss.
Behavioral Assessment
Mechanical Allodynia
Mechanical allodynia was assessed by measuring the hind paw withdrawal threshold (PWT) after von Frey filament stimulation. Rats stood on a wire mesh floor beneath an inverted plastic cage. The von Frey filament was applied to the hind paw plantar surface in ascending order of force (0.16, 0.4, 0.6, 1, 2, 4, 6, 8, 10 and 15 g) for up to 6 s per filament. Once a withdrawal response was established, the hind paw was re-tested, starting with the next lower von Frey filament, until no response occurred. The lowest amount of force required to elicit a positive response was designated as the PWT, represented in grams (g).21–23
Heat Hyperalgesia
Heat hyperalgesia of the hind paw was measured using methods reported by Hargreaves et al.24 In brief, rats were placed in a plastic cage on a glass plate, and the light source under the glass plate was aimed at the plantar surface of the hind paw. The time from the start of irradiation to the withdrawal reflex of the hind paw was defined as the paw withdrawal latency. The light source automatically cut off for 15 s to prevent tissue damage. The thermal stimulation intensity remained consistent throughout the experiment. The left and right hind paws of each rat were measured three times alternately, and the interval between each test was 5 min. The average of paw withdrawal latencies of the last two tests was taken as the thermal hyperalgesia threshold. All behavioral tests were conducted under blind conditions.
5-HT Analysis by High-Performance Liquid Chromatography (HPLC)
Rats were anesthetized with 10% chloral hydrate (300 mg/kg, intraperitoneally) and decapitated at 1 day before, 7, 14, and 21 days after drug administration. The dorsal part of each lumbar spinal cord segment was dissected. Spinal cord tissue was fully homogenized in an ice bath with 1 mL of 0.4 mol/L perchloric acid for every 100 mg of spinal cord tissue. Homogenate stood for 10 min before centrifugation at 4°C for 20 min. The supernatant was stored at −20°C until use. HPLC was carried out using an Agilent chromatograph equipped with a fluorescence detector (Agilent, Santa Clara, California, USA). A C18 column (4.6 mm×250 mm, 5 μm, Agilent) was used for the reserve phase. A mixture (9:1) of 0.1 mol/L potassium dihydrogen phosphate solution and methanol was used as a mobile phase. The mobile phase flow rate was 1.0 mL/min (λEx: 254 nm; λEm: 338 nm; column temperature: 25°C). Serotonin hydrochloride (H9523, Sigma, USA) was used as the internal standard. The supernatant of spinal cord tissue homogenate was mixed with methanol in a 1:1 ratio, precipitated for 30 min, and centrifuged at 4°C for 10 min. The supernatant was passed through a 0.45-μm filtration membrane and the injection sample volume was 20 μL. The analysis was performed in triplicate, and data are expressed as the mean ± standard deviation of three measurements.25
Western Blot Assay
The RVM tissues were removed from the anesthetized and decapitated rats as previously described.26 The proteins were extracted using a total protein extraction kit (Cat. No: SJ-200,501, ProMab) according to the protocol provided by the manufacturer. Equal amounts of total proteins (50 μg) from the RVM were separated on 10% SDS-PAGE gels and then trans-blotted to nitrocellulose membranes. The blot was incubated with sheep anti-Tph antibody (1:1000, Millipore, USA) overnight at 4°C. The membrane was washed with Tris-buffered saline and incubated for 1 h with anti-sheep IgG/HRP (1:2000, Santa Cruz Biotechnology, USA) in 5% milk/TBS. The loading and blotting of an equal amount of proteins were verified by reprobing the membrane with an anti-GAPDH antibody (1:1000, ProMab). The blots were finally visualized in enhanced chemiluminescence solution (Pierce, Rockford, IL, USA) and exposed to X-ray films. The developed X-ray films were scanned with a Bio-Rad scanner for data analysis.21,27
RVM Drug Microinjection
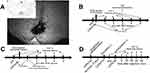
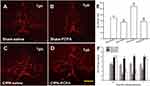
Rats were anesthetized with 10% chloral hydrate (300 mg/kg, intraperitoneally) and then placed on the stereotaxic apparatus. A midline incision was made after the infiltration of 2% lidocaine into the skin. A small hole was drilled in the skull to insert a microinjection needle into the target site. The coordinates for RVM were as follows: –10.5 mm caudal to bregma, midline, and –9.0 mm ventral to the surface of the cerebellum.13 Microinjection was performed by delivering 0.5 μL of the drug solution slowly over a 30 s period, using a 0.5-μL Hamilton syringe with a 32-gauge needle. The injection needle was left in place for at least 5 min before withdrawal. Microinjection sites in the RVM were verified by the administration of 0.5 μL of carbon black ink, and medullary sections were observed and photographed with an Olympus fluorescence microscope (Figure 1). In inhibitor experiments, the selective Tph inhibitor para-chlorophenylalanine (PCPA, #C6506, Sigma) was dissolved in normal saline, and the p38 MAPK inhibitor SB203580 (#S8307, Sigma) was dissolved in 2% dimethyl sulfoxide (DMSO).18,26,28,29
Immunohistochemistry
Animals were anesthetized using 10% chloral hydrate (300 mg/kg, intraperitoneally) and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde. The brain was removed, post-fixed in 4% paraformaldehyde, and immersed in a 10–30% sucrose gradient in phosphate buffer saline (PBS) at 4°C. Transverse brain sections of the RVM (20 μm) were cut in a cryostat according to the stereotaxic coordinates by Paxinos and Watson, and processed for immunofluorescence. All sections were blocked with 10% goat serum, and incubated for 24 h at 4°C with the primary antibody. The primary antibodies used in this study were as follows: sheep anti-Tph (1:500, Millipore), and rabbit anti-c-Fos (1:200, Santa Cruz Biotechnology, Inc.). The sections were rinsed in PBS and subsequently incubated with the corresponding secondary antibodies (Cy3-rabbit-anti-sheep [1:200] and fluorescein isothiocyanate-conjugated goat-anti-rabbit-immunoglobulin G [1:200]) for 2 h at room temperature. The immunofluorescent sections were imaged with an Olympus fluorescence microscope with a charge-coupled device spot camera for data analysis. The numbers of RVM Tph-positive neurons and spinal c-Fos neurons were counted in one out of every neighboring three sections by two researchers blind to the treatment conditions.26
Intrathecal Catheterization
Fourteen days after intraperitoneal injection of paclitaxel, rats received intrathecal catheterization. After being anesthetized with an intraperitoneal injection of 10% chloral hydrate (300 mg/kg), the rat’s lumbar area was prepared, and then disinfected with 75% ethanol. A longitudinal 1.5-cm incision was made in the skin at the L3–L4 level, and the spinous process of L3 was removed until the spinal dura mater was exposed. The intrathecal catheter (PE-10 tube, AniLab Software and Instruments Co. Ltd, Ningbo, China) was inserted 2.5 cm into the subarachnoid space. After entry into the subarachnoid space was confirmed by sudden tail movement, the catheter was situated at the lumbar enlargement of the spinal cord. The other end was tunneled subcutaneously toward the occiput, exposing the distal tip out of the posterior cervical area. After recovery from anesthesia, only rats that had normal motor function were chosen for further observation. Three days after catheterization, 10 μL of 2% lidocaine was injected intrathecally through the catheter to observe lower limb paralysis. Only the rats showing bilateral lower limb paralysis were selected for further experiments.12,21 Four days after catheterization, 5-HT3 receptor antagonist ondansetron was injected intrathecally, and its impact on pain behavior was determined. Ondansetron (O3639, Sigma) was dissolved in normal saline (20 μg in 20 μL/rat, intrathecally).30
Statistical Method
Data were expressed as mean ± SEM and analyzed using SPSS Statistics 22.0 (SPSS Inc., Chicago, USA). The mechanical allodynia and heat hyperalgesia data and the 5-HT levels at the spinal cord determined by HPLC obtained in the naive, sham and CIPN groups were analyzed by one-way ANOVA followed by post hoc comparison (Tukey’s honestly significant difference test). The other behavioral data and the data obtained in Western blot, HPLC and immunohistochemistry were compared by unpaired Student’s t-tests. P < 0.05 was considered statistically significant.
Results
Changes in Pain Thresholds of Paclitaxel-Injected Rats
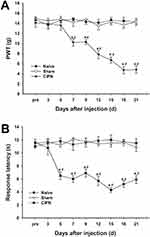
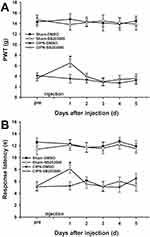
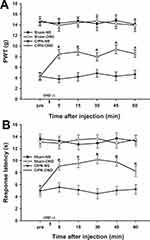
The possible effects of paclitaxel on mechanical allodynia and heat hyperalgesia were determined. Mechanical PWT was significantly decreased in paclitaxel-injected rats compared with the control groups. The PWT decreased significantly from day 7 to day 21 after paclitaxel injection (n = 6, P < 0.05) (Figure 2A). The response latencies to thermal stimuli were significantly shortened in paclitaxel-injected rats compared with those in control groups. The response latencies to thermal stimuli shortened significantly from day 5 to day 21 after injection (n = 6, P < 0.05) (Figure 2B). Paclitaxel-injected rats exhibited obvious mechanical allodynia and thermal hyperalgesia without significant weight loss or health changes until day 21. The results did not show significant changes in PWT and response latencies in the control groups (P > 0.05).
Upregulation of Descending Serotoninergic Projection in CIPN Rats
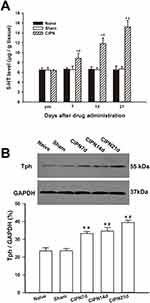
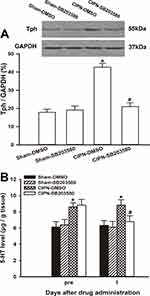
HPLC showed that the spinal content of 5-HT was significantly upregulated in the CIPN rats compared with the sham and naïve group on days 7, 14, and 21 after paclitaxel injection (day 7:F(2,6)=6.663, P=0.030; CIPN vs naive, P=0.048; CIPN vs sham, P=0.042; day 14:F(2,6)=32.937, P=0.001; CIPN vs naive, P=0.001; CIPN vs sham, P=0.001; day 21:F(2,6)=59.226, P<0.001; CIPN vs naive, P<0.001; CIPN vs sham, P<0.001). There was no significant change in the content of spinal 5-HT between the sham group and naïve group (P > 0.05) (Figure 3A). Western blotting showed that Tph expression was significantly upregulated in the RVM on days 7, 14, and 21 in CIPN rats compared with the sham and naïve groups (day 7: CIPN vs naive, P=0.003; CIPN vs sham, P=0.001; day 14: CIPN vs naive, P=0.005; CIPN vs sham, P=0.003; Day 21: CIPN vs naive, P=0.001; CIPN vs sham, P<0.001) (Figure 3B). There was no significant difference in Tph expression between the sham group and the naïve group (P=0.918).
Inhibition of Tph in the RVM Alleviates CIPN
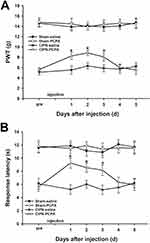
The results of the von Frey test showed that microinjection of 20 µg PCPA (the selective inhibitor of Tph) into the RVM significantly attenuated the chemotherapy-induced mechanical allodynia from day 1 to day 3 after administration (day1: CIPN-PCPA vs CIPN-saline, P<0.001; day2: CIPN-PCPA vs CIPN-saline, P<0.001; day3: CIPN-PCPA vs CIPN-saline, P=0.001) (Figure 4A). Heat hyperalgesia was also significantly alleviated after microinjection of PCPA into the RVM of CIPN rats compared with the RVM saline microinjection CIPN group (day1: CIPN-PCPA vs CIPN-saline, P<0.001; day2: CIPN-PCPA vs CIPN-saline, P<0.001; day3: CIPN-PCPA vs CIPN-saline, P<0.001) (Figure 4B). The baseline mechanical and thermal pain thresholds were not significantly altered by RVM microinjection of PCPA in the control groups (P > 0.05).
Inhibition of Descending Serotoninergic Projection by RVM Microinjection of PCPA
To determine the changes in Tph neurons in the RVM after PCPA microinjection, we examined the number of Tph neurons in the RVM via immunofluorescence on the third day after microinjection. Tph neurons were found throughout the RVM in both sham and CIPN rats. However, the number of RVM Tph neurons was dramatically reduced by PCPA compared with the saline microinjection group (sham-PCPA vs sham-saline, P=0.038; CIPN-PCPA vs CIPN-saline, P=0.001; CIPN-saline vs sham-saline, P=0.003) (Figure 5A–E). We also determined 5-HT content in the dorsal spinal cord at levels L4–L6 via HPLC. HPLC showed a time-dependent decrease of 5-HT concentration in the spinal dorsal horn after microinjection of PCPA. 5-HT content in the spinal dorsal horn was significantly lower in PCPA-treated rats than in saline-treated rats (day 1: sham-PCPA vs sham-saline, P=0.002; CIPN-PCPA vs CIPN-saline, P=0.002; day 3: sham-PCPA vs sham-saline, P=0.002; CIPN-PCPA vs CIPN-saline, P=0.001; day 5: sham-PCPA vs sham-saline, P=0.028; CIPN-PCPA vs CIPN-saline, P=0.410) (Figure 5F).
Intra-RVM Microinjection of SB203580 Alleviates CIPN Behaviors
Previous studies indicated that p38 MAPK was involved in both neuropathic pain and inflammatory pain. To investigate the role of p38 MAPK in the RVM in paclitaxel-induced pain, either selective p38 MAPK inhibitor SB203580 (50 μg) or DMSO was microinjected into the RVM of the experimental rats. The results of the von Frey test showed that paclitaxel-induced mechanical allodynia was significantly attenuated one day after RVM microinjection of SB203580 without altering the baseline mechanical pain threshold in the sham group (day 1: CIPN-SB203580 vs CIPN-DMSO, P<0.001) (Figure 6A). In addition, the thermal pain threshold was significantly reversed at one day after RVM microinjection of SB203580 (day 1: CIPN-SB203580 vs CIPN-DMSO, P<0.001) (Figure 6B). The pain thresholds were not altered by RVM microinjection of DMSO in both CIPN rats and sham rats (P > 0.05).
Inhibition of Descending Serotoninergic Projection by Intra-RVM Microinjection of SB203580
To investigate the effect of intra-RVM microinjection of SB203580 on the expression of Tph, the RVM tissue was removed from the experimental rats one day after drug administration. Western blotting showed that the protein level of Tph in SB203580-treated CIPN rats was significantly lower compared with the vehicle-treated CIPN rats (P =0.001). The expression of Tph was not significantly altered by SB203580 in sham rats (P > 0.05) (Figure 7A). The HPLC results showed that the 5-HT level in the dorsal spinal cord was significantly downregulated in SB203580-treated CIPN rats compared with DMSO-treated CIPN rats (P=0.046) (Figure 7B). 5-HT content in the dorsal spinal cord was not significantly changed in SB203580-treated sham rats (P > 0.05).
Reduction of c-Fos Expression in the Spinal Cord by Intra-RVM Microinjection of SB203580
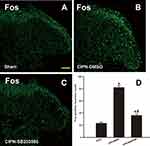
To further investigate whether RVM descending serotoninergic projection contributed to the activation of spinal pain-sensitive neurons, c-Fos expression was detected in the spinal dorsal horn. One day after the intra-RVM administration of SB203580, mechanical noxious stimuli were applied to the plantar surface of the rat’s hind paw, and the lumbar spinal cords were collected 2 hours later. The results of immunofluorescence showed that SB203580 significantly suppressed CIPN-induced c-Fos expression in the spinal dorsal horn (P<0.001), suggesting that pain-sensitive neurons in the spinal cord were inhibited by SB203580 (Figure 8).
Intrathecal Injection of Ondansetron Partially Reversed Paclitaxel-Induced Mechanical Allodynia and Thermal Hyperalgesia
5-HT from the RVM plays an important role in modulating spinal nociceptive signaling via the 5-HT receptor subtypes, including the 5-HT3 receptor. To investigate the role of the spinal 5-HT3 receptor in CIPN, the 5-HT3 receptor antagonist ondansetron was injected intrathecally. Mechanical allodynia and thermal hyperalgesia were significantly reversed from 5 min to 60 min after intrathecal injection of ondansetron in CIPN rats (P<0.001) (Figure 9). The mechanical and thermal pain thresholds were not changed by intrathecal injection of ondansetron in sham rats (P > 0.05).
Discussion
In this study, we showed that RVM Tph and spinal 5-HT were apparently upregulated in CIPN rats, and intra-RVM administration of PCPA significantly alleviated mechanical and thermal pain behaviors through selective depletion of RVM-spinal descending serotonergic projection. Additionally, intra-RVM microinjection of SB203580 attenuated paclitaxel induced pain and partially abolished the descending serotonergic projection. Moreover, the upregulated c-Fos expression in the spinal dorsal horn of the CIPN rats was significantly inhibited by RVM injection of SB203580. Intrathecal ondansetron partially relieved paclitaxel-induced pain behaviors, indicating 5-HT3 receptors were involved in descending serotonergic modulation of spinal nociceptive transmission. Our findings suggest that RVM-spinal descending serotonergic projection pathway activation is involved in the modulation of spinal nociceptive transmission of CIPN and activation of RVM p38 MAPK signaling pathway contributes to descending serotonergic facilitation of CIPN.
In recent years, researchers have examined the mechanisms involved in the development of CIPN. Most of these studies focused on the mechanisms at the peripheral and spinal levels.7 CIPN has been associated with a variety of alterations, including abnormalities in injured and uninjured afferents supplying the lesion site, upregulation of inflammatory mediators, altered ion channel activity, central sensitization, and autonomic nervous system alterations.1 However, the exact pathophysiology is still unclear, and various underlying mechanisms have been proposed for different classes of chemotherapy drugs. The cytotoxicity mechanisms of chemotherapy drugs are often linked to their neurotoxicity side-effect, implying the difficulty in eliminating toxicity without reducing the anti-cancerous efficacy of the drugs.7 The endogenous descending pain modulating system is an integral part of supraspinal pain signal inhibitory and facilitatory processing, particularly in persistent pain.14,31 The descending pain modulation system has been shown to contribute to the development of chronic pain in various conditions, including inflammatory, neuropathic, and cancer pain.9,32,33 Since CIPN is usually accompanied by inflammation and nerve damage, it is reasonable to speculate that descending pain modulation is involved in the pathogenesis of CIPN. The RVM is a key part of the endogenous descending pain modulating system that relays information about pain modulation from higher brain sites, and exerts bidirectional pain modulation for spinal pain transmission.14 The RVM is a major source of serotonergic projection to the spinal dorsal horn, and 5-HT is one of the major neurotransmitters involved in descending modulation of spinal nociceptive transmission.9 Changes in descending 5-HT release have been observed under pathological pain conditions.34 For example, spinal 5-HT level was increased in several models of neuropathic pain and inflammatory pain.35,36 Serotonin released from RVM neurons plays an important role in descending pain modulation during different persistent pain states, but its role in CIPN is not fully understood. In the present study, RVM Tph and spinal 5-HT were upregulated in CIPN rats, and intra-RVM administration of the Tph inhibitor PCPA significantly alleviated mechanical and thermal pain behaviors through selective depletion of RVM spinal descending serotonergic projection. Therefore, our findings suggest that RVM spinal descending serotonergic projection pathway activation is involved in the modulation of spinal nociceptive transmission in CIPN.
It has been established that the RVM descending serotonergic pathway exerts an inhibitory or facilitatory influence on spinal pain processing, depending on the activation of diverse 5-HT subtype receptors.37 5-HT in the spinal dorsal horn greatly contributes to pain modulation. Spinal 5-HT receptors mediate complex pro- and anti-nociceptive effects; in general, 5-HT2A/5-HT3/5-HT4 receptors are considered to be facilitatory whereas 5-HT1/2C/7 receptors are inhibitory. These receptors are present in the pre-synaptic terminals of primary afferent nerve fibers, inhibitory interneurons, excitatory interneurons and projection neurons, and modify nociceptive transmission. Several lines of evidence suggest that 5-HT receptor function changes in various neuropathic pain states.38 The 5-HT1A receptor is involved in central pain modulation mechanisms with a pivotal role in the inhibitory descending pain pathway. It is expressed in the raphe nucleus and in several areas involved in central pain modulation mechanisms. Full and partial 5-HT1A receptor agonists have shown to be effective in pain relieving.39 Previous findings have shown that 5-HT2C receptor activation in the spinal cord induced antiallodynic effects in neuropathic pain models, but mediated the nociceptive effects of oxaliplatin-induced neuropathy.40 It has been proposed that the balance between RVM descending serotonergic facilitation and inhibition shifts toward pronociception in persistent pain via enhanced activation of pronociceptive 5-HT receptors, including the 5-HT3 receptor.12 The presence of spinal 5-HT3 receptors has been reported in the superficial layers of the spinal dorsal horn, on terminals of excitatory interneurons and on neurokinin 1 projection neurons in laminae I/III. 5-HT3 receptors are also expressed on inhibitory GABAergic interneurons.37,41 Previous studies have reported key roles of the 5-HT2A and 5-HT3 receptors in descending pain facilitation in a lot of pain states. These data identified modality and intensity selective facilitatory roles of spinal 5-HT2A and 5-HT3 receptors on sensory neuronal processing within the pain ascending transmission pathway. It was reported that cold and heat stimuli transmission was facilitated via spinal 5-HT2A and 5-HT3 receptors, respectively, in spinal nerve ligation rats. The 5-HT3R-mediated sensitisation of transient receptor potential vanilloid 1 (TRPV1) in injured and uninjured primary afferent terminals likely led to sensitisation to mechanical and heat stimuli in neuropathic pain. In neuropathic pain animal models, many studies demonstrated that ablation of descending serotoninergic pathways inhibited pain hypersensitivity, and have also indicated that nerve injury induced descending facilitation by activating spinal 5-HT3 receptors.38,42 In mice with genetic deletion of the 5-HT3 receptor, the second phase nociceptive behavioral responses to mechanical and thermal stimuli in the formalin test, which involves centrally mediated mechanisms, were significantly alleviated.43,44 These studies implied that spinal 5-HT upregulated to bind to 5-HT3 receptors under conditions of tissue-injury-induced pain. 5-HT is synthesized in RVM descending neurons, and the 5-HT3 receptors are located on the nerve terminals of myelinated and unmyelinated primary afferent fibers (Aδ and C) in the superficial layers of the spinal dorsal horn.44,45 In this study, compared with control rats, Tph expression in the RVM was significantly upregulated in CIPN rats. Activation of Tph-positive neurons in the RVM resulted in 5-HT release into the spinal cord and binding to 5-HT3 receptors. The HPLC results in our study indicated that the release of 5-HT was increased in the spinal cord during the persistence of CIPN. An increase in c-Fos-immunoreactive neurons has been widely used as an indicator for the activation of pain-sensitive neurons.12 To confirm our observations of pain behaviors, we investigated c-Fos protein expression in the spinal cord. Consistent with upregulated 5-HT release and enhanced pain behaviors in CIPN rats, c-Fos expression was elevated in the spinal dorsal horn. Intrathecal injection of ondansetron partially relieved paclitaxel-induced pain behaviors, indicating that the 5-HT3 receptor is involved in descending serotonergic modulation of spinal nociceptive transmission. Therefore, these results imply that the activation of spinal 5-HT3 receptors is involved in the persistence of CIPN via descending facilitation of spinal processing of nociceptive information, which is consistent with the previous studies. In addition, the effects of other spinal 5-HT receptor subtypes in the development of spinal neuronal hyperexcitability during CIPN require further study.
The role of RVM 5-HT-containing neurons in chronic pain has been previously studied by inducing lesions in these neurons. Some studies have shown that RVM 5-HT-containing neurons played a role in descending pain facilitation, while other studies have reported that they participated in descending pain inhibition.37,41 Wei et al reported that descending 5-HT released from the RVM played a critical role in descending pain facilitation, rather than inhibition, during hyperalgesia and allodynia maintenance after peripheral inflammation and nerve injury via RNAi of Tph in the RVM.13 Wei et al’s results suggest that spinal projection of 5-HT from the RVM is necessary to maintain persistent pain states. In this study, we stereotactically microinjected the selective Tph inhibitor PCPA into the RVM of CIPN rats. Using this method, the synthesis of 5-HT in RVM neurons was selectively inhibited, thus reducing the descending projection of 5-HT to the spinal cord. As compared with the method of inducing lesions in RVM 5-HT-containing neurons using the selective serotonergic neurotoxin 5,7-DHT,46 or loss of serotonergic neurons by genomic knock-out of the transcription factor Lmx1b,47 our method only specifically eliminated the RVM spinal 5-HT projection without affecting the other functions of RVM 5-HT-containing neurons. 5.7-DHT and gene regulation methods could lead to lesions in the RVM 5-HT-containing neurons, which also damage other colocalized neurotransmitters and the multiple receptors expressed in serotonergic neurons. Thus, with our method, it is possible to accurately evaluate the role of RVM spinal descending 5-HT in CIPN pathogenesis.
It has been known that the descending pain modulatory system undergoes plastic changes following peripheral inflammation and nerve injury, and exerts bidirectional (facilitatory and inhibitory) influence on spinal nociceptive responses.9,14 Many studies have shown that peripheral tissue inflammation and nerve injury can activate mitogen-activated protein kinases (MAPKs) in some brain regions that are key components of the descending pain modulatory system.15,16 Activation of these MAPKs is thought to play an important role in the development of central nervous system plasticity, which itself has critical involvement in the dynamic changes that occur in the descending nociception modulatory system after peripheral tissue injury.17 Peripheral inflammation induced by complete Freund’s adjuvant activated p38 MAPK in RVM neurons.48 Carrageenan-induced inflammatory pain was alleviated by microinjection of the p38 MAPK inhibitor SB203580 into the RVM.26 It has been reported that intrathecal delivery of the Ras inhibitor attenuates hyperalgesia and allodynia in vincristine-induced neuropathic pain. Ras is a key element in activating the intracellular signal transduction pathway involving MAP-kinase family members, such as Ras/Raf/MEK/ERK2. MAP-kinase cascade activation might be an important mechanism in the onset of vincristine-related neuropathic pain.49 Paclitaxel is known to induce strong inflammatory responses in the central and peripheral nervous systems, which has been associated with the formation and sustention of persistent pain.5 Inflammatory events, such as an increased release of proinflammatory cytokines in the peripheral nerves, are also linked to paclitaxel-induced pain.50 In this study, intra-RVM administration of the p38 MAPK selective inhibitor SB203580 significantly alleviated CIPN caused by paclitaxel, and the analgesic effect was related both to the inhibition of Tph expression in the RVM and 5-HT release in the spinal cord. A previous study has demonstrated that phosphorylation of p38 MAPK can activate the transcription of Tph, thereby increasing the biosynthesis of 5-HT.19 In the RVM of CIPN rats, the expression of Tph was significantly increased, and intra-RVM injection of SB203580 selectively blocked the p38 MAPK pathway, thereby at least partially blocking the transcription of Tph in RVM serotonergic neurons. This in turn inhibited the expression of Tph and reduced 5-HT projection to the spinal cord. This may be one of the mechanisms by which the p38 MAPK pathway is involved in RVM serotonergic descending pain facilitation in CIPN. In addition, the role of other members of the MAPK superfamily in CIPN development requires further investigation.
Conclusion
Taken together, our data indicate that increased RVM descending serotonergic projection to the spinal dorsal horn contributes to paclitaxel-induced pain. This process can be attributed at least partially to the activation of the p38 MAPK pathway in the RVM. Descending serotonergic activation of spinal 5-HT3 receptors is involved in the persistence of CIPN via descending facilitation of spinal nociceptive transmission. Our findings shed new light on the supraspinal descending serotonergic mechanism underlying the persistence of CIPN caused by paclitaxel, and provide experimental evidence for the exploitation of new mechanism-based treatments for CIPN.
Acknowledgments
The present work was supported by the Development Project of Shandong Province (2018GSF118165) and the National Natural Science Foundation of China (NSFC, 81971010).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Boyette-Davis JA, Hou S, Abdi S, Dougherty PM. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Manag. 2018;8(5):363–375. doi:10.2217/pmt-2018-0020
2. Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on chemotherapy-induced peripheral neuropathy (CIPN): a narrative review. Br J Anaesth. 2017;119(4):737–749. doi:10.1093/bja/aex229
3. Ibrahim EY, Ehrlich BE. Prevention of chemotherapy-induced peripheral neuropathy: a review of recent findings. Crit Rev Oncol Hematol. 2020;145:102831. doi:10.1016/j.critrevonc.2019.102831
4. Beh ST, Kuo YM, Chang WW, et al. Preventive hypothermia as a neuroprotective strategy for paclitaxel-induced peripheral neuropathy. Pain. 2019;160(7):1505–1521. doi:10.1097/j.pain.0000000000001547
5. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
6. Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 2017;81(6):772–781. doi:10.1002/ana.24951
7. Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neurosci Lett. 2015;596:90–107. doi:10.1016/j.neulet.2014.10.014
8. Chen T, Taniguchi W, Chen QY, et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat Commun. 2018;9(1):1886. doi:10.1038/s41467-018-04309-2
9. Zhuo M. Descending facilitation. Mol Pain. 2017;13:1744806917699212. doi:10.1177/1744806917699212
10. Khasabov SG, Wang JC, Simone DA, Strichartz GR. A role for neurokinin-1 receptor neurons in the rostral ventromedial medulla in the development of chronic post thoracotomy pain. Pain. 2017;158(7):1332–1341. doi:10.1097/j.pain.0000000000000919
11. Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120(11):3779–3787. doi:10.1172/JCI43766
12. Huang ZX, Lu ZJ, Ma WQ, et al. Involvement of RVM-expressed P2X7 receptor in bone cancer pain: mechanism of descending facilitation. Pain. 2014;155(4):783–791. doi:10.1016/j.pain.2014.01.011
13. Wei F, Dubner R, Zou S, et al. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci. 2010;30(25):8624–8636. doi:10.1523/JNEUROSCI.5389-09.2010
14. Chen Q, Heinricher MM. Descending control mechanisms and chronic pain. Curr Rheumatol Rep. 2019;21(5):13. doi:10.1007/s11926-019-0813-1
15. Imbe H, Senba E, Kimura A, Donishi T, Yokoi I, Kaneoke Y. Activation of mitogen-activated protein kinase in descending pain modulatory system. J Signal Transduct. 2011;2011:468061. doi:10.1155/2011/468061
16. Hu ML, Zhou FY, Liu JJ, Ding Y, Zhong JM, Ding MX. Electroacupuncture inhibits the activation of p38MAPK in the central descending facilitatory pathway in rats with inflammatory pain. Evid Based Complement Alternat Med. 2017;2017:7531060. doi:10.1155/2017/7531060
17. Ni HD, Xu LS, Wang Y, et al. Astrocyte activation in the periaqueductal gray promotes descending facilitation to cancer-induced bone pain through the JNK MAPK signaling pathway. Mol Pain. 2019;15:1744806919831909. doi:10.1177/1744806919831909
18. Medeiros MA, Costa-e-Sousa RH, Olivares EL, Cortes WS, Reis LC. A reassessment of the role of serotonergic system in the control of feeding behavior. An Acad Bras Cienc. 2005;77(1):103–111. doi:10.1590/s0001-37652005000100008
19. Wood JL, Russo AF. Autoregulation of cell-specific MAP kinase control of the tryptophan hydroxylase promoter. J Biol Chem. 2001;276(24):21262–21271. doi:10.1074/jbc.M007520200
20. Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94(3):293–304. doi:10.1016/S0304-3959(01)00363-3
21. Bu HL, Xia YZ, Liu PM, et al. The roles of chemokine CXCL13 in the development of bone cancer pain and the regulation of morphine analgesia in rats. Neuroscience. 2019;406:62–72. doi:10.1016/j.neuroscience.2019.02.025
22. Fox A, Medhurst S, Courade JP, et al. Anti-hyperalgesic activity of the cox-2 inhibitor lumiracoxib in a model of bone cancer pain in the rat. Pain. 2004;107(1–2):33–40. doi:10.1016/j.pain.2003.09.003
23. Ye D, Bu H, Guo G, et al. Activation of CXCL10/CXCR3 signaling attenuates morphine analgesia: involvement of Gi protein. J Mol Neurosci. 2014;53(4):571–579. doi:10.1007/s12031-013-0223-1
24. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi:10.1016/0304-3959(88)90026-7
25. Wang CT, Mao CJ, Zhang XQ, et al. Attenuation of hyperalgesia responses via the modulation of 5-hydroxytryptamine signalings in the rostral ventromedial medulla and spinal cord in a 6-hydroxydopamine-induced rat model of Parkinson’s disease. Mol Pain. 2017;13:1744806917691525. doi:10.1177/1744806917691525
26. Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci. 2009;30(2):229–241. doi:10.1111/j.1460-9568.2009.06813.x
27. Liu X, Bu H, Liu C, et al. Inhibition of glial activation in rostral ventromedial medulla attenuates mechanical allodynia in a rat model of cancer-induced bone pain. J Huazhong Univ Sci Technolog Med Sci. 2012;32(2):291–298. doi:10.1007/s11596-012-0051-5
28. Gao J, Zhang JX, Xu TL. Modulation of serotonergic projection from dorsal raphe nucleus to basolateral amygdala on sleep-waking cycle of rats. Brain Res. 2002;945(1):60–70. doi:10.1016/s0006-8993(02)02625-2
29. Wang YM, Gao FJ, Lin SQ, et al. Activation of p38MAPK in spinal microglia contributes to autologous nucleus pulposus-induced mechanical hyperalgesia in a modified rat model of lumbar disk herniation. Brain Res. 2020;1742:146881. doi:10.1016/j.brainres.2020.146881
30. Oatway MA, Chen Y, Weaver LC. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 2004;110(1–2):259–268. doi:10.1016/j.pain.2004.03.040
31. Khasabov SG, Malecha P, Noack J, Tabakov J, Giesler GJ
32. Silva M, Costa-Pereira JT, Martins D, Tavares I. Pain modulation from the brain during diabetic neuropathy: uncovering the role of the rostroventromedial medulla. Neurobiol Dis. 2016;96:346–356. doi:10.1016/j.nbd.2016.10.002
33. Ni HD, Yao M, Huang B, et al. Glial activation in the periaqueductal gray promotes descending facilitation of neuropathic pain through the p38 MAPK signaling pathway. J Neurosci Res. 2016;94(1):50–61. doi:10.1002/jnr.23672
34. Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol. 2011;22(5–6):390–404. doi:10.1097/FBP.0b013e328349aae4
35. Mifflin KA, Benson C, Thorburn KC, Baker GB, Kerr BJ. Manipulation of neurotransmitter levels has differential effects on formalin-evoked nociceptive behavior in male and female mice. J Pain. 2016;17(4):483–498. doi:10.1016/j.jpain.2015.12.013
36. Lee JH, Li DX, Yoon H, et al. Serotonergic mechanism of the relieving effect of bee venom acupuncture on oxaliplatin-induced neuropathic cold allodynia in rats. BMC Complement Altern Med. 2014;14(1):471. doi:10.1186/1472-6882-14-471
37. Bardoni R. Serotonergic modulation of nociceptive circuits in spinal cord dorsal horn. Curr Neuropharmacol. 2019;17(12):1133–1145. doi:10.2174/1570159X17666191001123900
38. Patel R, Dickenson AH. Modality selective roles of pro-nociceptive spinal 5-HT2A and 5-HT3 receptors in normal and neuropathic states. Neuropharmacology. 2018;143:29–37. doi:10.1016/j.neuropharm.2018.09.028
39. Di Cesare Mannelli L, Ghelardini C, Micheli L, et al. Synergic stimulation of serotonin 5-HT1A receptor and alpha2-adrenoceptors for neuropathic pain relief: preclinical effects of 2-substituted imidazoline derivatives. Eur J Pharmacol. 2017;810:128–133. doi:10.1016/j.ejphar.2017.06.023
40. Baptista-de-Souza D, Di Cesare Mannelli L, Zanardelli M, et al. Serotonergic modulation in neuropathy induced by oxaliplatin: effect on the 5HT2C receptor. Eur J Pharmacol. 2014;735:141–149. doi:10.1016/j.ejphar.2014.04.028
41. Liu QQ, Yao XX, Gao SH, et al. Role of 5-HT receptors in neuropathic pain: potential therapeutic implications. Pharmacol Res. 2020;159:104949. doi:10.1016/j.phrs.2020.104949
42. Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci. 2017;18(11):2483. doi:10.3390/ijms18112483
43. Kayser V, Elfassi IE, Aubel B, et al. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A-/-, 5-HT1B-/-, 5-HT2A-/-, 5-HT3A-/- and 5-HTT-/- knock-out male mice. Pain. 2007;130(3):235–248. doi:10.1016/j.pain.2006.11.015
44. Zeitz KP, Guy N, Malmberg AB, et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22(3):1010–1019. doi:10.1523/JNEUROSCI.22-03-01010.2002
45. Maxwell DJ, Kerr R, Rashid S, Anderson E. Characterisation of axon terminals in the rat dorsal horn that are immunoreactive for serotonin 5-HT3A receptor subunits. Exp Brain Res. 2003;149(1):114–124. doi:10.1007/s00221-002-1339-7
46. Leong ML, Gu M, Speltz-Paiz R, et al. Neuronal loss in the rostral ventromedial medulla in a rat model of neuropathic pain. J Neurosci. 2011;31(47):17028–17039. doi:10.1523/JNEUROSCI.1268-11.2011
47. Zhao ZQ, Chiechio S, Sun YG, et al. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J Neurosci. 2007;27(22):6045–6053. doi:10.1523/JNEUROSCI.1623-07.2007
48. Imbe H, Okamoto K, Aikawa F, et al. Effects of peripheral inflammation on activation of p38 mitogen-activated protein kinase in the rostral ventromedial medulla. Brain Res. 2007;1134(1):131–139. doi:10.1016/j.brainres.2006.11.091
49. Jaggi AS, Singh N. Analgesic potential of intrathecal farnesyl thiosalicylic acid and GW 5074 in vincristine-induced neuropathic pain in rats. Food Chem Toxicol. 2012;50(5):1295–1301. doi:10.1016/j.fct.2012.01.038
50. Boyette-Davis JA, Walters ET, Dougherty PM. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag. 2015;5(4):285–296. doi:10.2217/pmt.15.19
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.