Back to Journals » OncoTargets and Therapy » Volume 12
Selecting treatment options in refractory metastatic colorectal cancer
Received 14 November 2018
Accepted for publication 13 February 2019
Published 27 March 2019 Volume 2019:12 Pages 2271—2278
DOI https://doi.org/10.2147/OTT.S194605
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Gaetano Romano
Margaret Byrne, Muhammad Wasif Saif
Northwell Health Cancer Institute, Donald and Barbara Zucker School of Medicine at Hofstra, Lake Success, NY, USA
Abstract: Survival of patients with metastatic colorectal cancer (mCRC) has significantly improved in the last decade. Survival gains are not driven by advances in first-line therapy but by incremental additional effects of subsequent treatment lines. To maximize outcomes, patients should receive all active agents. Identification of patient subgroups is increasing individualization of treatment. Novel oral agents, such as regorafenib and TAS-102, as well as promising immunotherapeutic agents have offered salvage treatment options for refractory mCRC. Although most therapeutic developments for mCRC in the chemorefractory setting focuses on new targets and/or more potent agents, reconsideration of established targets has gained importance with the growth of a rational pharmacogenomic approach to drug development, such as HER2. The authors describe treatment options for patients with refractory colon cancer following first- and second-line therapy.
Keywords: TKI, fluoropyrimidine, bevacizumab, cetuximab, panitumumab, PD-1 inhibitor, FOLFIRI, epidermal growth factor receptor, vascular endothelial growth factor receptor, platelet-derived growth factor receptors, KRAS, NRAS, HER2, advanced, colon cancer, refractory, Lonsurf, regorafenib
Introduction
Colon cancer is the third-leading cause of cancer-related death in the USA. In 2018, an estimated 97,000 patients will be diagnosed with colon cancer and 43,000 patients will be diagnosed with rectal cancers; together, colorectal cancer will account for >50,000 deaths.1,2 At diagnosis, only 20% of patients have metastatic disease; however, several patients will develop disease progression during the disease course.3 The optimal treatment strategy for patients with nonresectable disease is rapidly changing.
For several years, the backbone of therapy for patients with metastatic colorectal cancer (mCRC) was 5-fluorouracil (5-FU) with leucovorin, which only offered a response rate of ~20% and an overall survival (OS) of 6 months. In the 1990s, oxaliplatin and irinotecan were found to have activity in colon cancer and each drug combined with 5-FU improved OS to nearly 24 months.4–6 In the past several years, the discovery of targeted biologic agents, such as monoclonal antibodies to EGFR and VEGF, targeted kinases, and other cytotoxic agents, in addition to a better understanding of the molecular biology of cancer, have improved OS even further to nearly 30 months.7–9
The National Comprehensive Cancer Network currently recommends that patients with unresectable or metastatic colon cancer receive combination treatment, which usually includes 5-FU with oxaliplatin or irinotecan, in addition to a biologic agent depending on mutation status for first-line therapy.10,11 Unfortunately, a large majority of patients develop disease progression or metastatic disease at some point following treatment.7,12 In summary, the improvement in OS in this patient population represents incremental benefits of later treatments. A strategic approach to therapy is required to optimize patient outcomes. This paper will describe treatment options for patients with refractory colon cancer following first- and second-line therapy. It is important to recognize patients with oligometastatic disease who may benefit from local therapies as opposed to systemic therapies.
Treatment options for refractory metastatic colorectal cancer
The treatment of refractory mCRC includes two new US FDA-approved oral agents: regorafenib approved in 2012 and trifluridine/tipiracil (TAS-102) approved in 2015. Both agents were approved as a single agent in patients with refractory mCRC, each producing modest improvements in OS (Table 1).
Regorafenib
Regorafenib is a multi-targeted tyrosine kinase inhibitor that binds to at least 19 targets, including angiogenic, stromal, and oncogenic tyrosine kinase receptors.13,14
In the CORRECT trial, 760 patients with metastatic colon cancer with disease progression during or within 3 months following standard therapy were randomized to receive regorafenib 160 mg daily or oral placebo daily for 3 of 4 weeks. This study showed that regorafenib improved both progression-free survival (PFS) (1.9 months for regorafenib vs. 1.7 months for placebo, P<0.0001) and OS (6.4 months for regorafenib vs. 5.0 months for placebo, P=0.0052) for these patients.15,16
Another study, the CONCUR trial, showed similar results in an Asian population. In this study, patients with progressive metastatic disease who had received at least two previous lines of treatment were randomized to regorafenib vs. best supportive care and placebo. This study also showed both a PFS (3.2 months for regorafenib vs. 1.7 months for placebo, P<0.0001) and an OS advantage for those who received regorafenib (8.8 months for regorafenib vs. 6.3 months for placebo, P=0.0002).17
Unfortunately, regorafenib led to adverse events in the majority of patients in both the CORRECT and CONCUR trials (93% and 97%, respectively).15,17,18 The most common adverse events in patients who received regorafenib in the CORRECT trial were fatigue (67% of patients, 1% of patients with grade 3 or 4 fatigue), hand–foot skin reaction (50% of patients, 21% of patients with grade 3 or 4 hand-foot skin reaction), elevated liver function tests (LFTs) (40%–60%; 8%–13% with grade 3 or 4 elevated LFTs) (Table 2).15 As a whole, regorafenib can be difficult to tolerate and frequently requires dose modifications. Because of poor tolerability, patients who start regorafenib should have a follow-up appointment with a provider each week to evaluate for the development of adverse events.
For patients who receive regorafenib, it is important that they receive appropriate sequencing of treatment. The REVERCE trial was a phase II trial that evaluated 101 patients with KRAS wild-type (WT) metastatic colon cancer who failed previous therapy. Patients were randomized to regorafenib followed by cetuximab vs. cetuximab followed by regorafenib. This study showed that there is an OS benefit for patients who received regorafenib prior to cetuximab therapy (17.4 months vs. 11.6 months, P=0.0293).19
Alternate dosing of regorafenib
Optimal dosing of regorafenib remains an unanswered question, especially as many patients are required for discontinuing the treatment to maintain the quality of life of the patients. In both the CORRECT and CONCUR trials, patients were treated with the maximum tolerated dose of regorafenib, which may have contributed to the development of adverse events.15–17
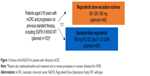
To answer this question, the Regorafenib Dose Optimization Study (ReDOS) was performed. The ReDOS study was a phase II dose-escalation study of regorafenib. In this study, patients were randomized 1:1:1:1 to receive regorafenib 80 mg daily with weekly dose increases if no adverse events vs. regorafenib 160 mg daily without any dose changes, in addition to prophylactic treatment of palmar-plantar-erythroderma (PPE) vs. reactive treatment of PPE (Figure 1). This study met its primary endpoint of proportion of patients who completed two cycles of protocol treatment and were able to initiate cycle 3 (43% of patients in the escalating dose group vs. 24% in the group who started full-dose therapy, P=0.0281). It showed that patients were able to complete more therapy if they were treated with escalating doses as opposed to starting at full dose.20 This suggests that starting a lower dose may increase tolerability of this drug without decreasing effectiveness.
TAS-102: a novel oral fluoropyrimidine
TAS-102 is an orally bioavailable fluororpyrimidine. It consists of two components: trifluridine, a purine analog, and tipiracil, a thymidine phosphorylase inhibitor that helps to prevent the breakdown of trifluridine.21,22
In the RECOURSE trial, 800 patients with refractory metastatic colon cancer who had previously received at least two lines of therapy were randomized in a 2:1 comparison to receive TAS-102 or best supportive care. This study showed that TAS-102 improved PFS (2.0 for TAS-102 vs. 1.7 for placebo, P<0.0001) and OS (7.1 months for TAS-102 vs. 5.3 months for placebo, P<0.0001).23
The TERRA study was similar to the RECOURSE trial but was performed in an Asian population with metastatic colorectal adenocarcinoma refractory to at least two therapies. Patients were randomized in a 2:1 comparison to TAS-102 vs. best supportive care and placebo. This study also showed the PFS benefit (2.0 months for TAS-102 vs. 1.8 for placebo, P<0.0001) and OS benefit for those who received TAS-102 (7.8 months for TAS-102 vs. 7.1 months for placebo, P=0.0035).24
Overall, the incidence of adverse events in patients who received TAS-102 was similar to those who received regorafenib; however, adverse events in the TAS-102 group were hematologic in nature: neutropenia (38% of patients) and lymphopenia (21%) (Table 2). Of note, 4% of patients treated with TAS-102 had febrile neutropenia. One patient suffered from treatment-related death due to sepsis. Hematologic toxicity was particularly prevalent among patients who had received multiple lines of therapy. For patients who had previously been treated with at least two lines of therapy, 53% of patients had a cycle delay of at least 4 days due to hematologic toxicity.23 An additional factor is that TAS-102 has a very complex dosing schedule that requires very detailed and specific dosing instructions.
The C-TASK FORCE study evaluated the efficacy and safety of TAS-102 and bevacizumab in patients with metastatic colon cancer who were refractory or intolerant of previous therapy. In this phase I/II study, patients received two drugs combination per study. Of the 16 patients in the phase II study, 9 did not have progression at 16 weeks, resulting in a 16-week PFS of 42.9%. The most common adverse events were neutropenia (72% of patients), leukopenia (44%), anemia (16%), febrile neutropenia (16%), and thrombocytopenia (12%). Overall, this study showed that TAS-102 with bevacizumab has promising activity and a manageable adverse effect profile, which suggests that this combination may become a potential treatment option for patients with refractory metastatic colon cancer.25
Can we select regorafenib and TAS-102 for the right patient?
Regorafenib and TAS-102 have been tested in very similar situations, but they have not been tested in a head-to-head study.
Sequencing the agents
A retrospective analysis evaluated efficacy and major toxicities associated with both drugs in a Japanese population using propensity score matching. The study found similar efficacy between the agents with regard to tumor response, PFS, and OS, regardless of treatment sequence. However, there were higher rates of dose reductions with regorafenib due to adverse events.26
Similarly, a recent systematic review performed indirect comparison between regorafenib and TAS-102. This study showed similar tumor response, PFS, and OS between the treatment groups. Patients who received regorafenib had a statistically significant increase in all adverse events (RD 0.35, 95% CI: 0.04–0.67, P=0.013) and in grade 3–5 adverse events (RD 0.22, 95 CI: 0.13–0.31, P<0.001). The subgroup analysis confirmed that regorafenib was associated with higher fatigue and hand–foot syndrome, whereas TAS-102 was associated with more anemia, neutropenia, and thrombocytopenia.27 Sequencing of these drugs should be determined based on patient characteristics and adverse event profiles.
In the RECOURSE study, ~20% of patients were previously treated with regorafenib and those who previously received regorafenib had similar response rates to those who had not received regorafenib.23 This suggests that TAS-102 has similar activity regardless of prior exposure to regorafenib.
In both the CORRECT and RECOURSE trials, all patients had previously been exposed to a biologic agent (anti-VEGF or anti-EGFR therapy based on mutation status) prior to treatment with regorafenib or TAS-102.15,23 However, in the studies performed in Asia (CONCUR and TERRA studies), only ~40%–50% of patients had been previously exposed to a biologic agent.17,24 These studies performed in Asian populations showed very similar outcomes to the CORRECT and RECOURSE trials, which suggests that moving regorafenib or TAS-102 to the forefront would not necessarily affect outcomes.15,23
We conclude that both regorafenib and TAS-102 are oral agents that appear to have similar tumor control, PFS, and OS. However, they have not been compared in a head-to-head study. With this in mind, using the adverse event profile can help to guide the appropriate therapies for patients (Table 3). In particular, regorafenib is a reasonable option for patients with good LFTs (Child-Pugh A or B) and good performance status. However, patients who receive regorafenib tend to have more dose reductions due to adverse events; principally, patients who receive regorafenib tend to have higher rates of hand–foot reaction and fatigue. Alternatively, patients treated with TAS-102 tend to have more difficulty with bone marrow toxicity; specifically, they may not tolerate TAS-102 if they have been heavily pretreated.
Anti-EGFR therapy rechallenge
Another treatment option for patients with refractory metastatic colon cancer is rechallenge with anti-EGFR therapy. In the phase II CRICKET trial, 28 patients with KRAS WT metastatic colon cancer were rechallenged with third-line cetixumab and irinotecan. Six patients had partial response and nine patients had stable disease. This study suggests that patients may benefit from rechallenge with anti-EGFR therapy. In particular, patient selection may be improved by only rechallenging patients who do not develop KRAS mutations.28
Similarly, in the E-Rechallenge trial, 33 patients with KRAS WT mCRC who were refractory to previous fluoropyrimidines, oxaliplatin, irinotecan, cetuximab, and bevacizumab and in whom previous treatment with cetuximab was effective in any earlier line (achieving complete response, partial response, or stable disease that persisted for ≥6 months) were rechallenged with cetuximab and oxaliplatin. Overall, 15.6% of the patients had a partial response, 40.6% had stable disease, and 43.8% had progressive disease. Median PFS was 88 days and OS was 262 days. There was no signal for increased adverse events. This study also suggests that patients who have had previous response to anti-EGFR therapy may benefit from rechallenge.29
Immunotherapy to treat mCRC
Another unique subset of colon cancer patients express deficient DNA mismatch repair (dMMR) and a high level of microsatellite instability-high (MSI-h), which is present in ~15% of patients.30,31 The presence of MSI-h in patients with metastatic colon cancer is a poor prognostic marker.32–35 A histopathologic characteristic is the presence of dense tumor-infiltrating lymphocytes, which likely contributes to high levels of tumor neoantigens and the corresponding high immunogenicity of these tumors.36–41 Early phase studies were performed in patients with 15 different MSI-h cancers, including mCRC. These studies showed an overall objective response rate of 39.6%. Of those who had an objective response, 78% had a response of at least 6 months (range from 1.6 to 22.7 months). In the subset of patients with MSI-h colon cancer, 36% (32/90 patients) had an overall response.42–48
Based upon knowledge of the immunogenic tumor microenvironment in MSI-h tumors, in addition to the observed dramatic response in this subset of patients with anti-PD-1 therapy, a trial of pembrolizumab was undertaken that enrolled three cohorts of patients: MSI-h colorectal cancers, MSI-h noncolorectal cancers, and microsatellite stable colorectal cancers. In this study, patients with MMR-deficient colorectal tumors had an improvement in immune-related overall response rate (40% vs. 0%) and immune-related PFS (78% vs. 11%) compared to patients with MMR-proficient colorectal cancers. Similarly, the PFS (not reached vs. 2.2 months) and OS (not reached vs. 5.0 months) were also improved in the patients with MMR-deficient colorectal cancers compared to patients with MMR proficient colorectal cancer. Of note, patients with MMR-deficient noncolorectal cancers had similar response rates to those with MMR-deficient colorectal cancers.44 This study showed that MMR status is predictive of the clinical benefit of pembrolizumab.
Based on positive results in early phase studies, a multi-center, open-label, phase II trial was started to evaluate the role of nivolumab in patients with recurrent or metastatic dMMR/MSI-h colon cancer who had previously been treated with at least one therapy, including fluororpyrimidine and oxaliplatin or irinotecan. Patients were treated with nivolumab 3 mg/kg every 3 weeks for four doses followed by nivolumab 3 mg/kg every 2 weeks until disease progression, discontinuation because of toxicity, or death. At a median follow-up of 12 months, 23 of 74 patients (31.1%) had a response and 51 of 74 patients (69%) had disease control for ≥12 weeks. Median duration of response was not reached. The most common grade 3 or 4 drug-related adverse events were increased concentrations of lipase (8% of patients) and amylase (3% of patients). None of the deaths were deemed to be treatment related by investigators.49 This study shows that nivolumab provides durable response rates and disease control in pretreated patients with dMMR colorectal disease.
In the same phase II trial, a subset of patients with dMMR/MSI-h colorectal cancer received ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four doses followed by nivolumab 3 mg/kg every 2 weeks until disease progression, discontinuation because of toxicity, or death. Of 119 patients, 65 patients (54.6%) achieved an objective response, including 3.4% with complete response and 51.3% with partial response. Disease control for at least 12 weeks was obtained in 95 patients (80%). Any-grade adverse events were reported in 73% of patients, with 49 patients (41%) with grade 1 or 2 events, 32 patients (27%) with grade 3 events, and 6 patients (5%) with grade 4 events. The most common adverse events included fatigue, diarrhea, and pruritis.50 This study showed that nivolumab, in addition to ipilimumab, may provide improved efficacy relative to single-agent immune checkpoint blockade without significantly affecting the safety profile.
These studies show that immune checkpoint inhibitors are a promising treatment option for patients with dMMR/MSI-h mCRC.
‘Old’ target and ‘novel’ treatment
With a better understanding of the biology and molecular subtypes of colorectal cancer, molecular targeted therapies have emerged as treatment options for patients with metastatic colon cancer.
Approximately 3%–4% of patients with colorectal cancer express HER2 amplifications.51,52 In a molecularly annotated platform of patient-derived xenografts, combination therapy of HER2 and EGFR inhibition induced long-lasting tumor regression.53
Based on these results, the HERACLES trial was performed. This was a phase II proof of concept trial that evaluated dual-targeted therapy with trastuzumab and lapatinib in 27 patients with treatment-refractory, KRAS WT, HER2-positive metastatic colon cancer. It showed a median PFS of 21 weeks and a median OS of 46 weeks. In particular, eight patients achieved an objective response rate. These results are regarded as extraordinary given the heavily pretreated population included in this study (median of five prior regimens). This was the first study to show that there is a genetically defined subpopulation of patients with colorectal cancer (5% of KRAS WT) with sensitivity to pharmacological blockade of a specific oncogenic product. Additionally, the results suggest that the extent of HER2 gene copy number elevation and HER2 expression might be associated with response to treatment.54 Further studies are needed to elucidate the role of HER2-directed therapies for colon cancer. Modern biomarkers platforms may also change the landscape of treatment for patients with refractory metastatic colon cancer. These platforms may help to detect new mutations, which may be a target for therapies in the future. Similarly, they may help to direct personalized oncological care.
Conclusion
A significant proportion of heavily pretreated patients with mCRC maintains good performance status and is eligible for further systemic treatment. The survival of patients with metastatic colon cancer has improved significantly in the past decade. These survival gains are not driven by changes in front-line therapy; rather, they represent later stage treatment lines. To maximize outcomes, patients should receive all active treatments if possible. We recognize the importance of personalizing treatment to each individual based on molecular subtyping, in addition to the likelihood of developing adverse effects. Immunotherapies are a promising treatment option that may lead to further survival gains for a subset of patients. Based on this, MSI testing should be considered standard of care for all patients with metastatic colon cancer.
Disclosure
Dr Saif has received research funding from Taiho and has also received honorarium for speaking for Lonsurf. The authors report no other conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. | ||
Shah MA, Renfro LA, Allegra CJ, et al. Impact of patient factors on recurrence risk and time dependency of oxaliplatin benefit in patients with colon cancer: analysis from modern-era adjuvant studies in the adjuvant colon cancer end points (ACCENT) database. J Clin Oncol. 2016;34(8):843–853. | ||
Colucci G, Gebbia V, Paoletti G, et al; Gruppo Oncologico Dell’Italia Meridionale. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23(22):4866–4875. | ||
Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. | ||
Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. | ||
Grothey A, Marshall JL. Optimizing palliative treatment of metastatic colorectal cancer in the era of biologic therapy. Oncology (Williston Park). 2007;21(5):553–564. | ||
Loree JM, Kopetz S, Raghav KP. Current companion diagnostics in advanced colorectal cancer; getting a bigger and better piece of the pie. J Gastrointest Oncol. 2017;8(1):199–212. | ||
Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. | ||
Benson AB 3rd, Venook AP, Al-Hawary MM, et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16(4):359–369. | ||
Benson AB 3rd, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(3):370–398. | ||
Loree JM, Kopetz S. Recent developments in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol. 2017;9(8):551–564. | ||
Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. | ||
Chu E. An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11(1):1–13. | ||
Grothey A, Cutsem EV, Sobrero A, et al; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. | ||
Strumberg D, Scheulen ME, Schultheis B, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106(11):1722–1727. | ||
Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–629. | ||
Grothey A, Sobrero AF, Siena S, et al. Time profile of adverse events (AE’s) from regorafenib (REG) treatment for metastatic colorectal cancer (mCRC) in the phase III CORRECT study. J Clin Oncol. 2013;31(15):3637. | ||
Shitara K, Yamanaka T, Denda T, et al. REVERCE: a randomized phase II study of regorafenib followed by cetuximab versus the reverse sequence for previously treated metastatic colorectal cancer patients. J Clin Oncol. 2018;36(4):557. | ||
Bekaii-Saab TS, Ou FS, Anderson DM, et al. Regorafenib dose optimization study (ReDOS): randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC)-an ACCRU Network study. J Clin Oncol. 2018;36(4 Suppl):611. | ||
Lenz HJ, Stintzing S, Loupakis F. TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev. 2015;41(9):777–783. | ||
Chen J, Han M, Saif MW. TAS-102 an emerging oral fluoropyrimidine. Anticancer Res. 2016;36(1):21–26. | ||
Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. | ||
Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA Study. J Clin Oncol. 2018;36(4):350–358. | ||
Kuboki Y, Nishina T, Shinozaki E, et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol. 2017;18(9):1172–1181. | ||
Moriwaki T, Fukuoka S, Taniguchi H, et al. Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): a Japanese Society for Cancer of the Colon and Rectum multicenter observational study. Oncologist. 2018;23(1):7–15. | ||
Abrahao ABK, Ko YJ, Berry S, Chan KKW, et al. A comparison of regorafenib and TAS-102 for metastatic colorectal cancer: a systematic review and network meta-analysis. Clin Colorectal Cancer. 2018;17(2):113–120. | ||
Rossini D, Cremoimi C, Del Re M, et al. Efficacy of anti-EGFR rechallenge in RAS and BRAF wt metastatic colorectal cancer: clinical and translational results of the phase II CRICKET study by GONO. Am Assoc Cancer Res; 2018. Availble from: http://cancerres.aacrjournals.org/content/78/13_Supplement/CT088. Accessed March 7, 2019. | ||
Osawa H. Phase II study of cetuximab rechallenge in patients with RAS wild-type metastatic colorectal cancer: E-Rechallenge trial. Ann Oncol; 2018. Available from: https://oncologypro.esmo.org/Meeting-Resources/ESMO-2018-Congress/Phase-II-Study-of-Cetuximab-Rechallenge-in-Patients-with-RAS-Wild-Type-metastatic-Colorectal-Cancer-E-Rechallenge-Trial. Accessed March 6, 2019. | ||
Kloor M, von Knebel Doeberitz M. The immune biology of microsatellite-unstable cancer. Trends Cancer. 2016;2(3):121–133. | ||
Ozcan M, Janikovits J, von Knebel Doeberitz M, Kloor M. Complex pattern of immune evasion in MSI colorectal cancer. Oncoimmunology. 2018;7(7):e1445453. | ||
Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol. 2014;25(5):1032–1038. | ||
Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009;100(2):266–273. | ||
Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20(20):5322–5330. | ||
Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117(20):4623–4632. | ||
Kloor M, Staffa L, Ahadova A, von Knebel Doeberitz M. Clinical significance of microsatellite instability in colorectal cancer. Langenbecks Arch Surg. 2014;399(1):23–31. | ||
Smyrk TC, Watson P, Kaul K, Lynch HT, et al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91(12):2417–2422. | ||
Guidoboni M, Gafà R, Viel A, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159(1):297–304. | ||
Giannakis M, Mu XJ, Shukla SA, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15(4):857–865. | ||
Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43–51. | ||
Saeterdal I, Bjorheim J, Lislerud K, et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A. 2001;98(23):13255–13260. | ||
Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417–424. | ||
O’Neil BH, Wallmark JM, Lorente D, et al. Safety and antitumor activity of the anti–PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12(12):e0189848. | ||
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. | ||
Le DT, Kavan P, Kim TW, et al. KEYNOTE-164: pembrolizumab for patients with advanced microsatellite instability high (MSI-H) colorectal cancer. J Clin Oncol. 2018;36(15 Suppl):3514. | ||
Cohen RB, Delord JP, Doi T, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the Phase 1b KEYNOTE-028 Study. Am J Clin Oncol. Epub 2018 Feb 21. | ||
Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21(19):4286–4293. | ||
Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. | ||
Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. | ||
Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. | ||
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. | ||
Kavuri SM, Jain N, Galimi F, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5(8):832–841. | ||
Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1(6):508–523. | ||
Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(6):738–746. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.