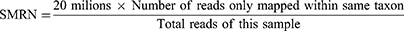Back to Journals » Infection and Drug Resistance » Volume 16
Secondary Infection Surveillance with Metagenomic Next-Generation Sequencing in COVID-19 Patients: A Cross-Sectional Study
Authors Chen R, Xie M, Wang S, Yu F, Zhang D, Yuan L, Zheng J, Wang J, Zhou J, Li B, Zheng S, Fan Y, Han D
Received 2 June 2023
Accepted for publication 6 September 2023
Published 29 September 2023 Volume 2023:16 Pages 6463—6472
DOI https://doi.org/10.2147/IDR.S424061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Renke Chen,1,* Mengxiao Xie,2,* Shenlong Wang,1 Fei Yu,2– 4 Dan Zhang,2– 4 Lingjun Yuan,2 Jieyuan Zheng,2 Jingchao Wang,2 Jieting Zhou,2 Binxiao Li,2 Shufa Zheng,2– 4 Yongsheng Fan,1 Dongsheng Han2– 4
1The First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 2Department of Laboratory Medicine, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 3Key Laboratory of Clinical in vitro Diagnostic Techniques of Zhejiang Province, Hangzhou, People’s Republic of China; 4Institute of Laboratory Medicine, Zhejiang University, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongsheng Han, Centre of Clinical Laboratory, First Affiliated Hospital, College of Medicine, Zhejiang University, 79 Qingchun Road, Hangzhou, 310003, People’s Republic of China, Email [email protected] Yongsheng Fan, School of Basic Medical Sciences, Zhejiang Chinese Medical University, 548 Binwen Road, Hangzhou, 310053, People’s Republic of China, Email [email protected]
Background: Metagenomic next-generation sequencing (mNGS) is a promising tool for improving antimicrobial therapy and infection control decision-making in complex infections. Secondary infection surveillance using mNGS in COVID-19 patients has rarely been reported.
Methods: Respiratory pathogen and antibiotic resistance prediction were evaluated by BALF mNGS for 192 hospitalized COVID-19 patients between December 2022 and February 2023.
Results: Secondary infection was confirmed in 83.3% (160/192) of the COVID-19 patients, with bacterial infections (45%, 72/160) predominating, followed by mixed bacterial and fungal infections (20%, 32/160), and fungal infections (17.5%, 28/160). The incidence of bacterial or viral secondary infection was significantly higher in patients who were admitted to the ICU, received mechanical ventilation, or developed severe pneumonia (all p< 0.05). Klebsiella pneumoniae (n=30, 8.4%) was the most prevalent pathogen associated with secondary infection followed by Acinetobacter baumannii (n=29, 8.1%), Candida albicans (n=29, 8.1%), Aspergillus fumigatus (n=27, 7.6%), human herpes simplex virus type 1 (n=23, 6.4%), Staphylococcus aureus (n=20, 5.6%) and Pneumocystis jiroveci (n=14, 3.9%). The overall concordance between the resistance genes detected by mNGS and the reported phenotypic resistance in 69 samples containing five clinically important pathogens (ie, K. pneumoniae, A. baumannii, S. aureus, P. aeruginosa and E. coli) that caused secondary infection was 85.5% (59/69).
Conclusion: mNGS can detect pathogens causing secondary infection and predict antimicrobial resistance for COVID19 patients. This is crucial for initiating targeted treatment and rapidly detect unsuspected spread of multidrug-resistant pathogens.
Keywords: COVID-19, SARS-CoV-2, metagenomic next-generation sequencing, antibiotic resistance
Introduction
The coronavirus disease 2019 (COVID-19) caused by the emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants continues to threaten human life worldwide. The disease severity of COVID-19 patients is very wide: from an asymptomatic carrier state to severe infection and critical illness with intensive care admission and/or mechanical ventilation requirement.
In general, critically ill patients are prone to secondary infection due to the long course of disease, invasive mechanical ventilation and the immuno-suppressed state.1 Previous studies have shown that secondary bacterial, fungal, and respiratory viral infection is very common in both adult and child severe and critically ill COVID-19 patients.2–5 A recent systematic review and meta-analysis showed that the pooled prevalence of secondary infections in COVID-19 patients was 24%, with the highest observed in ICU patients (41%).6 Sang et al found that the secondary infections measured by bacteria and fungi culture from respiratory tract, blood and other body fluid specimens were very common (86.6%) when COVID-19 patients were admitted to ICU for over 72 hours.7 COVID-19 patients often receive steroid therapy, which could exacerbate bacterial or fungal infections.8 Such secondary infections, especially secondary invasive fungal infection (IFIs) and bloodstream infections, increased morbidity and mortality of hospitalized COVID-19 patients.5,9 Therefore, the rapid comprehensive identification of the pathogens responsible for the development of secondary infections in COVID-19 patients is essential to implement individually tailored antibiotic therapy, improve antimicrobial stewardship and help prevent emergence and transmission of multi-drug-resistant (MDR) organisms.
Laboratory techniques for detecting the pathogens associated with secondary infections among COVID-19 patients within previous studies included microbial cultures from various samples (such as blood, cerebrospinal fluid, respiratory secretions, bronchoalveolar lavage fluid, sputum and urine), real-time reverse transcription–polymerase chain reaction (RT-PCR) tests for multiple respiratory viruses, serum tests for detection of galactomannan- (GM) and 1,3-β-d-glucan (BDG), and BioFire, FilmArray Pneumonia Panel Plus Assay.2,10 Metagenomic next-generation sequencing (mNGS) tests, as an advanced nucleic acid detection technology for pathogen identification, have the ability to detect broad-spectrum pathogens, predict antibiotic resistance, and provide results within 24 hours.11,12 Respiratory tract infection-based cohort studies have shown that the positive rate of mNGS (>60%) for respiratory tract pathogens is significantly higher than that of traditional microbial detection methods (30–50%).13,14 In particular, mNGS has a big advantage in the diagnosis of unexplained and co-infection.15 However, faced with high cost and the clinical utility that has not yet been fully verified, mNGS testing has not been routinely applied for the diagnosis of COVID-19 patients. Few studies have revealed the comprehensive spectrum of respiratory pathogens associated with secondary infection in COVID-19 patients using mNGS tests.16,17 In our clinical laboratory, we have established a sequencing platform for mNGS testing to provide patients with a real-time sequencing service within the hospital.18 In this study, we performed a retrospective study in hospitalized COVID-19 patients who had received mNGS tests to investigate the prevalence of secondary infections and to assess the performance of resistance prediction by mNGS for clinically important bacteria.
Methods and Materials
Patient Selection and Study Design
A cohort of 192 COVID-19 patients was included in this study. These patients underwent BALF mNGS tests to investigate potential secondary infections during their hospitalization in the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) between December 2022 and February 2023 (Figure 1A). Prior to SARS-COV-2 infection, they were not in any stage of infectious disease. The clinical treatment team will determine whether the microorganisms detected by mNGS are the pathogens causing the secondary infection based on clinical manifestations, microbiological results (culture, smear, serology, PCR and mNGS), hematological results, radiological findings, treatment response and disease outcomes. This study was approved by the FAHZU institutional review board (IIT20220714A). Patient informed consent statement is waived by FAHZU institutional review board as the data used in this study does not include patient privacy data.
 |
Figure 1 Pathogens associated with COVID-19 patients: (A) CT value distribution of SARS-COV-2 detected by PCR for each patient; (B) The SMRN value distribution of SARS-COV-2 detected by mNGS for each patient (converted to log10 value); (C) The heat map shows the number of patients with secondary infections caused by various pathogens (longitudinal axis) and the number of patients with different pathogen numbers (0–8) detected through mNGS (horizontal axis). The numbers in the figure refer to the number of patients; (D) The spectrum of pathogens detected by mNGS that cause secondary infections in COVID-19 patients. The full name of each pathogen is shown in Table S1. |
Routine RT-PCR for SARS-COV-2
For detection of SARS-CoV-2, a commercial real-time reverse-transcription PCR (rRT-PCR) was performed using a commercial quantitative RT-PCR test kit for both ORF1ab and N genes (BioGerm, Shanghai, China) in accordance with the manufacturer’s instructions. Samples with Ct values ≤38.0 for both ORFab1 and N genes were considered positive, specimens with Ct values >38.0 were repeated, specimens with repeated results of Ct values >38 and specimens with undetectable Ct values were considered negative.19
Microbial Culture and Phenotypic Resistance Testing
Clinical doctors prescribe routine microbiological testing (CMT) based on the specific needs of each patient. These tests include bacterial and fungal cultures, smears, acid fast staining, fluorescence staining, and Indian ink staining, as well as serological tests for pathogens such as Legionella spp., Cryptococcus spp., Candida spp., and Aspergillus spp., as well as polymerase chain reaction (PCR) for cytomegalovirus (CMV), Epstein-Barr virus (EBV), and multiple respiratory viruses. The phenotypic resistance of the cultivated bacteria or fungi are tested using Kirby-Bauer (K-B) or broth microdilution methods.
BALF mNGS Testing for Pathogen Identification
BALF mNGS tests for both microbial DNA and RNA were performed for each patient. A 200ul BALF sample is used for nucleic acid extraction. For DNA extraction, host cells and DNA are removed with Tween 20 (Sigma) and Benzonase (Qiagen), and microbial DNA are extracted with the QIAamp® UCP Pathogen DNA Kit (Qiagen) following the manufacturer’s instructions. RNA extraction was extracted with the QIAamp® Viral RNA Kit (Qiagen), and ribosomal RNA was removed with the Ribo-Zero rRNA Removal Kit (Illumina, San Diego, CA, USA). After nucleic acid extraction, RNA samples undergo reverse transcription to form cDNA, and then the steps of library construction and sequencing are carried out in the same way as DNA samples. In detail, Libraries were constructed using a Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA) and sequenced on Illumina Nextseq CN500 sequencer for 50 cycles of single-end sequencing (SE-50), generating approximately 20 million reads for each library.12 For quality control, negative and positive samples were run as in our previous studies.12,18 The bioinformatics analysis and interpretation of the results were the same as in our recent published article.12 The detected microbes and their stringent mapped read numbers (SMRNs) were reported sent to providers (or treating team). SMRN value was calculated as below:
Resistance Gene Prediction
Unassembled mNGS reads were directly aligned to the ARG reference database (CARD: https://card.mcmaster.ca/) using BLASTN with parameters (-megablast, -evalue 1e-5) for ARG annotation and subtyping which aimed at accurately characterizing ARG subtypes. Then, we filtered out the false-positive ARGs with a greedy LCA algorithms. In brief, we select the hit with highest score. If there were multiple alignment with same alignment score, all hits were kept. The lowest common ancestor was calculated at ARG subtype or family level. Next, the score index was further calculated based on identified ARG subtype/variation features or ARG family with weight coefficients for each strain using an in-house python script. The maximal Youden index was used to estimate the cutoff value for reporting resistance using receiver operating characteristic (ROC) analysis through short-read simulation experiments based on our in-house training data set. For mNGS data, the species attribution of ARGs was mainly determined based on whether the calculated copy number of ARGs was within the normal range for the assumed affiliation of the gene-species. If the calculated copy number was normal, we accepted the null hypothesis; otherwise, it was rejected.
Statistical Analysis
The demographic data were summarized using descriptive statistics. Secondary infection rates were compared using chi-square tests. Data analyses were performed using SPSS 18 software (SPSS Inc., Chicago IL, USA) and GraphPad version 8.0.1 software (GraphPad Software, San Diego, CA, USA) with a p value ≤0.05 as the significance threshold, and all tests were two-tailed.
Results
Clinical Characteristics of COVID-19 Patients
In total, there were 140 (72.9%) males, 75 (39.1%) immunocompromised and 122 (63.5%) invasively ventilated COVID-19 patients (Table 1). A 57.8% (111/192) of the patients were admitted to ICUs, 68.8% (132/192) were diagnosed severe pneumonia and 25.5% (49/192) had developed sepsis. Hypertension (49%, 94/192) and diabetes (36.5%, 70/192) are the most common underlying diseases. Cardiovascular, gastrointestinal and neurologic symptoms occurred in 57.3% (110/192), 39.1% (75/192) and 36.5% (70/192) of the patients, respectively. All the patients were positive for SARS-COV-2 detected by PCR when they were admitted to the hospital, with the median CT values of the ORF1ab gene and N gene were 27 [interquartile range (IQR) 23–31] and 27 (IQR 23–31), respectively (Figure 1A). mNGS tests were also positive for SARS-COV-2 in every tested sample, with the median stringent mapped read number (SMRN) value 781 (IQR 30-15061) (Figure 1B).
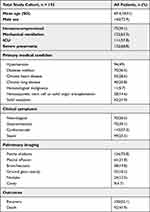 |
Table 1 Demographic and Clinical Characteristics of Patients Included in This Study |
Incidence of Secondary Infection and Associated Pathogens
Based on mNGS testing and clinical diagnosis, 83.3% (160/192) of the patients were considered to have developed secondary infections (Figure 1C). Bacterial infections (45%, 72/160) were predominant, followed by mixed bacterial and fungal infections (20%, 32/160), and fungal infections (17.5%, 28/160). Mixed bacterial, fungal and viral infections were confirmed in 11 patients. A 57.3% (110/192) of the patients were infected with one or two pathogens, and only five cases were identified with six or more pathogens (Figure 1C).
A total of 357 pathogens (71 species) causing secondary infections were identified by mNGS tests (Figure 1D), including 210 bacteria (44 species), 114 fungi (19 species), and 33 Viruses (8 species). The most prevalent bacteria were Klebsiella pneumoniae (n=30), Acinetobacter baumannii (n=29), Staphylococcus aureus (n=20), Pseudomonas aeruginosa (n=15), Streptococcus pneumoniae (n=13), Enterococcus faecalis (n=12), and Corynebacterium striatum (n=10) (Figure 1D). Ureaplasma urealyticum, Mycobacterium tuberculosis complex (MTBC), Legionella pneumophila and several anaerobic bacteria were also culprits of secondary infections (Figure 1D). Candida albicans (n=29), Aspergillus fumigatus (n = 27) and Pneumocystis jiroveci (n=14) were the predominant fungi, and human herpes simplex virus type 1 (HSV1) (n=23) was the most prevalent virus in patients with secondary infections.
The Incidence of Secondary Infections in Patients with Different Medical Conditions
We found a higher rate of secondary infections in patients with poor underlying conditions [ie, admitted to the ICU (87.4%), immunocompromised (85.3%), suffering from diabetes (90%) or hypertension (87.2%), requiring mechanical ventilation (86.9%) or developing severe pneumonia (88.6%)] and in patients who had developed clinical complications such as sepsis (93.9%), multiple organ failure (91.1%), neurological (90%), gastrointestinal (86.8%) and cardiovascular (89.1%) symptoms than in other patient groups (Figure 2A). Further analysis showed that the positive rate of bacterial pathogens in patients admitted to the ICU, with mechanical ventilation, developing severe pneumonia, sepsis, or cardiovascular symptoms was significantly higher than that of patients without these medical conditions (all p<0.05) (Figure 2B, F–H, L). Patients admitted to the ICU, with mechanical ventilation or developing severe pneumonia were associated with a higher likelihood of acquiring a secondary viral infection (all p<0.05) (Figure 2B, F and G). There was no statistical difference in the rate of fungi infection in each patient group (Figure 2B–L).
The Performance of Resistance Gene Detection by mNGS Testing
In this study, we evaluated the concordance of the resistance genes detected by mNGS and the reported phenotypic resistances by microbial culture for five clinically important pathogens (ie, K. pneumoniae, A. baumannii, S. aureus, P. aeruginosa and E. coli). There was concordance between the resistance genes and the reported phenotypic resistance in 19 samples containing K. pneumoniae [ie, 12 carbapenem-resistant Klebsiella pneumoniae (CRKP), three extended-spectrum beta-lactamase-K pneumoniae (ESBL-KP) and 4 drug-susceptible K. pneumoniae] (Figure 3). All the identified resistance genes of the 12 CRKP were blaKPC genes. The three ESBL-KP could be explained by the identified ESBL genes of blaSHV, blaCTX-M and blaTEM. However, resistance phenotypes could not be genotypically predicted in four CRKP and one ESBL-KP due to low read count by mNGS. A 77.8% (14/18) of the carbapenem-resistant A. baumannii (CRAB) were found to contain carbapenemase resistance genes (CRGs), including blaOXA-23, blaOXA-51 and blaNDM gene. The drug-resistant phenotypes and genotypes of all 15 culture-positive S. aureus were completely consistent, specifically, including (1) mecA genes detected in 11 MRSA isolates, (2) ErmB gene detected in one erythromycin-resistant MSSA isolate, and (3) no resistance gene was detected in the three drug-susceptible S. aureus (Figure 3). For the eight culturable P. aeruginosa, mNGS accurately predicted seven phenotypes, including two CRPA (blaNDM genes were detected) and five sensitive strains (no resistance genes were identified). No resistance gene was detected in one CRPA (Figure 3). Two ESBL genes (blaCTX-M and blaTEM) were identified in all four samples containing E. coli that was phenotypically reported as an ESBL (Figure 3). Overall, the concordance between the reported phenotypes of the above five pathogens and the genotypes of mNGS was 85.5% (59/69) (Table 2).
 |
Table 2 The Concordance of the Resistance Genes Detected by mNGS and the Phenotypic Resistances Reported by Microbial Culture for Five Pathogens |
Discussion
Secondary infection and antimicrobial resistance complicate the management of hospitalized COVID-19 patients and will very likely lead to poor outcomes.2,3 Therefore, rapid pathogen identification is urgently needed in this patient population to improve empirical treatment decisions and enhance patient survival. Established practice has initially demonstrated that mNGS has the potential to simultaneously identify pathogens and predict drug resistance within 24h.12,20,21 In this study, based on BALF mNGS, we evaluated the secondary infection of 192 COVID-19 patients who were infected SARS-COV-2 during the first nationwide outbreak of Omicron variant of SARS-CoV-2 in China between December 2022 and February 2023. The results showed that secondary infection occurred in 83.3% (160/192) of the patients, and those with poor underlying conditions were more likely to be susceptible to secondary infections. mNGS identified a wide range of pathogens including clinically rare pathogens. Mixed infections with two or more pathogens were present in nearly half of the cases (47.4%, 91/192) (Figure 1C). Importantly, the concordance between the resistance genes detected by mNGS and the reported phenotypic resistance can reach 85.5% (59/69) for multidrug-resistant bacteria commonly found in the clinic. The availability of such data in real time has the potential to fundamentally help clinicians initiate targeted antimicrobial therapy earlier and control the spread of multidrug-resistant bacteria.
This study revealed that secondary infections is very common in hospitalized COVID-19 patients. The rate of secondary infections (83.3%) found in this study was similar to that (86.6%) identified in a multicenter retrospective study from China,7 but higher than those reported in most studies based on conventional microbial methods.1,2,22 The higher secondary infection rate in this study may be related to the following factors: (1) all of the 192 patients infected SARS-COV-2 during the first nationwide outbreak of Omicron variant of SARS-CoV-2 in China between December 2022 and February 2023. At that time, limited medical resources were occupied by a large number of hospitalized SARS-COV-2 patients, which prevented most people from receiving high-quality individualized medical care and management. This situation increases the possibility of secondary infections during their hospitalization; (2) Most of the patients in this study were male over 60 years of age (56.8%, 109/192) and most of them had severe pneumonia with mechanical ventilation (68.9%, 75/109). In addition, steroid therapy was an indispensable option for these patients. All of the above factors have been demonstrated in previous studies associated with increased risk of secondary infection;1,7,8,22 (3) Benefit from the broad pathogen detection capabilities of mNGS. This study revealed that mNGS is able to detect pathogens that are difficult to identify or not covered by conventional microbial detection methods, such as U. urealyticum, L. pneumophila, several anaerobic bacteria, P. jiroveci and various viruses (Figure 1C).
mNGS has become a useful tool for solving complex infections. However, to our knowledge, no study has used mNGS to comprehensively assess the spectrum of pathogens causing secondary infections in COVID-19 patients, although a proof-of-concept study had suggested that mNGS is suitable for secondary infection and antibiotic resistance assessment of COVID-19 patients.17 In our study, nearly half of the COVID-19 patients (47.4%, 91/192) had secondary infections caused by mixed infections of multiple pathogens. Rapid identification of each pathogen causing a mixed infection based on unbiased mNGS is essential to improve antimicrobial stewardship and infection control investigations, as some pathogens in mixed infections may be missed if conventional microbial identification methods such as culture and targeted PCR are used for diagnosis.12 It is important to note, however, that mNGS results are often interfered with by colonizing and contaminating microorganisms. Therefore, cautious interpretation and infectious disease consultation is important for mNGS results.12
In order to fight against the existing or potential secondary infection, 64.4% of hospitalized patients with COVID-19 took antibiotics,23 while in critically ill patients, this proportion was as high as 97.5%.24 Excessive use of antibiotics is a recipe for the rapid rise or spread of resistant bacteria such as K. pneumoniae and A. baumannii, especially given the crowded conditions.7 A recent special report from the US Centers for Disease Control and Prevention found a 15% increase in the rate (per discharge or admission) of resistant organisms including carbapenem-resistant Acinetobacter, MRSA, CRE, and ESBL associated with the COVID-19 pandemic.25 The latest review showed that the most commonly reported resistant Gram-negative bacteria contributing to the secondary infections of COVID-19 patients was A. baumannii, followed by K. pneumonia, E. coli, and P. aeruginosa. Commonly reported Gram-positive bacteria were S. aureus and E. faecium.23 These bacteria are also the most common culprits of secondary infections in the population studied in this study (Figure 1D). Therefore, accurate and rapid prediction of drug resistance of these pathogens is essential for rational selection of infection treatment regimens, improving patient prognosis and controlling the spread of drug-resistant organisms. In the present study, the overall concordance between the resistance genes detected by mNGS and the reported phenotypic resistance in 69 samples containing five pathogens (ie, K. pneumoniae, A. baumannii, S. aureus, P. aeruginosa and E. coli) that causing secondary infection was 85.5% (59/69) (Table 2). This finding again demonstrates the clinical utility of mNGS for rapid prediction of bacterial drug resistance.17 With the advancement of technology, mNGS has the hope of stably and quickly identifying more common and clinically significant drug resistance genes, which will be very helpful in improving treatment of bacterial and fungal infections, improving antimicrobial stewardship and help identify target infection control interventions.
There are limitations to this study. First, the collected cases in this study originated from the nationwide outbreak of COVID-19 in China. The high secondary infection rate of this group patients was caused by a serious shortage of medical resources and the inability to implement standardized anti-infection treatment normally. Further, multicenter research based on mNGS is beneficial for a more comprehensive understanding of the secondary infection incidence of COVID-19. Second, we were unable to analyze the genotypic and phenotypic relationships of pathogens other than the five most common pathogens due to insufficient sample size or lack of positive culture results.
Providing clinicians with a rapid and accurate mNGS testing is of great value in improving antimicrobial stewardship and identifying transmission of MDR organisms, as well as targeting infection control interventions. This study demonstrates mNGS is well suitable for secondary infection surveillance and resistance prediction in long-stay COVID-19 patients, especially during the COVID-19 pandemic where the capacity challenges and disease severity may result in unpredictable epidemiology and high levels of antibiotic resistance. With the continuous mutation and prevalence of SARS-COV-2 in the population, more studies should be performed to evaluate the secondary infection of COVID-19 patients based on unbiased mNGS tests.
Acknowledgments
This study was supported by Zhejiang Provincial Natural Science Foundation (grant number LY23H200001).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests in this work.
References
1. Alshrefy AJ, Alwohaibi RN, Alhazzaa SA, et al. Incidence of bacterial and fungal secondary infections in covid-19 patients admitted to the ICU. Int J Gen Med. 2022;15:7475–7485. doi:10.2147/IJGM.S382687
2. Alhumaid S, Alabdulqader M, Al DN, et al. Global coinfections with bacteria, fungi, and respiratory viruses in children with SARS-cov-2: a systematic review and meta-analysis. Trop Med Infect Dis. 2022;7(11):380.
3. Krumbein H, Kümmel LS, Fragkou PC, et al. Respiratory viral co‐infections in patients with covid‐19 and associated outcomes: a systematic review and meta‐analysis. Rev Med Virol. 2023;33(1):e2365. doi:10.1002/rmv.2365
4. Moreno-García E, Puerta-Alcalde P, Letona L, et al. Bacterial co-infection at hospital admission in patients with covid-19. Int J Infect Dis. 2022;118:197–202. doi:10.1016/j.ijid.2022.03.003
5. Thompson GR, Miceli MH, Jiang J, et al. Secondary invasive fungal infection in hospitalised patients with COVID-19 in the United States. Mycoses. 2023;66(6):527–539. doi:10.1111/myc.13579
6. Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-cov-2 and other pathogens: a systematic review and meta-analysis. PLoS One. 2021;16(5):e0251170. doi:10.1371/journal.pone.0251170
7. Sang L, Xi Y, Lin Z, et al. Secondary infection in severe and critical covid-19 patients in China: a multicenter retrospective study. Ann Palliat Med. 2021;10(8):8557–8570. doi:10.21037/apm-21-833
8. Moorthy A, Gaikwad R, Krishna S, et al. SARS-cov-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021;20(3):418–425. doi:10.1007/s12663-021-01532-1
9. Monaco M, Floridia M, Giuliano M, et al. Hospital-acquired bloodstream infections in patients deceased with covid-19 in Italy (2020–2021). Front Med. 2022;9:1041668. doi:10.3389/fmed.2022.1041668
10. Kolenda C, Ranc AG, Boisset S, et al. Assessment of respiratory bacterial coinfections among severe acute respiratory syndrome coronavirus 2-positive patients hospitalized in intensive care units using conventional culture and biofire, filmarray pneumonia panel plus assay. Open Forum Infect Dis. 2020;7(11):ofaa484. doi:10.1093/ofid/ofaa484
11. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341–355. doi:10.1038/s41576-019-0113-7
12. Han D, Yu F, Zhang D, et al. The real-world clinical impact of plasma mngs testing: an observational study. Microbiol Spectr. 2023;11(2):e0398322. doi:10.1128/spectrum.03983-22
13. Graf EH, Simmon KE, Tardif KD, et al. Unbiased detection of respiratory viruses by use of RNA sequencing-based metagenomics: a systematic comparison to a commercial PCR panel. J Clin Microbiol. 2016;54(4):1000–1007. doi:10.1128/JCM.03060-15
14. Xie Y, Du J, Jin W, et al. Next generation sequencing for diagnosis of severe pneumonia: china, 2010–2018. J Infect. 2019;78(2):158–169. doi:10.1016/j.jinf.2018.09.004
15. Diao Z, Han D, Zhang R, Li J. Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. J Adv Res. 2022;38:201–212. doi:10.1016/j.jare.2021.09.012
16. Babiker A, Bradley HL, Stittleburg VD, et al. Metagenomic sequencing to detect respiratory viruses in persons under investigation for covid-19. J Clin Microbiol. 2020;59(1). doi:10.1128/JCM.02142-20
17. Charalampous T, Alcolea-Medina A, Snell LB, et al. Evaluating the potential for respiratory metagenomics to improve treatment of secondary infection and detection of nosocomial transmission on expanded covid-19 intensive care units. Genome Med. 2021;13(1):182. doi:10.1186/s13073-021-00991-y
18. Diao Z, Lai H, Han D, Yang B, Zhang R, Li J. Validation of a metagenomic next-generation sequencing assay for lower respiratory pathogen detection. Microbiol Spectr. 2023;11(1). doi:10.1128/spectrum.03812-22
19. Yu F, Xie G, Zheng S, et al. Assessment of the diagnostic ability of four detection methods using three sample types of covid-19 patients. Front Cell Infect Microbiol. 2021;11. doi:10.3389/fcimb.2021.685640
20. Liu H, Zhang Y, Yang J, Liu Y, Chen J, Szymczak WA. Application of mngs in the etiological analysis of lower respiratory tract infections and the prediction of drug resistance. Microbiol Spectr. 2022;10(1):e0250221. doi:10.1128/spectrum.02502-21
21. Serpa PH, Deng X, Abdelghany M, et al. Metagenomic prediction of antimicrobial resistance in critically ill patients with lower respiratory tract infections. Genome Med. 2022;14(1):14. doi:10.1186/s13073-022-01016-y
22. De Bruyn A, Verellen S, Bruckers L, et al. Secondary infection in covid-19 critically ill patients: a retrospective single-center evaluation. BMC Infect Dis. 2022;22(1). doi:10.1186/s12879-022-07192-x
23. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi:10.1001/jama.2020.1585
24. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi:10.1001/jamainternmed.2020.0994
25. Langford BJ, Soucy JR, Leung V, et al. Antibiotic resistance associated with the covid-19 pandemic: a systematic review and meta-analysis. Clin Microbiol Infect. 2023;29(3):302–309. doi:10.1016/j.cmi.2022.12.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.