Back to Journals » Cancer Management and Research » Volume 15
Sebaceous Carcinoma of the Submandibular Gland a Case Report and Review of the Literature
Authors Ju W, Luo G, Shi Y, Zhou F, Li M, Xu J, Yan Z, Yang X
Received 7 November 2022
Accepted for publication 17 January 2023
Published 5 February 2023 Volume 2023:15 Pages 123—130
DOI https://doi.org/10.2147/CMAR.S392573
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Wei Ju,1,2,* Guan-fa Luo,1,* Yuan-yuan Shi,3 Fei-jun Zhou,1 Meng-qi Li,1 Jian-hui Xu,1 Zhi-xin Yan,2 Xi-hu Yang1
1Department of Oral and Maxillofacial Surgery, Affiliated Hospital of Jiangsu University, Zhenjiang, Jiangsu, 212001, People’s Republic of China; 2Department of Burn and Plastic Surgery, Affiliated Hospital of Jiangsu University, Zhenjiang, Jiangsu, 212001, People’s Republic of China; 3Department of Pathology, Affiliated Hospital of Jiangsu University, Zhenjiang, Jiangsu, 212001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xi-hu Yang, Email [email protected]
Objective: Sebaceous carcinoma (SC) of the submandibular gland is extremely rare. Owing to the low morbidity and nonspecific clinical manifestations, diagnosis is commonly delayed, which increases metastasis and mortality. To date, there have been five reported cases of SC of the submandibular gland. Here, we present a new case and review the relevant literature.
Methods and Results: A 36-year-old woman presented with an enlarged left submandibular gland. Clinical features included a non-tender solitary nodular mass with normal overlying skin. There were no special findings on computed tomography or ultrasound examination except for a swollen mass in the left submandibular gland. The patient underwent surgical resection. Pathological examination confirmed the diagnosis of SC with nerve infiltration. Immunohistochemical examination of this case showed positive staining for P63, P40, CK7, CK8/18, MLH1, MSH2, MSH6, and PMS2. The specimen was negative for androgen receptor, CEA, S-100, CK5/6, SOX-10, SOX-11, SMA, and GCDFP-15. The KI-67 labeling index was determined to be 15%. PAS and anti-epithelial membrane antigen were positive in partial area. The patient is still undergoing follow-up, and no metastasis or recurrence has been observed for 2 months.
Conclusion: This case highlighted the fact that despite its rarity, SC should be considered as a differential diagnosis for masses located in the head and face. Early and accurate diagnosis, followed by wide surgical excision, has a favorable prognosis. Therefore, clinicians should be familiar with the clinical and pathological features of this disease.
Keywords: sebaceous carcinoma, salivary gland neoplasms, submandibular gland, androgen receptor, P63, P40
Sebaceous carcinoma (SC) generally develops in the periocular glands or skin, and carcinoma originating from the salivary glands is extremely rare.1,2 The etiology and pathogenesis of SC are not yet clear, but they may be related to Muir–Torre syndrome (MTS), gene mutations, long-term ultraviolet (UV) damage, immunosuppression, or viral infections.3–5 These tumors generally develop in middle-aged to elderly people; the mean age for extraocular SC is 65 years (range: 9–93 years), and no noteworthy gender predilection has been identified.6–8 At early stages of this disease, the clinical manifestations of SC are nonspecific, which delays diagnosis and may result in increased metastasis and mortality. Therefore, it is critical to understand the epidemiology and biology of this rare cancer to improve early detection; however, at present, only about 50 cases of this rare cancer have been reported in the English literature.9,10 Here, we report the case of a young-aged woman who was diagnosed with SC of the submandibular gland and review the relevant literature. The clinicopathological and immunohistochemical (IHC) features are also discussed.
Case Presentation
A 36-year-old woman presented to our institution in 2022 with the chief complaint of a non-tender solitary nodular mass in the left submandibular region that had developed more than 2 months prior. The patient had no fever, chills, cough, or other oral lesions. Her face showed basic symmetry and the overlying skin was normal in appearance. Anti-inflammatory drugs were administered orally, but no significant improvement was observed.
Physical examination revealed a 1.5×1 × 1 cm3 pea-sized firm, fixed mass with clear boundaries that was not encapsulated located on the inner side of the left submandibular gland region. The mass exerted no pressure effect on the surrounding organs. The patient had no remarkable medical or family history. No other abnormalities were observed in other organ systems. Laboratory examination revealed no abnormalities.
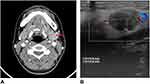
Computed tomography (CT) revealed that the left submandibular gland was slightly swollen and strongly enhanced in the intravenous contrast condition compared with the contralateral side. A nodular lesion in the left submandibular gland was observed with a diameter of approximately 1.2 cm, uniform reinforcement, and clear boundary (Figure 1A). The CT scan revealed no evidence of other lesions in the head or neck region. Ultrasound examination revealed a mixed-component nodule in the left submandibular gland (Figure 1B). The hypoechoic mass was rich in short rods and punctate blood flow signals.
Diffuse enlargement of the left submandibular gland was observed intraoperatively. The left submandibular gland was completely resected under general anesthesia, and the surgical margins were negative. Hematoxylin and eosin-stained sections of the surgical specimen revealed tumor cell nests infiltrating normal salivary gland tissues. Malignant cells were characterized by pleomorphic nuclei with prominent nucleoli and a moderate number of mitoses in the foamy cytoplasm (Figure 2A). There was evidence of perineural invasion (Figure 2B), but no significant vascular invasion was observed. IHC analysis of the specimen showed strong positivity for P63, P40, CK7, CK8/18, MLH1, PMS2, MSH2, and MSH6. The KI-67 labeling index was determined to be 15%. The partial area was positive for PAS and anti-epithelial membrane antigen (EMA). The tumor was negative for androgen receptor (AR), CEA, S-100, CK5/6, BCL-2, SOX-10, SOX-11, SMA, CD117, DOG-1, Galectin-3, GCDFP-15, and GFAP (Figure 2C–J).
The morphological and IHC findings were compatible with the diagnosis of SC. The patient is still undergoing follow-up, and at 2 months since diagnosis, no metastasis or recurrence has been observed.
Discussion
SC originating from the salivary glands is rare, and to date, approximately 50 cases have been reported in the English literature. Most of these occur in the parotid gland, and only five have been reported to originate from the submandibular gland.6,11 The onset age of salivary gland SC has been reported to range from 9 to 93 years, with a bimodal age distribution (a minor peak in the 3rd decade and a major peak in the 6th and 7th decades).8,12 The clinical data of salivary gland SC cases since 2010 are summarized in Table 1. Clinically, the symptoms of SC vary from an indolent, slow-growing, painless, solitary nodule to a painful, rapidly progressing swelling accompanied by facial paralysis. Therefore, it was frequently misdiagnosed as common benign conditions, resulting in delayed treatment and management. Risk factors for the development of SC include prior radiation exposure, MTS, immunosuppression, DNA mismatch repair, and long-term UV damage.13,14 Suspected SC should be assessed based on the history of visceral malignancy and other risk factors.15 The lesion in this case occurred in the major salivary gland without past tumor history or risk factor exposure, making it difficult to diagnose. Additionally, the patient’s age was in the minor peak of the usual onset range, making the diagnosis more difficult.
 |
Table 1 Reported Cases of Salivary Sebaceous Carcinoma (from 2010 to 2022) |
Sebaceous gland tissue can be detected in the normal major salivary glands.6 However, the origin of sebocytes in the parotid gland is unclear. This may occur as a result of differentiation of pluripotent stem cells or ductal cells. SCs can be categorized into well-differentiated and poorly-differentiated varieties based on the extent of sebocyte differentiation.16 Poorly-differentiated lesions pose a challenge to pathologists as they lack any conspicuous sebocyte differentiation. Microscopic examination of the well-differentiated specimen showed that the sebocytes and duct epithelial cells formed many irregular, asymmetric sebaceous lobules. Typically, malignant cells exhibit significant cytoplasmic vacuolation and hyperchromatism.6 Pleomorphic cells further show variable degrees of mitotic activity and nuclear atypia.
Due to their origins and differentiation, there are histologic overlaps and discrepancies between different cases of SC (Table 2). IHC markers, such as GCDFP-15, EMA, AR, CK7, P40, P63, and adipophilin, can be helpful in confirming the diagnosis.6,17,18 The P63 antibody typically stains myoepithelial and basal cells and the proliferative cells of the sebaceous glands. P40 is a short isoform target of P63 that can be utilized as a marker of the sebaceous lineage to evaluate sebocyte differentiation.18 One study examining periocular SC found that AR is useful in the diagnosis of poorly-differentiated tumors. Higher AR expression increases the risk of progression and recurrence, as circulating dihydrotestosterone promotes the growth of sebaceous glands.19 However, AR expression was negative in some metastatic salivary gland SCs.6 EMA is expressed primarily in the sebocytes in both the cytoplasm and membrane, but is negative in most basaloid peripheral cells. Extraocular SC is obviously correlated with mismatch repair (MMR) proteins (MSH2, MLH1, MSH6, and PMS2) in previous studies, suggesting that the MMR pathway is primarily responsible for the pathogenesis of SC.20,21 Detection of MMR protein loss by IHC for SC diagnosis has a sensitivity of 81–85%.22 In this case, IHC staining showed normal nuclear expression of MLH1 and MSH2 in tumor cells; P40 and P63 were positive, and granular expression of EMA was observed. The tumor cells were negative for S-100 and AR, and the KI-67 proliferation index was approximately 15%.
 |
Table 2 IHC Makers of Salivary Gland Sebaceous Carcinoma |
Because several types of malignant tumors arise in the salivary glands, imaging examinations alone cannot lead to a histological diagnosis, but ultrasonography or CT scan of the lymph node region can be used to assess the recurrence or clinical stage. Salivary gland SC should also be distinguished from epithelial-myoepithelial carcinoma, poorly-differentiated squamous carcinoma, pleomorphic adenoma, mucoepidermoid tumors, and lymphadenoma or lymphadenocarcinoma. Surgical resection remains the primary treatment modality, although this results in a degree of functional and esthetic morbidity, and local recurrence and metastasis occur in a significant proportion of patients.23,24 The role of radiation therapy in primary SC is uncertain. There is no specificity in the early stages of SC, thus requiring a high level of suspicion by clinicians for timely diagnosis and treatment. Metastatic spread can occur to either regional nodes or distant sites, such as the lungs and brain. Thus, close follow-up is critical to investigate potential recurrence.
Conclusions
This case highlights that any rapidly growing skin or subcutaneous mass should raise a suspicion of SC, although its incidence is low. The symptoms, signs, and CT examinations are not specific compared with those of carcinoids. Immediate biopsy or pathological detection is essential to ensure a timely diagnosis. Management options should be considered based on histological features as well as the extent of tumor spread for a favorable prognosis.
Ethics and Consent Statements
This study was approved by the Ethics Committee of the Affiliated Hospital of Jiangsu University. The patient has provided informed consent for case details of this manuscript and accompanying images to be published. The case details of this manuscript were approved by our institution.
Funding
This study was supported by National Natural Science Foundation of China (82173380), Doctoral Program for Entrepreneurship and Innovation of Jiangsu Province (JSSCBS20211596), and Social development project of Zhenjiang Key Research and development Program (SH2021073).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Owen JL, Kibbi N, Worley B, Reynolds KA, Poon E, Alam M. Sebaceous carcinoma: evidence-based clinical practice guidelines. Lancet Oncol. 2019;20(12):e699–e714. doi:10.1016/S1470-2045(19)30673-4
2. Takata T, Ogawa I, Nikai H. Sebaceous carcinoma of the parotid gland. An immunohistochemical and ultrastructural study. Virchows Arch Pathol Anat Histopathol. 1989;414(5):459–464. doi:10.1007/BF00718631
3. North JP, Golovato J, Vaske CJ, et al. Cell of origin and mutation pattern define three clinically distinct classes of sebaceous carcinoma. Nat Commun. 2018;9(1):1894. doi:10.1038/s41467-018-04008-y
4. Sargen MR, Starrett GJ, Engels EA, Cahoon EK, Tucker MA, Goldstein AM. Sebaceous carcinoma epidemiology and genetics: emerging concepts and clinical implications for screening, prevention, and treatment. Clin Cancer Res. 2021;27(2):389–393. doi:10.1158/1078-0432.CCR-20-2473
5. D’Arcy ME, Castenson D, Lynch CF, et al. Risk of rare cancers among solid organ transplant recipients. J Natl Cancer Inst. 2021;113(2):199–207. doi:10.1093/jnci/djaa078
6. Soares CD, Morais TML, Carlos R, et al. Sebaceous adenocarcinomas of the major salivary glands: a clinicopathological analysis of 10 cases. Histopathology. 2018;73(4):585–592. doi:10.1111/his.13664
7. Toberer F, Haenssle HA, Rütten A, et al. Angiogenesis in ocular and extraocular sebaceous carcinoma. Acta Derm Venereol. 2019;99(13):1270–1274. doi:10.2340/00015555-3342
8. Erickson LA. Sebaceous Carcinoma. Mayo Clin Proc. 2021;96(8):2285–2287. doi:10.1016/j.mayocp.2021.06.009
9. Giridhar P, Kashyap L, Mallick S, Dutt Upadhyay A, Rath GK. Impact of surgery and adjuvant treatment on the outcome of extraocular sebaceous carcinoma: a systematic review and individual patient’s data analysis of 206 cases. Int J Dermatol. 2020;59(4):494–505. doi:10.1111/ijd.14739
10. Pratt D, Lynch DW. Sebaceous adenocarcinoma in parotid gland of a 65-year-old. SD Med. 2022;75(4):158–160.
11. Ohara N, Taguchi K, Yamamoto M, Nagano T, Akagi T. Sebaceous carcinoma of the submandibular gland with high-grade malignancy: report of a case. Pathol Int. 1998;48(4):287–291. doi:10.1111/j.1440-1827.1998.tb03907.x
12. Holzgreve A, Pfluger T, Schmid I, et al. 18F-FDG PET/CT for response assessment in pediatric sebaceous carcinoma of the parotid gland. Diagnostics. 2020;10(11):908. doi:10.3390/diagnostics10110908
13. Sargen MR, Cahoon EK, Lynch CF, Tucker MA, Goldstein AM, Engels EA. Sebaceous carcinoma incidence and survival among solid organ transplant recipients in the United States, 1987–2017. JAMA Dermatol. 2020;156(12):1307–1314. doi:10.1001/jamadermatol.2020.3111
14. Hazawa M, Lin DC, Handral H, Xu L, Chen Y. ZNF750 is a lineage-specific tumour suppressor in squamous cell carcinoma. Oncogene. 2017;36(16):2243–2254. doi:10.1038/onc.2016.377
15. Neelakantan IV, Di Palma S, Smith CE, McCoombe A. Parotid sebaceous carcinoma in patient with Muir Torre syndrome, caused by MSH2 mutation. Head Neck Pathol. 2016;10(3):354–361. doi:10.1007/s12105-015-0670-9
16. Plaza JA, Chung C, Salim S, Gru A, Sangueza M. Sebaceous carcinomas: a clinicopathological comparison of ocular and extraocular variants. Am J Dermatopathol. 2021;43(11):763–772. doi:10.1097/DAD.0000000000001812
17. Boecker W, Reusch M, Mielke V, et al. Spatial analysis of p63, K5 and K7 defines two groups of progenitor cells that differentially contribute to the maintenance of normal sebaceous glands, extraocular sebaceous carcinoma and benign sebaceous tumors. J Dermatol. 2019;46(3):249–258. doi:10.1111/1346-8138.14765
18. Jain D, Mathur SR, Sharma MC, Iyer VK. Cytomorphology of sebaceous carcinoma with analysis of p40 antibody expression. Diagn Cytopathol. 2015;43(6):456–461. doi:10.1002/dc.23250
19. Mulay K, Shah SJ, Aggarwal E, White VA, Honavar SG. Periocular sebaceous gland carcinoma: do androgen receptor (NR3C4) and nuclear survivin (BIRC5) have a prognostic significance? Acta Ophthalmol. 2014;92(8):e681–7. doi:10.1111/aos.12466
20. Na HY, Park JH, Shin SA, et al. Targeted sequencing revealed distinct mutational profiles of ocular and extraocular sebaceous carcinomas. Cancers. 2021;13(19):4810. doi:10.3390/cancers13194810
21. Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312(1):25–31. doi:10.1007/s00403-019-01971-4
22. Tetzlaff MT, Singh RR, Seviour EG, et al. Next-generation sequencing identifies high frequency of mutations in potentially clinically actionable genes in sebaceous carcinoma. J Pathol. 2016;240(1):84–95. doi:10.1002/path.4759
23. Orr CK, Yazdanie F, Shinder R. Current review of sebaceous cell carcinoma. Curr Opin Ophthalmol. 2018;29(5):445–450. doi:10.1097/ICU.0000000000000505
24. Siguan APT, Yray MDS. Sebaceous carcinoma of the axilla. J Surg Case Rep. 2020;2020(12):rjaa513. doi:10.1093/jscr/rjaa513
25. Kumabe A, Kawase T, Miura K, et al. Sebaceous carcinoma of the parotid gland: f-18 FDG PET/CT findings. Clin Nucl Med. 2010;35(4):260–262. doi:10.1097/RLU.0b013e3181d18f0d
26. Manteghi A, Zwillenberg S, Arguello-Guerra V. Sebaceous carcinoma of the parotid gland: a case report and review of the literature. Ear Nose Throat J. 2014;93(6):E29–32. PMID: 24932826.
27. Das K, Karmakar A. Sebaceous carcinoma of parotid gland. Gomal J Med Sci. 2014;12(2):122–123.
28. Takada Y, Kawamoto K, Baba S, Takada T, Inoue T, Tomoda K. Sebaceous carcinoma of the parotid gland: a case report. Case Rep Oncol. 2015;8(1):106–112. doi:10.1159/000379742
29. Kressin MK, Coogan AC. Sebaceous carcinoma of the parotid gland. Diagn Cytopathol. 2013;41(9):803–805. doi:10.1002/dc.22892
30. Khmou M, Laadam K, Cherradi N. Parotid gland, an exceptional localization of sebaceous carcinoma: case report. BMC Clin Pathol. 2016;16:10. doi:10.1186/s12907-016-0031-y
31. Marnoucheel A, Maghous A, Kadiri S, et al. Sebaceous carcinoma of the parotid gland: a case report and review of the literature. J Med Case Rep. 2016;10:174. doi:10.1186/s13256-016-0946-z
32. Syder NC, Rabi S, Hu JC. Sebaceous carcinoma arising from heterotopic salivary gland tissue in a patient with Muir-Torre syndrome. Dermatol Surg. 2021;47(12):1659–1660. doi:10.1097/DSS.0000000000003259
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.