Back to Journals » Journal of Inflammation Research » Volume 15
SCD1-Fatty Acid Desaturase Inhibitor MF-438 Alleviates Latent Inflammation Induced by Preservative-Free Prostaglandin Analog Eye Drops
Authors Pacwa A, Machowicz J, Wojtyniak A , Pietrucha-Dutczak M, Toropainen E, Koskela A, Mrukwa-Kominek E, Lewin-Kowalik J, Smedowski A
Received 4 November 2021
Accepted for publication 17 December 2021
Published 8 February 2022 Volume 2022:15 Pages 793—806
DOI https://doi.org/10.2147/JIR.S347784
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Video abstract of "SCD1 inhibitor MF-438 alleviates latent inflammation" [ID 347784].
Views: 267
Anna Pacwa,1,2 Joanna Machowicz,1 Alicja Wojtyniak,1 Marita Pietrucha-Dutczak,1 Elisa Toropainen,3,4 Ali Koskela,4 Ewa Mrukwa-Kominek,5,6 Joanna Lewin-Kowalik,1,2 Adrian Smedowski1,2,6
1Department of Physiology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 2GlaucoTech Co, Katowice, Poland; 3School of Pharmacy, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland; 4Department of Ophthalmology, University of Eastern Finland, Kuopio, Finland; 5Department of Ophthalmology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 6Department of Ophthalmology, Professor K. Gibinski University Clinical Center, Medical University of Silesia, Katowice, Poland
Correspondence: Adrian Smedowski
Department of Physiology, Medical University of Silesia, Medykow 18, Katowice, 40-752, Poland, Tel +48 530813996, Fax +48 322525087, Email [email protected]
Introduction: Prostaglandin analogs are the first line of treatment in patients with glaucoma. Recently, many preservative-free prostaglandin analogs have been marketed to increase their tolerance in chronic use. However, potentially safer formulations have been reported to induce inflammation within ocular surface and adnexa, associated with pronounced activation of tissue macrophages.
Aim: We aimed to evaluate the effect of a Stearoyl-CoA desaturase-1 (SCD1) inhibitor, MF-438, on the differentiation of monocytes exposed to eye drop detergents, representing saturated fatty acid derivatives.
Methods: A culture of human peripheral blood monocytes was exposed to eye drops containing fatty acid derivatives (eye drop detergents), pf-latanoprost (Monoprost®, hydroxystearate macrogolglycerol – MGHS40) or pf-tafluprost (Taflotan®, polysorbate 80 – PS80), as well as pf-latanoprost+MF-438, MGHS40, and PS80. For the negative control C(-), monocytes were cultured in basal medium, and for the positive controls, monocytes were stimulated with Lipopolysaccharide (LPS) and Interferon γ (IFNγ) (M1 macrophages) or Interleukin-4 (IL-4) (M2 macrophages). The concentration of desaturase in the cell homogenates was determined by ELISA. The number of cells was counted under a microscope at 20x magnification.
Results: The following concentrations of SCD1 (ng/mL) were measured: 7.8± 0.3 – pf-latanoprost group; 1.5± 0.4 – pf-tafluprost group; 6.8± 0.7 – MGHS40 group; 0.4± 0.002 – PS80 group; 0.9± 0.02 – pf-latanoprost+MF-438 group; 5.4± 1.6 – C(-) control; 0.5± 0.04 – M1 control; 2.2± 0.13 – M2 control. The percentages of macrophages in culture were 33.6%, 17.6%, 33%, 0%, 13.5%, 18.6%, 36.3%, and 39.3% for the pf-latanoprost, pf-tafluprost, MGHS40, PS80, pf-latanoprost+MF-438, C(-), M1, and M2 cultures, respectively. There was a strong correlation between SCD1 concentration and macrophage count in the culture (r=0.8, p< 0.05).
Conclusion: Inhibition of SCD1 in monocytes prevents their transformation into macrophages after exposure to saturated fatty acid derivatives contained in eye drops, which may contribute to the limitation of latent inflammation within ocular adnexa and could possibly translate into better tolerability of the topical treatment.
Keywords: prostaglandin analogs, Stearoyl-CoA desaturase-1, latent inflammation, macrophages
Introduction
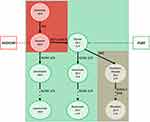
Glaucoma is a chronic optic neuropathy that requires continuous topical treatment to control disease progression.1 Topical eye drops are the first line of treatment, with prostaglandin analogs (PA) as a recommended first choice in therapy.2 Historically, all eye drop formulas have contained preservatives to make the eye drops sterile for long-term application. Unfortunately, preservatives in eye drops have been identified to have detrimental effects on the eye surface, leading to chronic inflammation, redness and dry-eye syndrome, which all affect patient compliance.3–5 To improve the tolerance of topical treatment, preservative-free (pf) formulas have been developed. Despite the introduction of pf formulas, the most commonly used preservative-free prostaglandin analogs (pfPA) contain other excipients (ie, detergents), which may also have negative effects on eye surface structures.6–8 Detergents are derivatives of fatty acids, which can be saturated, for example macrogol-glycerol hydroxystearate 40 (MGHS40, stearate derivative) or unsaturated, such as polysorbate 80 (PS80, oleate derivative). Delivery of these excipients to the eye surface enables penetration of PA through the water/lipid barriers, such as tear film and corneal epithelium, but on the other hand, these substances themselves are absorbed into the bloodstream, where they undergo further modifications, ie, within blood monocytes.3,6,9,10 Both saturated and unsaturated derivatives may induce different metabolic pathways and activate different enzymes within blood monocytes.10 Saturated fatty acid derivatives activate pathways, among which Stearoyl-CoA desaturase-1 (SCD1) has been shown to be associated with the transformation of monocytes into macrophages, while unsaturated fatty acid derivatives activate the Delta-6-desaturase (D6D), which is less likely linked to monocyte transformation10 (Figure 1). For example, it is known that the delivery of saturated fatty acids and their accumulation within adipose tissue may result in the development of latent inflammation, as shown by tissue macrophage infiltration with only a low-grade increase in proinflammatory markers.10,11
In our previous study, we detected the presence of latent inflammation within rabbit ocular adnexa and ocular surface after exposure to pfPA eye drops containing MGHS40.6 Macrophage infiltration was absent after treatment with pfPA eye drops containing PS80. Since we suspected that this phenomenon is related to the impact of the stearate derivative MGHS40 on blood monocyte enzymes that can induce their maturation and migration, we aimed to investigate this hypothesis in the current study. Using an in vitro culture of human peripheral blood monocytes (HPBMs), we showed that the transformation of monocytes into macrophages induced by MGHS40 is correlated with the content of SCD1 desaturase and that inhibition of this enzyme may be used to control the transformation process. Moreover, we were able to distinguish morphological subtypes of macrophages (ie, M1- and M2-like cells) that were differentiated from monocytes depending on the applied eye drop excipients.
Methods
Chronic topical treatment may induce latent inflammation within ocular adnexa. The aim of this study was to evaluate the effect of SCD1 inhibitor (MF-438) on the maturation of monocytes exposed to saturated fatty acid derivatives from eye drops.
Study Groups and Experimental Design
We used an in vitro culture of human peripheral blood monocytes (Sigma, Saint Louis, MO, USA) seeded at a density of 1.5^105 cells per well in 24-well plates. The cells were cultured in basal Blood Cell Culture Medium (Sigma) supplemented with the following excipients: pf-latanoprost (Monoprost®, Thea, France), pf-tafluprost (Taflotan®, Santen, Japan), polysorbate 80 (Sigma), macrogol-glycerol-hydroxystearate-40 (Sigma), and pf-latanoprost+MF-438 (SCD1 inhibitor, Calbiochem, San Diego, CA, USA). As a negative control, we used cells cultured in unsupplemented Blood Cell Culture Medium (Sigma). There were two positive control groups: M1 macrophages cultured in ImmunoCult SF Macrophage Medium supplemented with macrophage colony-stimulating factor (M-CSF), lipopolysaccharide (LPS) and interferon γ (IFNγ), and M2 macrophages cultured in ImmunoCult SF Macrophage Medium supplemented with M-CSF and Interleukin-4 (IL-4) (all from STEMCELL Technologies, Canada).
To optimize the concentration of the applied supplements, we based our calculations on their concentration in the original eye drop formulations and the pharmacokinetic principles of topical application, where it is known that approximately 50% of the applied dose is absorbed systemically to conjunctival vessels and bloodstream.7,12 Based on this, we calculated the desired concentrations of the chosen excipients per volume of culture medium, to mimic conditions that are observed in vivo. However, the systemic absorption of topically applied ocular dugs represents very complex problem, since extraocular structures, especially nasolacrimal drainage and nasal mucosa may determine the level of systemic absorption via non-conjunctival route.
The cells were first seeded in the culture wells and were grown for 24 h in basal medium to let them attach and adapt to culture conditions. After 24 h, the culture medium was exchanged, and the cells were divided into 8 groups: negative control, M1-positive control (supplement concentrations based on the manufacturer’s instructions), M2-positive control (supplement concentrations based on the manufacturer’s instructions), pf-latanoprost (3 μL of commercially available eye drops per 3 mL of culture medium), pf-tafluprost (3 μL of commercially available eye drops per 3 mL of culture medium), PS80 (2.5 μg of excipient per 3 mL of culture medium), MGHS40 (150 μg of excipient per 3 mL of culture medium), pf-latanoprost+MF-438 (3 μL of commercially available eye drops + 3 μL of inhibitor per 3 mL of culture medium – the final concentration of MF-438 was 1 µmol/mL). MF-438 is a thiadiazol–pyridazine compound that acts as an inhibitor of SCD1. Drugs from this group are used in cancer therapies and for the treatment of infectious diseases caused by viruses, bacteria and parasites. The cells were cultured for 7 days to observe their transformation into macrophages. The supplemented culture medium was exchanged every third day. We used partial medium exchange (500 μL out of a total of 1 mL in each well) due to the poor attachment of the cells to the bottom of the wells. The culture medium was collected for subsequent analyses, and after 7 days of culture, the cells were collected and homogenized.
Concentration Optimization and Cytotoxicity Assays
To optimize the culture conditions, based on our previous experience, we tested concentrations of excipients ranging from 0.1% to 0.5% to evaluate cell survival. We measured the culture medium for Lactate dehydrogenase (LDH) activity as a marker of cell toxicity.7 LDH release into the culture medium from the monocyte in vitro culture was detected by a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. LDH activity was quantified using a plate reader with a measurement wavelength of 490 nm and a reference wavelength of 655 nm.
Assessment of Concentrations of Desaturases and Inflammatory Markers
Desaturases SCD1 and D6D are monocyte microsomal enzymes involved in their maturation and migration process in response to fatty acid derivatives. The concentrations of these enzymes were analyzed in cell culture homogenates using ELISA kits (MyBiosource, San Diego, CA, USA) according to the manufacturer’s instructions.
Matrix metalloproteinase-9 (MMP-9), interleukin (IL) 1β, 6, 10, 12, 23, and tumor necrosis factor alpha (TNFα) are pro-inflammatory cytokines, whose release increases in response to inflammation and cell/tissue injury.13,14 The concentrations of these cytokines (pg/mL) in cell homogenates were measured by a commercially available LUMINEX assay (BioTechne, Minneapolis, MN, USA) according to the manufacturer’s instructions. Procalcitonin is a novel and highly sensitive inflammatory marker released by macrophages and monocytes in response to a stressor.15 The level of procalcitonin was detected similarly to the other inflammatory cytokines using LUMINEX (BioTechne).
LUMINEX Assay
We used a Magnetic Luminex Assay for IL-23, procalcitonin and MMP-1 and the Human High Sensitivity Cytokine Kit A for the other cytokines listed above. Dedicated 96-well microplates with a precoated magnetic microparticle cocktail were used for the assay. Then, 100 μL of cell homogenate and 25 μL of microparticle cocktail were mixed and incubated in the plates for 3 hours at room temperature on a horizontal shaker with the speed of 50 rpm. After washing away any unbound residues, 50 μL of biotinylated antibody cocktail specific to the analytes was added to each well and incubated for 1 hour at room temperature. Following another wash, 50 μL of streptavidin–phycoerythrin conjugate was added to each well and incubated for 30 min to bind biotinylated antibody. After the final wash, the plates were read using a BioRad Bio-PLexAnalyzer (BioRad, Hercules, CA, USA).
Cell Count
Cell counts were performed using ImageJ software from five visual fields of each well under 20× magnification (AxioVert, Zeiss, Germany). Small cells with round shapes were recognized as monocytes, and large, elongated, ameboid or spindled cells with processes and intracellular granules were recognized as macrophages. M1-like macrophages were characterized mostly by spindle-shaped cell bodies, whereas M2-like macrophages were mostly ameboid and contained yellow granules. The phenotype comparison was based on the cell features in the M1 and M2 controls.
Rabbit Eyelid Staining
Rabbit eyelids exposed to 50 μL pf-tafluprost or pf-latanoprost once daily for 8 weeks were obtained from Prof. Kai Kaarniranta (University of Eastern Finland, Kuopio, Finland). For this experiment, female albino New Zealand rabbits (about 3–4 kg weight) were used in the study. All animal procedures were conducted in accordance with the ARVO statement for the use of Animals in Ophthalmic and Vision Research and experiments were approved by the National Animal Experiment Board (ELLA, Finland) and carried out in accordance with guidelines of the Finnish Act on Animal Experimentation. Paraffin sections of tarsal glands were blocked with 10% normal goat serum (NGS) in 0.05% TBS/0.1% Triton for 30 min, followed by incubation with primary antibodies (against CD206 or CD80, both from Santa Cruz Biotechnology, Dallas, TX, USA) at +4 °C overnight. The tissue was then incubated with an AlexaFluor species-matched secondary antibody (Thermo, Waltham, MA, USA) for 3 hours at room temperature. Specimens were counterstained with DAPI (4′,6-diamidyno-2-fenyloindol, Thermo) and investigated under a fluorescent microscope (Axio Scope. A1, Zeiss, Germany).
Statistical Analysis
Statistical analysis was performed with IBM SPSS (Armonk, NY, USA). The descriptive statistics are shown as the mean±standard deviation (SD). Two-sided comparisons between groups were performed using independent or paired-samples Student’s t-tests, with p values <0.05 considered statistically significant. Multiple group comparisons were performed using repeated-measures ANOVA followed by Tukey’s post hoc test. Pearson’s correlation test was applied for correlation analysis.
Results
The Application of Different Excipients Affected the Cellular Morphology of Cultured Blood Monocytes in the Concentration Assay
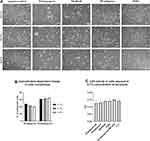
Cell morphology alterations were observed after 24 h of exposure to the tested excipients. The cell shape change from round to polypoidal was observed, especially in pf-latanoprost and MGHS40-treated cells, when compared with pf-tafluprost- and PS80-treated cells. Figure 2A shows the cell morphologies on the third day of culture. Additionally, we observed that an increase in pf-latanoprost concentration stimulated transformation into polypoidal cells, while an increase in pf-tafluprost concentration decreased this phenomenon (Figure 2B). Since concentrations of 0.3% and 0.5% resulted in pronounced cell death, especially in the pf-latanoprost group, we selected a 0.1% concentration for subsequent studies. For this concentration, the LDH assay of the culture medium showed no significant differences in LDH activity after 7 days of exposure to the excipients (p>0.05, ANOVA) (Figure 2C).
Seven-Day Treatment of Peripheral Blood Monocytes with 0.1% Concentrations of the Excipients Resulted in Transformation into Different Morphological Subtypes of Macrophages
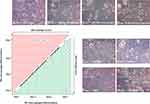
Based on the cell phenotype analysis, we distinguished four morphological types of cells that were present in our culture (Figure 3). Monocytes appeared as small, round cells with no spindles and with visible kidney-shaped nuclei. They were observed in all culture groups, especially in groups with lower macrophage counts (pf-tafluprost, PS80 and negative control). M0-like macrophages were characterized by a rosette-shaped morphology with long spindles and intracellular granulations. They were observed in all groups, except pf-tafluprost, the negative control and PS80. The absence of the M0 type was accompanied by the absence of the M1 type. The M1-like cells had elongated cell bodies with long spindles, and M2-like cells were amoeboid and contained yellow granulations (Figure 3).
The applied excipients polarized the differentiation of monocytes toward an M1 or M2 morphology, depending on the applied derivatives (Figure 4). The exclusive presence of M0 macrophages and monocytes was observed until Day 5 in all groups, and the presence of monocytes was observed only throughout the entire experiment in the group treated with PS80. M1 differentiation was pronounced on Day 7 in an increasing manner in the pf-latanoprost, MGHS40 and M1-positive control groups, while M2 differentiation was pronounced in the pf-tafluprost, MGHS40 and M2-positive control groups (Figure 4).
Overall, the highest percentage of macrophages was observed in M2- and M1-positive controls (39.2% and 36.3%, respectively), followed by the pf-latanoprost (33.6%) and MGHS40 (32.9%) groups. The application of MF-438 (SCD1 inhibitor) reduced the percentage of macrophages in the pf-latanoprost culture by approximately threefold compared with pf-latanoprost alone (13.5% vs 33.6%, p<0.01, Student’s t-test, Figure 5). Pf-tafluprost induced monocyte differentiation comparable to that of the negative control (17.6% and 18.6%, respectively), while PS80 completely inhibited monocyte/macrophage turnover (Figure 5).
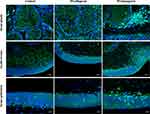
Immunostaining of Rabbit Eyelid Cross-Sections Revealed That Infiltration of Different Classes of Macrophages Depended on the Applied Excipient
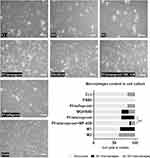
In the cross sections of rabbit eyelids, we observed M2 macrophage (positive for CD206) infiltration after both pf-tafluprost and pf-latanoprost treatment and M1 macrophages (positive for CD80) present predominantly in tissue exposed to pf-latanoprost (Figure 6). CD80-positive cells were observed within the tarsal glands, eyelid stroma and tarsal epithelium of rabbits exposed to commercially available eye drops (Figure 6).
ELISA Analysis Revealed Different Concentrations of SCD1 Desaturase After Exposure to Various Excipients
ELISA analysis of cell homogenates showed the highest concentration of SCD1 desaturase in the pf-latanoprost and MGHS40 groups, whereas MF-438 treatment decreased the SCD1 concentration by approximately sevenfold (p<0.001, Student’s t-test) (Figure 7). Pf-tafluprost and PS80 treatment resulted in significantly lower concentrations of SCD1 in treated cells (p<0.05 Student’s t-test) (Figure 7). There was a significant positive correlation between the concentration of SCD1 and the percentage of macrophages in culture (p=0.02, r2=0.8, Pearson’s correlation) (Figure 7). The concentration of SCD1 in M1- and M2-positive controls was not related to the percentage of macrophages due to different turnover stimuli; thus, these groups were excluded from correlation analysis. The concentration of D6D desaturase showed no significant differences between groups (p>0.05, ANOVA).
Multiplex Analysis Revealed Detectable Fluctuations in IL-1β, IL-6 and TNFα After Exposure to Various Excipients
Due to test sensitivity, we were able to detect only IL-1β, IL-6 and TNFα. For these cytokines, there were significant differences between groups. The IL-6 concentration was significantly higher in the pf-latanoprost group (2812.5±296 ng/mL) than in the pf-tafluprost group (1993±45 ng/mL) (p<0.01, Student’s t-test). Treatment with the SCD1 inhibitor decreased the IL-6 concentration in the pf-latanoprost group from 2812.5±296 ng/mL to 1555.75±134 ng/mL (p<0.01, Student’s t-test) (Figure 7). For TNFα and IL-1β concentrations, we did not detect significant differences (p>0.05, ANOVA), except in the M1 control group, which additionally confirmed the proinflammatory features of these cells (Figure 7).
Discussion
Macrophages are monocyte-derived phagocytic cells that reside in various tissues. These cells, which form by the polarization of monocytes, can exhibit beneficial and harmful features due to the presence of different classes of macrophages (ie, M1 or M2).16 Since the differentiation of monocytes into macrophages may involve various metabolic pathways, there are many possible therapeutic targets that can be used for potential treatments regulating this process. On the other hand, tissue microenvironments and multiple substances can affect macrophage polarization and alter their final function.17,18 The principal theory of macrophage polarization divides macrophages into two basic classes: M1 (proinflammatory) macrophages, which are typically activated and produce proinflammatory cytokines involved in phagocytosis and inflammation, and M2 (wound healing) macrophages, which are alternatively activated and involved in collagen production and wound healing.19,20 This classification also distinguishes M0 macrophages that represent immature forms that can differentiate into other subtypes.17,19,20 M1 macrophages are activated classically by IFNγ and LPS stimulation, while M2 activation requires IL-4 and IL-13.17,19,20 M1 cells secrete iNOS, TNFα, IL-1, IL-6, IL-12, IL-23, MCP-1 and IFNγ, cytokines involved in inflammation, tumor resistance and graft rejection, while the M2 cell secretome contains IL-10, arginase-1, TGFβ1, VEGF, and Ym1, representing the activity profile of immunoregulation, matrix deposition, tissue remodeling and wound healing.17 With the further development of understanding of macrophages, M2 cells have been divided into further subtypes (M2 a, b, c, d) depending on specific stimulating factors.18 The M1/M2 classification is based on in vitro features of macrophages grown under controlled conditions that cannot always be translated into living organisms; thus, there is a tendency to distinguish M1/M2 stimuli leading to polarization of certain immune cells rather than M1/M2 macrophages themselves; however, there is a large heterogeneity in current classifications.18
In addition to external stimuli, the polarization of macrophages also depends on the activity of intracellular molecular pathways that can affect their differentiation. Out of many pathways, enzymes involved in fatty acid metabolism are crucial regulators of macrophage polarization.10 Stearoyl-coenzyme A desaturase 1 catalyzes the production of monounsaturated fatty acids from saturated substrates. The distinct effects of alteration within this pathway on macrophage turnover are related to the accumulation of SCD1 substrates or to the concentration of SCD1 itself that leads to macrophage chemotaxis and the production of proinflammatory cytokines.21 SCD1 has been widely investigated for its role in inflammation. Surprisingly, the final outcome of SCD1 alteration depends on the tissue location and the local content and type of stimulus affecting the activity of this enzyme. The blockade of the expression and/or activity of SCD1 results in the accumulation of its substrates, palmitate and stearate first of all, that attract macrophages via TLR4 stimulation. This has been documented to be a possible proinflammatory mechanism in pancreatic β-cells, skin, muscles and atherosclerotic vascular endothelium.22–25 Complete knockout of the SCD1 gene results in deficiency of the sebum and atrophy of tarsal glands in the eyelids.26 In parallel, the inhibition of SCD1 shows beneficial effects in improving metabolic syndrome, reducing insulin resistance and adipose tissue inflammation and preventing obesity.27–30 All of the above-described outcomes of SCD1 dysregulation are related to disturbed metabolism and the balance between saturated and unsaturated fatty acids rather than to altered levels of SCD1 itself. However, there are reports that directly link the concentration and activity of the SCD1 enzyme with the polarization of macrophages into pro- or anti-inflammatory responses. The increase in SCD1 has been directly linked with impairment of wound healing properties of central nervous system macrophages (microglia), and inhibition of SCD1 increases remyelination of axons after brain injury.31 In this study, the authors showed that when SCD1 was increased, CNS macrophages shifted their morphology from foamy to spindle, which was associated with the promotion of proinflammatory cytokine release. In another study, Wallner et al proved that the activity of SCD1, not D6D desaturase, is associated with monocyte-macrophage transformation.10
In our study, preservative-free prostaglandin analogs induced the transformation of monocytes into M1- and M2-like macrophages in the case of pf-latanoprost and M2-like macrophages in the case of pf-tafluprost. This phenomenon was likely linked to the derivatives of fatty acids (MGHS40 and PS80), which represent the eye drop detergents, activating different desaturases. SCD1 desaturase, activated by the saturated derivative MGHS40 present in pf-latanoprost, was correlated with macrophage transformation, and chemical inhibition of this enzyme (using MF-438) decreased the macrophage count in the culture. This inhibition also decreased the release of the proinflammatory cytokine IL-6. The differentiation of macrophages from monocytes in vitro is a burden with high spontaneity; thus, the description and characterization of macrophage subtypes varies in the literature. In our study, we based characterization on cell morphology and comparison with model cells from M1 or M2 controls differentiated using a manufacturer predesigned kit. Similar to Eligini et al,32 we observed two major morphological types of cells differentiating from peripheral blood monocytes, spindle and round. Spindle cells with elongated shapes were morphologically similar to M1-control macrophages, which also secreted high levels of IL-6 and TNFα. This subtype of macrophages in the Eligini study was linked with a greater ability to produce superoxide anions and increased mRNA levels of proinflammatory chemokines, ie, CCL18 and CCL24, which suggests polarization toward the M1 type. Round, “fried-egg shape” cells containing lipid granules showed morphological similarities with M2-control macrophages. They did not show either increased IL-6 or TNFα content in the culture medium. In the Eligini study, these cells were characterized as less efficient in producing superoxide anions and expressing the anti-inflammatory proteins CD163 and IL-10.32 Detergents included in PA formulas enable PA solubility, which is the mechanism of their penetration through water/lipid ocular barriers. Of the analyzed solubilizers, MGHS40, a derivative of a saturated fatty acid (stearate), and PS80, a derivative of monounsaturated fatty acids (oleate), are the most commonly used. Since the eye drops after topical application are systemically absorbed from ocular surface blood vessels, the excipients are metabolized in circulating blood cells (ie, blood monocytes). The application of eye drops containing saturated fatty acid (stearate) derivatives may have detrimental effects on inflammatory cell maturation. Stearate may stimulate TLR4 receptors and induce chemotaxis of macrophages; on the other hand, it can induce the SCD1 enzyme within blood monocytes, which is directly linked with macrophage polarization. Based on our research, the polarization in this case is mostly toward proinflammatory M1 cells. The impact of SCD1 induction and the related generation of macrophages has been well described for adipose tissue inflammation, obesity and insulin resistance pathogenesis.10 The new population of macrophages induced by SCD1 does not necessarily cause active inflammation but rather latent inflammation, which is described as inflammatory cell infiltration with low-grade production of inflammatory cytokines.
The limitation of this study is related to the calculation of systemic absorption via conjunctival vessels after topical eye drops application. We are aware that nasolacrimal route, mostly nasal mucosa circulation, may represent the major absorption route; thus, our calculation could be overestimated.33 To minimize this probability, we conducted dose tittering study, to optimize subtoxic dose of used excipients in vitro. We know also that therapeutic doses of analyzed eyedrops induce similar transformation of macrophages in vivo in rabbits.6 Since the current study is rather focused on describing molecular mechanism of macrophages transformation associated with exposition to eye drops detergents, the calculation burden with overdose do not affect the principal aim of this study.
Conclusions
In our study, we proved that inhibition of SCD1 in cell culture supplemented with MGHS40-containing eye drops abolished the proinflammatory features of macrophages, as indicated by decreased IL-6 secretion, reduced total macrophage count in culture and reduced polarization toward M1-type cells. We expect that inhibition of SCD1 desaturase in in vivo conditions could similarly limit the latent inflammation induced by detergents present in preservative-free prostaglandin analogs and could be the way to improve eye drop tolerance. Such therapeutic approach, including the SCD1 inhibitor in eye drop formula, would have basic advantage in potential increase of the treatment tolerability. Using fixed-dose combination would not affect the dosage frequency of the eye drops. On the other hand, SCD1 inhibitors may not be selective enough; thus, it would require further in vivo experiments to identify potential side effects.
Data Sharing Statement
Data that support findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Iwona Matuszek and Joanna Mazela for technical help. We give special thanks to Professor Kai Kaarniranta for providing rabbit eyelid samples that greatly increased the quality of this study.
Funding
This study was supported by Santen grant SUM CTT/1202/2017.
Disclosure
Anna Pacwa, Joanna Lewin-Kowalik, and Adrian Smedowski are employees of GlaucoTech Co. The authors report no other potential conflicts of interest for this work.
References
1. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi:10.1016/S0140-6736(17)31469-1
2. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi:10.1001/jama.2014.3192
3. Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi:10.1016/j.preteyeres.2010.03.001
4. Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86(7):716–726. doi:10.1111/j.1755-3768.2008.01250.x
5. Kim SH, Kwon D, Lee S, et al. Concentration-and time-dependent effects of benzalkonium chloride in human lung epithelial cells: necrosis, apoptosis, or epithelial mesenchymal transition. Toxics. 2020;8(1). doi:10.3390/toxics8010017
6. Trzeciecka A, Paterno JJ, Toropainen E, et al. Long-term topical application of preservative-free prostaglandin analogues evokes macrophage infiltration in the ocular adnexa. Eur J Pharmacol. 2016;788:12–20. doi:10.1016/j.ejphar.2016.06.014
7. Smedowski A, Paterno JJ, Toropainen E, Sinha D, Wylegala E, Kaarniranta K. Excipients of preservative-free latanoprost induced inflammatory response and cytotoxicity in immortalized human HCE-2 corneal epithelial cells. J Biochem Pharmacol Res. 2014;2(4):175–184. doi:10.1016/j.pestbp.2011.02.012
8. Kim JH, Kim EJ, Kim YH, et al. In vivo effects of preservative-free and preserved prostaglandin analogs: Mouse Ocular Surface Study. Korean J Ophthalmol. 2015;29(4):270–279. doi:10.3341/kjo.2015.29.4.270
9. Juretić M, Cetina-čižmek B, Filipović-Grčić J, Hafner A, Lovrić J, Pepić I. Biopharmaceutical evaluation of surface active ophthalmic excipients using in vitro and ex vivo corneal models. Eur J Pharm Sci. 2018;120:133–141. doi:10.1016/j.ejps.2018.04.032
10. Wallner S, Grandl M, Konovalova T, et al. Monocyte to macrophage differentiation goes along with modulation of the plasmalogen pattern through transcriptional regulation. PLoS One. 2014;9(4):e94102. doi:10.1371/journal.pone.0094102
11. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi:10.1038/nature05485
12. Farkouh A, Frigo P, Czejka M. Systemic side effects of eye drops: a pharmacokinetic perspective. Clin Ophthalmol. 2016;Volume 10:2433–2441. doi:10.2147/OPTH.S118409
13. Lemp MA, Baudouin C, Baum J, et al. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international Dry Eye WorkShop (2007). In: Ocular Surface. Vol. 5. ETHIS COMMUNICATIONS, INC.; 2007:75–92. doi:10.1016/s1542-0124(12)70081-2
14. Hassan W, Ding L, Gao RY, Liu J, Shang J. Interleukin-6 signal transduction and its role in hepatic lipid metabolic disorders. Cytokine. 2014. doi:10.1016/j.cyto.2013.12.017
15. Taylor R, Jones A, Kelly S, Simpson M, Mabey J. A review of the value of procalcitonin as a marker of infection. Cureus. 2017;9(4). doi:10.7759/cureus.1148
16. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
17. Lee KY. M1 and M2 polarization of macrophages: a mini-review. Med Biol Sci Eng. 2019;2(1):1–5. doi:10.30579/mbse.2019.2.1.1
18. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6. doi:10.12703/P6-13
19. Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. doi:10.1016/j.ejphar.2020.173090
20. Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(Lps+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Front Immunol. 2019;10(MAY). doi:10.3389/fimmu.2019.01084
21. Liu X, Strable MS, Ntambi JM. Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Adv Nutr. 2011;2(1):15–22. doi:10.3945/an.110.000125
22. Thörn K, Hovsepyan M, Bergsten P. Reduced levels of SCD1 accentuate palmitate-induced stress in insulin-producing β-cells. Lipids Health Dis. 2010;9(1):108. doi:10.1186/1476-511X-9-108
23. MacDonald MLE, Van Eck M, Hildebrand RB, et al. Despite antiatherogenic metabolic characteristics, SCD1-deficient mice have increased inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(3):341–347. doi:10.1161/ATVBAHA.108.181099
24. Peter A, Weigert C, Staiger H, et al. Induction of stearoyl-CoA desaturase protects human arterial endothelial cells against lipotoxicity. Am J Physiol Endocrinol Metab. 2008;295(2):E339–E349. doi:10.1152/ajpendo.00022.2008
25. Hulver MW, Berggren JR, Carper MJ, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2(4):251–261. doi:10.1016/j.cmet.2005.09.002
26. Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131(9):2260–2268. doi:10.1093/jn/131.9.2260
27. Brown JM, Rudel LL. Stearoyl-coenzyme A desaturase 1 inhibition and the metabolic syndrome: considerations for future drug discovery. Curr Opin Lipidol. 2010;21(3):192–197. doi:10.1097/MOL.0b013e32833854ac
28. Feng D, Wang Y, Mei Y, et al. Stearoyl-CoA desaturase 1 deficiency protects mice from immune-mediated liver injury. Lab Invest. 2009;89(2):222–230. doi:10.1038/labinvest.2008.105
29. Liu X, Miyazaki M, Flowers MT, et al. Loss of stearoyl-CoA desaturase-1 attenuates adipocyte inflammation: effects of adipocyte-derived oleate. Arterioscler Thromb Vasc Biol. 2010;30(1):31–38. doi:10.1161/ATVBAHA.109.195636
30. Ravaut G, Légiot A, Bergeron KF, Mounier C. Monounsaturated fatty acids in obesity‐related inflammation. Int J Mol Sci. 2021;22(1):1–22. doi:10.3390/ijms22010330
31. Bogie JFJ, Grajchen E, Wouters E, et al. Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain. J Exp Med. 2020;217(5). doi:10.1084/jem.20191660
32. Eligini S, Brioschi M, Fiorelli S, Tremoli E, Banfi C, Colli S. Human monocyte-derived macrophages are heterogenous: proteomic profile of different phenotypes. J Proteomics. 2015;124:112–123. doi:10.1016/j.jprot.2015.03.026
33. Agrahari V, Mandal A, Agrahari V, et al. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016;6(6):735–754. doi:10.1007/s13346-016-0339-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.