Back to Journals » Infection and Drug Resistance » Volume 16
Scalp Infection Caused by Mycobacterium abscessus Manifested as Patchy Alopecia in an Immunocompetent Female
Authors Zhang X, Feng Y, Li D, Han J, Shi D
Received 15 April 2023
Accepted for publication 10 August 2023
Published 18 August 2023 Volume 2023:16 Pages 5413—5419
DOI https://doi.org/10.2147/IDR.S416974
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xiaoyu Zhang,1 Yahui Feng,2 Dongmei Li,3 Jingjian Han,4 Dongmei Shi2,5
1School of Clinical Medicine, Weifang Medical University, Weifang, People’s Republic of China; 2Laboratory of Medical Mycology, Jining No. 1 People’s Hospital, Jining, People’s Republic of China; 3Department of Microbiology & Immunology, Georgetown University Medical Center, Washington, DC, USA; 4Department of Medical Cosmetology, Jining No. 1 People’s Hospital, Jining, People’s Republic of China; 5Department of Dermatology, Jining No. 1 People’s Hospital, Jining, People’s Republic of China
Correspondence: Dongmei Shi, Laboratory of Medical Mycology & Department of Dermatology, Jining No. 1 People’s Hospital, No. 6 Jiankang Road, Jining, Shandong Province, 272067, People’s Republic of China, Tel +8618678769757, Email [email protected] Jingjian Han, Department of Medical Cosmetology, Jining No. 1 People’s Hospital, No. 6 Jiankang Road, Jining, Shandong Province, 272067, People’s Republic of China, Email [email protected]
Abstract: Mycobacterium abscessus (M. abscessus) is a fast-growing, non-tuberculous mycobacterium (NTM) that can cause human infections varying from superficial infection to pulmonary or even systemic infections. The latter is more commonly appeared in immunocompromised patients. The skin infection caused by M. abscessus often appears after trauma or surgical procedure. It is often manifested by subcutaneous nodules, papules, erythema, tender erythematous or violaceous plaques, cellulitis, abscesses, ulcerations, and draining sinuses. Herein, we present a non-typical cutaneous manifestation of M. abscessus infection in a 46-year-old woman who presented with alopecia on the scalp with no itching or pain. The pathogen was isolated and identified as M. abscessus by morphology and DNA sequencing. To our best knowledge, there was no report that this organism could cause skin lesions mimicking patchy alopecia. After 3 months of antibacterial treatment, the cutaneous lesion disappeared, and new growth of hair occurred in this patient.
Keywords: Mycobacterium abscessus, alopecia, non-tuberculous mycobacteria
Introduction
Mycobacterium abscessus (M. abscessus) is a member of the common rapidly growing nontuberculous mycobacteria (NTM). It naturally inhabits various environments and mainly causes pulmonary infection and systemic and/or disseminated infection. Today, skin infections and soft tissue infections caused by NTM, such as M. abscessus, are increasingly recognized worldwide.1 Their clinical manifestations vary with host immune status and affected anatomic sites, ranging from a single lesion to multiple lesions. The skin lesions are even a part of disseminated infection, especially in those immunosuppressed individuals. The skin infections caused by M. abscessus often occur after traumas and surgical or cosmetic procedures. The lesions manifest as subcutaneous nodules, papules, erythemas, tender erythematous or violaceous plaques, cellulitis, abscesses, ulcerations, and draining sinuses. Diagnosis may sometimes be difficult since there is no symbolic symptom in M. abscessus infection. Since M. abscessus is one of the most antibiotic-resistant pathogens, the misdiagnosis then may pose significant challenges to treating infection caused by this organism.
Here, we report an adult female with a scalp infection caused by M. abscessus. The patient was primarily misdiagnosed as alopecia due to patchy alopecia manifestation. The patient received a surgical excision in a local hospital in order to rule out a possible skin tumor. This case taught us that skin infections by M. abscessus, even other NTM, can lead to hair loss without itching or pain that mimics alopecia areata. The early diagnosis could be a life-saving measure for the high-risk hosts.
Case
A 46-year-old woman consulted a dermatologist with a one-month history of hair loss in the forehead area. One month ago, the patient noticed a hair loss area on the scalp without itching and pain. She did not receive any treatment, but the area of hair loss gradually enlarged. The patient denied any history of trauma or medical procedure but had temperately lived in Zhuhai (the southern city of China) for a couple of weeks before the onset of the lesion.
The physical examination found that the surface of the alopecia lesion on the forehead area was smooth without erythema, ulcer, scale, or broken hair. However, when palpating, a subcutaneous swelling (1.5×2 cm) below the alopecia lesion was noticed that had no obvious boundary or fluctuation (Figure 1A). There was no regional lymphadenopathy. The whole blood test and other routine laboratory tests were all within normal ranges.
The hairs taken closely around the lesion were stained with a calcofluor white. Hyphae and spores of fungi were not observed under fluorescent microscopy. To rule out the possibility of a skin tumor, the lump was biopsied. During the surgery, red gelatinous discharge in the subcutaneous lump was removed by curettage (Figure 1B). The red gelatinous material was also submitted for pathogen culture, and the biopsied skin was used for histological detection.
The red gelatinous material was cultured on Sabouraud-glucose Agar (SDA) with Chloramphenicol, Columbia Blood Agar Plate (CBA), and Middle Brook 7H11 Agar (M7H11) at 30°C (SDA, CBA, and M7H11, Hope Bio-Technology Co., Ltd., Qingdao, Shandong, China). There was no growth on both SDA and CBA after 1 week. However, round milky white yeast-like colonies of varying sizes were found on M7H11 Agar (Figure 2).
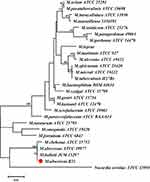
The isolate was identified by sequencing the ITS1/ ITS4 region of the rRNA gene. The DNA sequencing of the isolate was aligned with reference sequences in GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi), and our target sequence obtained 100% coverage and 100% homology with M. abscessus (No. MN922566.1). Based on the morphological characteristics and molecular sequence, the isolate was identified as M. abscessus. The sequencing of our isolate can be accessed in GenBank with registration number No. OP781845. The strain (No. B21) was stored in the Laboratory of medical mycology, Jining No. 1 People’s Hospital. We constructed a phylogenetic tree (Figure 3) based on ITS and β-tubulin sequences using the maximum-likelihood method in MEGA 7.0. The type strain of Nocardia seriolae ATCC 43993 was used as an outgroup.
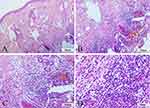
The histological examination of the skin tissue revealed notable granulomas feature which manifested as a mixed inflammatory cells infiltration in both the dermis and subcutaneous fat. These infiltrates consist mainly of abundant lymphocytes and plasma cells, along with a smaller number of epithelioid histiocytes and scarce neutrophils (Figure 4A–D). There were no obvious abnormalities in the epidermis.
The patient was diagnosed with cutaneous M. abscessus infection based on histology and pathogen identification. The patient was empirically given oral clarithromycin prior to the drug sensitivity testing. The drug sensitivity testing showed that our isolate was sensitive to clarithromycin and amikacin but resistant to doxycycline and sulfamethoxazole, which was similar to the reports in other literatures.2
Since the patient responded well to the primary treatment, clarithromycin (oral 500 mg twice a day) was continued for 3 months. Concurrently, Benzalkonium chloride solution was applied topically to the affected area. By the end of the treatment, the lump was not palpable, and new hair grew except for the presence of an inconspicuous scar in the surgical area (Figure 1C). The lesion did not recur within a 6-month follow-up.
Discussion
M. abscessus can be easily found in soil, decaying vegetation, and water in our living environments.3,4 Therefore, infection may occur after contact with sewage water containing M. abscessus. It is usually a nonpathogenic organism for immunocompetent hosts, but M. abscessus can cause some life-threatening conditions in those immunocompromised patients,5–7 which include a broad spectrum of infections in lung, skin and soft tissue, central nervous systems, or even death. Skin or soft tissue infections often occur after trauma or surgery and injection activities with nonsterile or contaminated needles, scalpels, or other devices. The localized cutaneous infections can be easily disseminated, particularly in an immuno-compromised host.8
Skin and soft tissue infections by M. abscessus are the most frequently involved organs after the lung. The clinical manifestations of skin and soft tissue infection caused by this pathogenic bacteria are presented mainly by subcutaneous fluctuating or hard nodules, papules, erythema, plaques, cellulitis, abscesses, ulcerations, and draining sinuses.9,10 The routes of M. abscessus infections can be through two mechanisms: (1) direct contact with pathogenic bacteria in contaminated water and environment or materials for sealing the wounds, such as surgery adhesives; (2) secondary to disseminated diseases.11–13 The trauma or medical events can be accidental skin damage or any surgery from cosmetic procedure to transplantation.9,14,15 Indeed, skin infections related to cosmetic procedures are increasingly reported, which include liposuction, breast augmentation, drug injection, tattoo, acupuncture, and other cosmetic-related operations.12,16–18 The water supply system has been assumed to be the source of some skin infections and outbreaks.19,20 The outbreaks reported in Canada, Italy, and the United States have been associated with the hands and feet of children who had a history of contact the swimming or wading pools.21–23 Our patient had no clear history of trauma and had not received any medical incidents. But she had a traveling experience before the onset of the infection. We suspect that she may have been exposed to this pathogen during swimming in the sea of the southern part of China. Cases of M. abscessus infection with scalp abscess are rare. We found only 2 cases in the PubMed database when searching the keywords “Mycobacterium abscessus” and “hair” up to March 2023. Their main characteristics are summarized in Table 1.24,25 The two reported cases both have explicit medical behaviors, which provided good diagnostic clues for clinicians. However, our patients do not have any memorable medical behaviors or trauma, which has posed specific difficulties in diagnosing the disease. This also leads us to reflect on whether there are diagnostic errors in the rare reports of scalping infections caused by branched bacteria. In addition, the initial manifestation of infection in our case is hair loss, which has not been mentioned in previous cases. Our case has enriched the manifestations of this pathogenic bacterial scalp infection and provided guidance for clinical diagnosis and treatment. We believe the lack of knowledge of this acid-fast bacillus infection may be the reason for few reports on the scalp abscesses of M. abscessus.
 |
Table 1 The Reported Cases of Scalp Mycobacterium abscessus Infection |
The misdiagnosis rate of this disease by clinicians is exceptionally high if based on clinical manifestations only. The reported clinical manifestations of NTM infection include superficial abscesses and nodules, which are firm or soft, non-tender or mildly tender.10 These skin lesions may become softer and even rupture with the progress of the infection. In our case, M. abscessus infection initially appeared as alopecia areata, and a soft subcutaneous swollen (cyst) was developed at the later stage of infection. Therefore, our case teaches us a lesson that NTM could be microbial pathogens in every scalp abscess infection.
The mechanisms of hair loss caused by M. abscessus infection are unclear. The histopathological findings of M. abscessus, similar to other NTMs, are nonspecific. Rodriguez et al summarized histopathological changes of M. abscessus infection into three categories:26 1) in a typical model, the abscess is surrounded by macrophages and epithelioid cells, accompanied by mixed granuloma and nodular or diffuse inflammatory infiltration; 2) in immunosuppressed patients, abscess mainly formed by neutrophils and accompanied by a small amount of granuloma reaction; and 3) in a granulomatous inflammation, as shown in our case, the infiltrate is composed of lymphocytes, plasma cells and epithelioid cells, and neutrophils are rare. In addition, the lipid vacuoles in pathological tissue have been noted.26,27 Rodriguez et al believed that some fat vacuoles could be moved upwards by the underneath inflammatory infiltrate.26 The mechanism of hair loss of the lipedematous scalp (LS) is not clear at present. However, the thickening and upward movement of fat and the change of microenvironment around hair follicles have been proposed to LS.28,29 We speculate that microenvironment changes or inflammation may lead to atrophy or destruction of hair follicles, which may also contribute to alopecia. In addition, the pressure on the hair follicles from the subcutaneous fat layer may interfere with the nutrition supply to the hair matrix, which then causes hair loss. From the clinical manifestations, more gray hairs shown in newly growing hairs than those surrounding hairs may suggest somehow damage in hair follicles.
As a common NTM in the environment, M. abscessus is increasingly isolated in abscess lesions following a trauma incidence. For skin infection, the exposed limbs are the primary location of infection. Scalp infection is rare that brings big challenges to doctors and patients. Hair loss on the scalp is a consequence of microbial growth, the immune environment, and histopathological features around hair follicles.
Ethics Approval
Our institutions do not require ethical approval to report individual cases or series of cases.
Informed Consent Statement
Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Acknowledgments
We are grateful to the patient reported in this article and her family for their genuine support.
Funding
This work was supported in part by grants from the National Nature Science Foundation of China (NM 82272358) and the Key Research and Development Plan of Jining (NM 2021YXNS121), Shandong, China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mougari F, Guglielmetti L, Raskine L, Sermet-Gaudelus I, Veziris N, Cambau E. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14(12):1139–1154. doi:10.1080/14787210.2016.1238304
2. Shen Y, Wang X, Jin J, et al. In vitro susceptibility of Mycobacterium abscessus and Mycobacterium fortuitum isolates to 30 antibiotics. Biomed Res Int. 2018;2018:4902941. doi:10.1155/2018/4902941
3. Brown-Elliott BA, Wallace RJ. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15(4):716–746. doi:10.1128/CMR.15.4.716-746.2002
4. Wentworth AB, Drage LA, Wengenack NL, Wilson JW, Lohse CM. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88(1):38–45. doi:10.1016/j.mayocp.2012.06.029
5. Bala K, Kumari S, Guleria R, Singh U. Recurrent bilateral breast abscess due to Mycobacterium abscessus in an immune-competent woman. BMJ Case Rep. 2020;13(10):e235857. doi:10.1136/bcr-2020-235857
6. Benwill J, Babineaux M, Sarria JC. Pulmonary Mycobacterium abscessus in an AIDS patient. Am J Med Sci. 2010;339(5):495–496. doi:10.1097/MAJ.0b013e3181d96ad7
7. Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36(1):91–99. doi:10.1016/j.ccm.2014.11.002
8. Petrini B. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS. 2006;114(5):319–328. doi:10.1111/j.1600-0463.2006.apm_390.x
9. Fang RY, Sun QN. Mycobacterium abscessus infections following injection of botulinum toxin. J Cosmet Dermatol. 2020;19(4):817–819. doi:10.1111/jocd.13094
10. Uslan DZ, Kowalski TJ, Wengenack NL, Virk A, Wilson JW. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142(10):1287–1292. doi:10.1001/archderm.142.10.1287
11. To K, Cao R, Yegiazaryan A, Owens J, Venketaraman V. General overview of nontuberculous mycobacteria opportunistic pathogens: mycobacterium avium and Mycobacterium abscessus. J Clin Med. 2020;9(8):2541. doi:10.3390/jcm9082541
12. Kothavade RJ, Dhurat RS, Mishra SN, Kothavade UR. Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur J Clin Microbiol Infect Dis. 2013;32(2):161–188. doi:10.1007/s10096-012-1766-8
13. Tejura N, Bontempo G, Chew D. Disseminated Mycobacterium abscessus infection secondary to an infected vascular stent: case report and review of the literature. Open Forum Infect Dis. 2018;5(9):ofy207. doi:10.1093/ofid/ofy207
14. Wongkitisophon P, Rattanakaemakorn P, Tanrattanakorn S, Vachiramon V. Cutaneous Mycobacterium abscessus infection associated with mesotherapy injection. Case Rep Dermatol. 2011;3(1):37–41. doi:10.1159/000324766
15. Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32(1). doi:10.1128/CMR.00069-18
16. Moreno-Izquierdo C, Zurita J, Contreras-Yametti FI, Jara-Palacios MA. Mycobacterium abscessus subspecies abscessus infection associated with cosmetic surgical procedures: cases series. IDCases. 2020;22:e00992. doi:10.1016/j.idcr.2020.e00992
17. Koh SJ, Song T, Kang YA, et al. An outbreak of skin and soft tissue infection caused by Mycobacterium abscessus following acupuncture. Clin Microbiol Infect. 2010;16(7):895–901. doi:10.1111/j.1469-0691.2009.03026.x
18. Bechara C, Macheras E, Heym B, Pages A, Auffret N. Mycobacterium abscessus skin infection after tattooing: first case report and review of the literature. Dermatology. 2010;221(1):1–4. doi:10.1159/000313974
19. Thomson RM, Carter R, Tolson C, Coulter C, Huygens F, Hargreaves M. Factors associated with the isolation of Nontuberculous mycobacteria (NTM) from a large municipal water system in Brisbane, Australia. BMC Microbiol. 2013;13:89. doi:10.1186/1471-2180-13-89
20. Dickison P, Howard V, O’Kane G, Smith SD. Mycobacterium abscessus infection following penetrations through wetsuits. Australas J Dermatol. 2019;60(1):57–59. doi:10.1111/ajd.12915
21. Dytoc MT, Honish L, Shandro C, et al. Clinical, microbiological, and epidemiological findings of an outbreak of Mycobacterium abscessus hand-and-foot disease. Diagn Microbiol Infect Dis. 2005;53(1):39–45. doi:10.1016/j.diagmicrobio.2005.03.010
22. Sinagra JL, Kanitz EE, Cerocchi C, et al. Mycobacterium abscessus hand-and-foot disease in children: rare or emerging disease. Pediatr Dermatol. 2014;31(3):292–297. doi:10.1111/pde.12333
23. Carter KK, Lundgren I, Correll S, et al. First United States outbreak of Mycobacterium abscessus hand and foot disease among children associated with a wading pool. J Pediatric Infect Dis Soc. 2019;8(4):291–296. doi:10.1093/jpids/piy036
24. Eustace K, Jolliffe V, Sahota A, Gholam K. Cutaneous Mycobacterium abscessus infection following hair transplant. Clin Exp Dermatol. 2016;41(7):768–770. doi:10.1111/ced.12900
25. Mohanty M, Mishra B, Sirka CS, Mohapatra PR. Case report: multiple scalp abscesses due to Mycobacterium abscessus infection following triamcinolone injection in an immunocompetent person. Am J Trop Med Hyg. 2022;107(3):592–594. doi:10.4269/ajtmh.22-0126
26. Rodríguez G, Ortegón M, Camargo D, Orozco LC. Iatrogenic Mycobacterium abscessus infection: histopathology of 71 patients. Br J Dermatol. 1997;137(2):214–218. doi:10.1046/j.1365-2133.1997.18081891.x
27. Sardiña LA, Kaw U, Jour G, et al. Diagnosis of Mycobacterium abscessus/chelonae complex cutaneous infection: correlation of tissue culture and skin biopsy. J Cutan Pathol. 2020;47(4):321–327. doi:10.1111/cup.13623
28. Dhurat RS, Daruwalla SB, Ghate SS, Jage MM, Sharma A. Distinguishing lipedematous scalp, lipedematous alopecia, and diffuse alopecia areata. Skin Appendage Disord. 2019;5(5):316–319. doi:10.1159/000495947
29. Yasar S, Gunes P, Serdar ZA, Tosun I. Clinical and pathological features of 31 cases of lipedematous scalp and lipedematous alopecia. Eur J Dermatol. 2011;21(4):520–528. doi:10.1684/ejd.2011.1385
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.