Back to Journals » Drug Design, Development and Therapy » Volume 17
Sanqi Qushi Granule Alleviates Proteinuria and Podocyte Damage in NS Rat: A Network Pharmacology Study and in vivo Experimental Validation
Authors Wang L , Liu H, Wang Y, Hong X, Huang X, Han M, Wang D, Shan W, Li P, Gu H, Liu B , Bao K
Received 15 February 2023
Accepted for publication 26 May 2023
Published 19 June 2023 Volume 2023:17 Pages 1847—1861
DOI https://doi.org/10.2147/DDDT.S403617
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Lijuan Wang,1,* Huoliang Liu,2,* Yi Wang,1 XiaoFan Hong,1 Xiaoyan Huang,1,3,4 Miaoru Han,1 Dan Wang,1 Wenjun Shan,1 Ping Li,1,3 Haowen Gu,1 Bo Liu,3,5 Kun Bao1,3,4,6
1Second Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 2The Affiliated TCM Hospital of Guangzhou Medical University, Guangzhou, People’s Republic of China; 3State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou, People’s Republic of China; 4Guangdong-Hong Kong-Macau Joint Laboratory on Chinese Medicine and Immune Disease Research, Guangzhou, People’s Republic of China; 5Guangzhou Key Laboratory of Chirality Research on Active Components of Traditional Chinese Medicine, Guangzhou, People’s Republic of China; 6Guangdong Provincial Key Laboratory of Chinese Medicine for Prevention and Treatment of Refractory Chronic Disease, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Liu; Kun Bao, Email [email protected]; [email protected]
Background: Nephrotic syndrome (NS) and its numerous complications remain the leading causes of morbidity and mortality globally. Sanqi Qushi granule (SQG) is clinically effective in NS. However, its potential mechanisms have yet to be elucidated.
Methods: A network pharmacology approach was employed in this study. Based on oral bioavailability and drug-likeness, potential active ingredients were picked out. After acquiring overlapping targets for drug genes and disease-related genes, a component-target-disease network and protein–protein interaction analysis (PPI) were constructed using Cytoscape, followed by GO and KEGG enrichment analyses. Adriamycin was injected into adult male Sprague-Dawley (SD) rats via the tail vein to establish NS model. Kidney histology, 24-hr urinary protein level, creatinine (Cr), blood urea nitrogen (BUN), triglyceride (TG), total cholesterol (TC), and low-density lipoprotein (LDL-C) level were assessed. Western blotting, immunohistochemistry, and TUNEL staining were applied.
Results: In total, 144 latent targets in SQG acting on NS were screened by a network pharmacology study, containing AKT, Bax, and Bcl-2. KEGG enrichment analysis suggested that PI3K/AKT pathway was enriched primarily. In vivo validation results revealed that SQG intervention ameliorated urine protein level and podocyte lesions in the NS model. Moreover, SQG therapy significantly inhibited renal cells apoptosis and decreased the ratio of Bax/Bcl-2 protein expression. Moreover, we found that Caspase-3 regulated the PI3K/AKT pathway in NS rats, which mediated the anti-apoptosis effect.
Conclusion: By combining network pharmacology with experimental verification in vivo, this work confirmed the treatment efficacy of SQG for NS. SQG protected podocyte from injury and inhibited kidney apoptosis in NS rats via the PI3K/AKT pathway at least partially.
Keywords: Sanqi Qushi granule, nephrotic syndrome, podocyte injury, kidney apoptosis, network pharmacology, PI3K/AKT pathway
Introduction
Nephrotic syndrome (NS) manifests a range of clinical conditions of massive proteinuria, edema, hypoproteinemia, and hyperlipidemia.1 Patients with NS are generally more susceptible to hypertension, thrombotic events, and serious infections.2 Prednisone is often the first-line therapy for NS.3 Management of NS is very challenging because 10%–20%of patients develop steroid-resistant nephrotic syndrome (SRNS).3 Moreover, an overwhelming majority of individuals with SRNS progress to Chronic Kidney Disease (CKD) and End-Stage Renal Disease (ESRD) within a few years.2,4 Currently, there is no specific treatment to curb disease progression into ESRD.2 Hence, it is urgent to develop effective drugs for NS.
Sanqi Qushi Granule (SQG) is derived from Sanqi oral solution, which is a hospital preparation for clinical use in Guangdong Provincial Hospital of Chinese Medicine.5 Our group confirmed that Sanqi oral solution could exert protective effects in podocyte and mitigate proteinuria.6,7 Further, the Sanqi Qushi Granule was tolerable, and clinically effective in lowering proteinuria for individuals with NS. However, the underlying mechanisms of SQG on NS are yet to be elucidated.
Network pharmacology is a novel approach for complicated mechanistic studies integrating systems biology, bioinformatics, cheminformatics, and other associated domains. It can disclose the central action targets and herb compounds with construction of drug-gene-disease network.8,9 In this study, we aim to validate the efficacy of SQG on NS rats and investigate the potential mechanisms with network pharmacology and experimental verification.
Methods and Reagents
Acquisition of Active Ingredients in SQG
The active ingredients of SQG were obtained from the Traditional Chinese Medicine Systems Pharmacology database (TCMSP, http://tcmspw.com) and existing literature. TCMSP has access to Chinese herbal medicines system information, encompassing drugs, targets, and their relations. Oral bioavailability (OB) and drug-likeness (DL) are two of main pharmacokinetic parameters for drugs properties. According to the selection criteria (an OB value of ≥30% and a DL value of ≥0.18), the screened compounds were allowed for further analysis.
Identification of NS-Related Genes
The targets associated with NS in humans were collected from Online Mendelian Inheritance in Man database (OMIM, http://omim.org/, updated in 2022), DisGeNET Database (https://www.disgenet.org/) and Gene Cards database (https://www.genecards.org/). “Nephrotic syndrome” was indicated to search as the keyword in this study. After taking the intersection of the predicted genes from three databases, target genes can be obtained.
PPI Network Analysis
The PPI network analysis was performed using the STRING database (http://string-db.org) to explore a functional interaction between proteins. Afterward, these interactions were visualized by importing all data into Cytoscape 3.9. 1 software. Within this network, nodes were indicated as targets and edges represented interaction relationships.
GO and KEGG Pathway Enrichment Analysis
Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment were carried out using Metascape (http://www.metascape.org/) database. The results of both two enrichment analyses focus on elucidating the core signaling pathway of SQG acting on NS.
Animals and Experiments
A group of adult male Sprague-Dawley (SD) rats weighting 180–220g were provided by Animal Research Laboratory of Guangdong Province (Guangzhou, China). The ethical approval was given by Research Institute of the Animal Protection and Use Committee of Guangdong Provincial Hospital of Chinese Medicine [No.2018069]. Adaptive feeding for 7 days, six randomly selected rats were assigned to the blank control group (Control) and were given 0.9% saline through the tail vein. Adriamycin (#HY-15142/CS-1239, MCE, United States) was injected into the tail vein of the remaining 12 rats only once to construct a NS model. After 3 weeks, the 12 rats were randomly assigned to the Model group (Model) and the SQG intervention group (SQG). Subsequent experimental interventions of a 4-week period are presented below. In case of model group and control group, the rats were only administered with saline. The daily dose of SQG decoction (11.02 g/Kg) was equal to the clinical dosage. This optimal dosage was screened based on a previous in vivo efficacy experiment, in which the results showed the efficacy of low-dose group was better and data are not yet published as shown in Figure S1.
SQG Aqueous Extract Preparation
All the raw herb of SQG were purchased from pharmacy in Guang Dong Provincial Hospital of Chinese Medicine, conforming with the criteria of 2020 edition Chinese Pharmacopoeia. The dosage proportions of Hedysarum Multijugum Maxim. (No.200800061, Gansu, China; 30 g), Panax Notoginseng (Burk.) F. H. Chen Ex C. Chow (No.2101011631, Yunnan, China; 5 g), Isaria cicadae Miquel (No.2208011101, Jiangsu, China; 10 g), Atractylodes Macrocephala Koidz. (2208011101, Jiangsu, China; 10 g), Radix Paeoniae Rubra (No. 2011004, Inner Mongolia, China; 10 g), and Smilacis Glabrae Rhixoma (2210001, Guangdong, China; 30 g), Curcumae Rhizoma (200602321, Guangxi, China; 10 g). The seven herbs were mixed and decocted twice, adding 8 times the amount of distilled water, each time for 2 h, followed by filtration. Subsequently, the aqueous extract of SQG was concentrated through an evaporator (IKA, Germany).
Biochemistry of Blood and Urine
Urine samples were collected employing metabolic cages for 24 hr. Rats were fasted with free access to water at time of urine collection. The volumes of the urine were calculated and recorded. After 15-min centrifugation at room temperature (3000 rpm), the supernatant was kept at −80°C for subsequent test. The blood was obtained via an abdominal aorta and left standing for 1 hr. After 5-min centrifugation (12,000 rpm, 4°C), the serum was isolated and preserved at −80°C to perform biochemical assays. All blood and urine samples were assayed in the Department of Clinical Laboratory in Guangdong Provincial Hospital of Chinese Medicine by an automatic biochemical analyzer (Roche cobas C702).
Histology
Kidney tissue of rats was fixed using 10% paraformaldehyde for 24 hr, followed by embedding with paraffin. Then, they were cutting into 3μm thick sections. Next, renal slices were deparaffinized and rehydrated in xylene and alcohol series, respectively. Finally, paraffin sections were stained with hematoxylin and eosin.
Immunohistochemistry
The slices of kidney tissue were dewaxed and rehydrated, followed by antigen retrieval with Tris-EDTA (PH9.0) at a high pressure. Hydrogen peroxide (3%) was used to block the endogenous peroxidase for 15 min. After 30-min blocking with 10% goat serum at 37°C, the renal slices were incubated using following primary antibodies nephrin (1:250, ab216341, Abcam, UK) overnight at 4°C. Next, 1-hr secondary antibody incubation was performed. Then, the renal tissue sections were detected using Max VisionTM HRP kit (MXB Biotechnologies, Fujian, China) and stained with hematoxylin (Leagene, Beijing, China). The stainings were visualized by microscope (Bio-Rad, Laboratories, Hercules, CA, United States), and quantitation was conducted by Image J.
Western Blot
The total protein was extracted from kidney tissues by adding a RIPA lysis buffer mixed with PMSF and phosphatase inhibitor cocktail. The Pierce™ BCA protein assay kit (23227, Thermo Fisher Scientific, Rockford, IL, United States) was used to detect the protein concentration of samples by following kit instruction manual. In equal amounts, the total protein was separated on the 10% SDS-PAGE gel and transferred onto the 0.45μM polyvinylidene fluoride membrane (Millipore, Burlington, United States). After blocking with 5% non-fat skimmed milk at room temperature, the membranes undergent overnight incubation with the corresponding primary antibody at 4°C (1:1000, p-PI3K, ab191606, Abcam; 1:1000, PI3K, 4257, CST; 1:1000, p-AKT, 4060, CST; 1:1000, AKT, 4685, CST; 1:1000, Bax, 2772, CST; 1:500, Bcl-2, sc-7382, Santa Cruz Biotechnology; 1:1000, Podocin, sc-518088, Santa Cruz Biotechnology; 1:250, Nephrin, ab216341, Abcam, UK; 1:5000, β-actin, AC038, ABclonal, China). The next day, the membranes were washed with TBST buffer three times, followed by 1-hr incubation at room temperature with a related secondary antibody. Subsequently, ECL reagent was used to visualize the protein bands (Millipore, Burlington, United States).
TUNEL Staining
In nucleus, genomic DNA fragmentation is a sign of apoptosis. TUNEL relies on the binding of the exposed 3’-OH ends of DNA to fluorescent probe Cy3 labeled dUTP, in the catalytic effect of Terminal Deoxynucleotidyl Transferase (TdT). Apoptosis DNA fragments in renal tissue were assessed by the TUNEL (Beyotime, Shanghai, China) assay kit, in accordance with the kit’s instructions. In brief, the slices (kidney, 3μm) were dewaxed, rehydrated, following by a 30-min incubation using protease K (no DNase 20μg/mL, Roche, Germany) at 37°C. After washing with PBS 3 times, the renal slices were incubated in a TUNEL reaction solution at 37°C avoiding light for 60 min. Subsequently, the staining was viewed under a microscope (Olympus, Japan).
Statistical Analysis
Results were graphed with GraphPad Prism 8.4.3. All data in this study were indicated as mean ± s.d. One-way ANOVA analysis was applied for data analysis. A p value < 0.05 was considered statistically significant.
Results
Bioactive Compounds in SQG
Among seven drugs of Huangqi, Sanqi, Baizhu, Chanhua, Chishao, Ezhu, Tufuling, 16, 7, 4, 22, 13, 1 and 15 compounds, respectively, were obtained by ADME screening (Supplementary Table S1). After removing duplicates, 69 active ingredients in SQG remained.
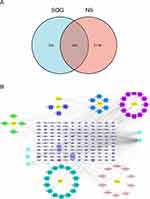
Active Ingredient-NS Target Network
Targets of the main ingredients in SQG were predicted in SwissTarget Prediction database and TCMSP database. After removing duplicates, the predicted targets contained 466 genes in total (Supplementary Table S2). Meanwhile, we also searched three different databases and identified therapeutic targets for NS: 2051 from GeneCard, 181 from OMIM, and 384 from Disgenet. Subsequently, 2262 unduplicated disease targets for NS were acquired in total (Supplementary Table S3). Ultimately, we obtained 144 overlapping targets, by taking the intersection of genes for NS and SQG (Figure 1A and Supplementary Table S4). We constructed “Active ingredient-NS Target Network” to clarify the potential mechanism of SQG against NS using Cytoscape software (Figure 1B). In this network, quercetin (MOL000098) is most closely related to the genes of NS, and the remaining compounds followed in turn: beta-sitosterol (MOL000358), stigmasterol (MOL000449), baicalein (MOL002714), naringenin (MOL004328), which are ordered by their degrees.
PPI Analysis
We imported 144 overlapping targets into the STRING database to build the PPI network and visualized it using Cytoscape (Figure 2). The PPI network displayed 534 proteins nodes and 2365 edges. The higher the protein degree is, the more critical role the protein plays in this process. The proteins ranked in the top 10 with high degree value were identified as hub genes, containing TP53, AKT1, MAPK1, HSP90AA1, SRC, MAPK14, RELA, TNF, ESR1, MAPK8.
GO and Pathway Enrichment Analysis
GO and KEGG pathway enrichment analyses were conducted by importing 144 overlapping genes into Metascape Database. The GO enrichment analysis results were presented, covering 1633 biological processes (BPs), 146 molecular functions (MFs), and 92 cellular components (CCs) term. The top 10 most enriched BP, CC, and MF terms are plotted in Figure 3A. BP enrichment analysis contains responses to oxidative stress, cellular responses to chemical stress, responses to peptides, responses to lipopolysaccharide, and so on. The CC terms mainly enriched membrane raft, membrane microdomain, vesicle lumen, and so on. The terms of MF contain steroid binding, DN−binding transcription factor binding, heme binding, and so on. The KEGG analysis yielded 165 pathways, of which the top 30 are displayed in Figure 3B. Further, the results indicated PI3K-Akt signaling pathway and apoptosis may be the underlying mechanism of SQG against NS.
 |
Figure 3 GO and pathway enrichment analysis of SQG active components in the treatment of common targets of NS. (A) The GO enrichment analysis. (B) KEGG pathway enrichment analysis. |
Urine Protein Quantitation and Serum Biochemical Indexes
Proteinuria is a key feature of nephrotic syndrome. As illustrated in Figure 4A, 24-h urinary protein levels with the exception of control group were increased significantly and ADR-treated rats developed heavy proteinuria. The SQG invention reduced proteinuria markedly (p < 0.01). After SQG treatment, Cr, BUN, TG, TC, and LDL-C levels distinctly decreased, compared with the control group in Figure 4B–F. The above results revealed that SQG had a therapeutic effect on NS rats similarly.
Histopathological Analysis
The pathological changes in the kidney were visualized by light microscopy. As shown in Figures 4G, there was no visible kidney injury in control group. Whereas, inflammatory infiltrates, tubular dilatation, and edema were significantly observed in rats with NS. While with SQG intervention, less inflammatory cell infiltrates were found, compared with NS rats without SQG treatment. The SQG group showed tubular dilatation and edema distinctly diminished, too. It can be seen that SQG could attenuate renal damages in NS rats induced by ADR.
SQG Attenuated Glomerular Podocyte Lesions in NS Rats
Podocytes, namely the visceral glomerular epithelial cell, play a role in maintaining glomerular filtration function. Once podocyte damage has occurred, proteinuria developed directly. Nephrin and podocin were expressed in a podocyte slit-pore diaphragm, which contributes to maintaining podocyte morphology. Decreased levels of nephrin were observed in NS rats with immunochemical staining, as shown in Figure 5A and B. However, after SQG administration, the expression of nephrin was significantly restored. The Western blot analysis of nephrin and podocin protein expression demonstrated similar trends in Figure 5C–E. Collectively, SQG treatment protected podocyte from damage in rats with NS.
Effects of SQG on Kidney Podocyte Apoptosis
Kidney tissues were subjected to TUNEL staining in this study (Figure 6A and B). The results exhibited considerable glomerular podocyte apoptosis in all NS rats. Compared with the model group, podocyte apoptosis in glomerulus was pronouncedly inhibited with SQG treatment. We further performed Western blot analysis for apoptosis-associated protein expressions (Bax, Bcl-2) in kidney tissues from three groups. As shown in Figures 6C–F, rats in model group had higher protein expression of Bax, together with reduced protein expression of Bcl-2, than rats in control group. This predicted apoptotic signaling pathway was activated, triggering cell death. In contrast, SQG administration downregulated protein expression of Bax and upregulated protein expression of Bcl-2, leading to inhibition of apoptosis.
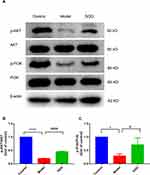
Activated PI3K/AKT Pathway by SQG
To explore the anti-apoptotic effects of SQG on ADR-induced rats of NS, we detected whether the PI3K/AKT signaling pathway is activated by WB. As indicated in Figure 7, the protein of p-PI3K and p-AKT were down-regulated in model group, and these were restored after SQG treatment.
Discussion
Nephrotic syndrome has a high prevalence globally, which often initiates various complications despite marked advances in its treatment.10 It is urgent to develop a promising drug for NS. In this study, we validated SQG efficacy for alleviating albuminuria and its protective role on podocyte in vivo experiment. Combined with network pharmacology method, in-depth study exploration proposed that SQG could inhibit podocyte apoptosis through the PI3K/AKT signaling pathway in NS rats.
A classical adriamycin-induced nephropathy (AN) rat model was adopted, which is characterized by resembling phenotypes to clinical nephrotic syndrome including severe proteinuria and histopathological damages.11,12 AN rat model was built by a single adriamycin tail vein injection and applied extensively in nephrology studies due to its stability and similarity to human nephrotic syndrome.13–15 After modeling, the rats developed heavy proteinuria, impaired renal function, and notable morphological changes, such as tubular lumen dilatation, leukocytic cell infiltration, and vacuolar degeneration in our study the model of NS rats was successfully established. Proteinuria is a hallmark of NS. The albuminuria highly represents podocyte damage and glomerular filtration dysfunction.16,17 Studies have demonstrated that proteinuria has a direct nephrotoxicity on tubular cells, which initiates interstitial inflammation and fibrosis, ultimately contributing to the onset of irreversible kidney injury.18,19 A rapid reduction of proteinuria is essential for containing progression to end-stage renal failure. Thus, one of the main objectives in the treatment of NS is to attenuate or eliminate proteinuria rapidly. In our study, SQG administration appreciably relieves proteinuria.
Podocytes are highly specialized cells in the glomerulus with limited proliferation and division capacity.20 It, as the glomerular filtration barrier, together with silt diaphragms are highly associated with the product of proteinuria.21 Once podocyte is damaged or dead, the protein leaks past the glomerular basement membrane into urine.22 Therefore, protecting podocyte against injury can attenuate proteinuria and delaying disease progression.23 The results of our study indicated that the expression of nephrin and podocin, marker of podocyte injury, were restored after SQG intervention, compared with NS rats.
With disease progression, renal function may worsen, followed by concentrations elevation of urea nitrogen and creatinine in blood. Hyperlipidemia is another clinical manifestation of NS with an increase in low-density lipoprotein and cholesterol.21 It was revealed that due to deposition of lipoprotein on glomeruli and tubules, renal cells are prone to apoptosis and renal insufficiency develops ultimately.24 Apoptosis of kidney cell is possibly associated with proteinuria and aberrant lipid metabolism.25,26 In this study, after modeling, the levels of BUN, Cr, TG, TC, and LDL-C in NS rats were higher than in control group. However, treatment with SQG improved renal functions and ameliorated lipid metabolism disorder in NS rats.
Moreover, apoptosis, which plays a significant role in the genesis and progression of NS, is also discovered to be remarkably increased in the renal cells of NS models.27,28 The present TUNEL staining indicated that compared with control group, a large amount of apoptosis occurred in glomeruli and tubules, when rats were injected with ADR, consistent with previous study. However, in SQG treatment group, the apoptosis was alleviated dramatically. Simultaneously, we detected the expression of a cluster of key apoptosis-associated proteins, including Bax, Bcl-2 by Western blot analysis to measure cell survival. The data showed that SQG treatment downregulated the Bax/Bcl-2 ratio treated with ADR. This implied that SQG could suppress apoptosis in the impaired kidney to exert reno-protective effect.
To elucidate the upstream modulation mechanism of apoptosis, a KEGG analysis was performed. Several signaling pathways are highly enriched, particularly PI3K/AKT pathway. The PI3K/AKT pathway serves as an important anti-apoptotic signaling pathway, which plays an important role in promoting apoptosis and autophagy.29 AKT is activated when PI3K-dependent generation of PIP3 binds to AKT PH domain at the plasma membrane.30,31 Activated AKT can phosphorylate Forkhead box O (FOXO) proteins, leading to growth inhibition and apoptosis.32,33 Furthermore, the Thr23 site phosphorylation of κB kinase is inhibited by activation of AKT, which is involved in the gene regulation of apoptosis.34 In addition, p-Akt phosphorylates Bcl-2-associated protein, enhancing the antiapoptotic effects of Bcl-2.35 The PI3K/Akt pathway is crucial for treatment in nephrology. Reportedly, Pulsed Focused Ultrasound alone has a beneficial therapeutic effect, improving renal histological injury, diminishing inflammation and apoptosis in the kidney, through PI3K/Akt signaling.36
In the network of components-target, the major active components of SQG are quercetin, beta-sitosterol, stigmasterol, baicalein, and naringenin ranked by degree. These active constituents have never been investigated in MN. However, Reportedly, stigmasterol could trigger PI3K/AkT, AkT/mTOR, JAK/STAT, and VEGFR-2 signaling pathways.37 Naringenin could activate the PI3K/Akt pathway and increase the expression of Bcl-xL and Bcl-2, inhibiting neuroapoptosis and ameliorating cognitive impairment in rats.38 Intriguingly, quercetin suppressed the PI3KR1 gene, which activated the PI3K/AKT signaling pathway, thus modulating lipid metabolism.39 The results in our study indicated that the p-PI3K and p-AKT expression with SQG intervention was higher than that without SQG intervention. The SQG treatment could activate PI3K/AKT signaling pathway. In this way, SQG treatment may exert anti-apoptotic effect to attenuate NS in ADR-induced rats. Yet, more detailed mechanisms focusing on pharmacology still remain unelucidated. In summary, the results of this study demonstrated that at least to some extent, SQG could suppress apoptosis and alleviate proteinuria via the PI3K/AKT signaling pathway in rats with NS.
Conclusion
In conclusion, this work demonstrated that SQG has the potential to alleviate proteinuria, protect podocytes from injury and inhibit kidney apoptosis through the PI3K/AKT pathway. Furthermore, the network pharmacology-based framework explained in our study offers novel insights into the treatment of NS with TCM.
Data Sharing Statement
The original contributions presented in this study are included in this article and Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics Statement
The Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine granted exemption from review for available data from public databases involving in our research. And in vivo experiment was conducted after approval by the Research Institute of the Animal Protection and Use Committee of Guangdong Provincial Hospital of Chinese Medicine (No. 20180691). We adhered strictly to the guidelines for ethical review of animal welfare (GB_T 35892-2018).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The work is supported by the National Natural Science Foundation of China (No. 81974565), the Special project of State Key Laboratory of Dampness Syndrome of Chinese Medicine (No. SZ2021ZZ36, No. SZ2021ZZ09 and No. SZ2021ZZ33), the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Lab) (No. 2020B1212030006), the Natural Science Foundation of Guangdong Province (No.2022A1515011628 and 2022A1515010103), Basic and Applied Basic Research Project of Guangzhou (No. 202201020488), Special Fund for International Cooperation Base of Traditional Chinese Medicine of National Administration of Traditional Chinese Medicine (No. 0610-2240NF021548).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tsuji K, Kitamura S, Wada J. MicroRNAs as biomarkers for nephrotic syndrome. Int J Mol Sci. 2020;22(1):88. doi:10.3390/ijms22010088
2. Warejko JK, Tan W, Daga A, et al. Whole exome sequencing of patients with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2018;13(1):53–62. doi:10.2215/cjn.04120417
3. Basu B, Angeletti A, Islam B, Ghiggeri GM. New and old anti-CD20 monoclonal antibodies for nephrotic syndrome. where we are? Front Immunol. 2022;13:805697. doi:10.3389/fimmu.2022.805697
4. Saleem MA. Molecular stratification of idiopathic nephrotic syndrome. Nat Rev Nephrol. 2019;15(12):750–765. doi:10.1038/s41581-019-0217-5
5. Xu P, Li S, Tian R, et al. Metabonomic analysis of the therapeutic effects of Chinese medicine sanqi oral solution on rats with exhaustive exercise. Front Pharmacol. 2019;10:704. doi:10.3389/fphar.2019.00704
6. Wang X, Liu J, Tian R, et al. Sanqi oral solution mitigates proteinuria in rat passive Heymann nephritis and blocks podocyte apoptosis via Nrf2/HO-1 pathway. Front Pharmacol. 2021;12:727874. doi:10.3389/fphar.2021.727874
7. Tian R, Wang L, Chen A, et al. Sanqi oral solution ameliorates renal damage and restores podocyte injury in experimental membranous nephropathy via suppression of NFκB. Biomed Pharmacother. 2019;115:108904. doi:10.1016/j.biopha.2019.108904
8. Wang X, Wang ZY, Zheng JH, Li S. TCM network pharmacology: a new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med. 2021;19(1):1–11. doi:10.1016/s1875-5364(21)60001-8
9. Nogales C, Mamdouh ZM, List M, Kiel C, Casas AI, Schmidt H. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43(2):136–150. doi:10.1016/j.tips.2021.11.004
10. Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol. 2018;14(1):57–70. doi:10.1038/nrneph.2017.155
11. Burke JF Jr, Laucius JF, Brodovsky HS, Soriano RZ. Doxorubicin hydrochloride-associated renal failure. Arch Intern Med. 1977;137(3):385–388. doi:10.1001/archinte.1977.03630150079022
12. Bertani T, Poggi A, Pozzoni R, et al. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest. 1982;46(1):16–23.
13. Guo J, Ananthakrishnan R, Qu W, et al. RAGE mediates podocyte injury in Adriamycin-induced glomerulosclerosis. J Am Soc Nephrol. 2008;19(5):961–972. doi:10.1681/asn.2007101109
14. Tomita N, Hotta Y, Naiki-Ito A, et al. Protective effects of tadalafil on damaged podocytes in an Adriamycin-induced nephrotic syndrome model. J Pharmacol Sci. 2022;149(2):53–59. doi:10.1016/j.jphs.2022.03.003
15. Xiao M, Bohnert BN, Grahammer F, Artunc F. Rodent models to study sodium retention in experimental nephrotic syndrome. Acta Physiol. 2022;235(3):e13844. doi:10.1111/apha.13844
16. Sun Y, Cui S, Hou Y, Yi F. The updates of podocyte lipid metabolism in proteinuric kidney disease. Kidney Dis. 2021;7(6):438–451. doi:10.1159/000518132
17. Garg P. A review of podocyte biology. Am J Nephrol. 2018;47(1):3–13. doi:10.1159/000481633
18. El Karoui K, Viau A, Dellis O, et al. Endoplasmic reticulum stress drives proteinuria-induced kidney lesions via Lipocalin 2. Nat Commun. 2016;7:10330. doi:10.1038/ncomms10330
19. Zandi-Nejad K, Eddy AA, Glassock RJ, Brenner BM. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int Suppl. 2004;(92):S76–89. doi:10.1111/j.1523-1755.2004.09220.x
20. Kim JH, Hwang KH, Dang BTN, et al. Insulin-activated store-operated Ca(2+) entry via Orai1 induces podocyte actin remodeling and causes proteinuria. Nat Commun. 2021;12(1):6537. doi:10.1038/s41467-021-26900-w
21. Zhou XJ, Klionsky DJ, Zhang H. Podocytes and autophagy: a potential therapeutic target in lupus nephritis. Autophagy. 2019;15(5):908–912. doi:10.1080/15548627.2019.1580512
22. Kasztan M, Fox BM, Speed JS, et al. Long-term endothelin-A receptor antagonism provides robust renal protection in humanized sickle cell disease mice. J Am Soc Nephrol. 2017;28(8):2443–2458. doi:10.1681/asn.2016070711
23. Hurcombe JA, Hartley P, Lay AC, et al. Podocyte GSK3 is an evolutionarily conserved critical regulator of kidney function. Nat Commun. 2019;10(1):403. doi:10.1038/s41467-018-08235-1
24. Lau A, Wang S, Liu W, Haig A, Zhang ZX, Jevnikar AM. Glycyrrhizic acid ameliorates HMGB1-mediated cell death and inflammation after renal ischemia reperfusion injury. Am J Nephrol. 2014;40(1):84–95. doi:10.1159/000364908
25. Shi S, Yu L, Chiu C, et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol. 2008;19(11):2159–2169. doi:10.1681/asn.2008030312
26. Kho MC, Park JH, Han BH, et al. Plantago asiatica L. ameliorates puromycin aminonucleoside-induced nephrotic syndrome by suppressing inflammation and apoptosis. Nutrients. 2017;9(4):386. doi:10.3390/nu9040386
27. Wang XW, Tian RM, Yang YQ, et al. Tripterygium glycoside fraction n2 ameliorates Adriamycin-induced nephrotic syndrome in rats by suppressing apoptosis. J Ethnopharmacol. 2020;257:112789. doi:10.1016/j.jep.2020.112789
28. Chen J, Yuan S, Zhou J, et al. Danshen injection induces autophagy in podocytes to alleviate nephrotic syndrome via the PI3K/AKT/mTOR pathway. Phytomedicine. 2022;107:154477. doi:10.1016/j.phymed.2022.154477
29. Zhang L, Shi X, Huang Z, et al. Network pharmacology approach to uncover the mechanism governing the effect of radix achyranthis bidentatae on osteoarthritis. BMC Complement Med Ther. 2020;20(1):121. doi:10.1186/s12906-020-02909-4
30. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi:10.1126/science.296.5573.1655
31. Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–269. doi:10.1016/s0960-9822(06)00122-9
32. Lee JT, Shan J, Zhong J, et al. RFP-mediated ubiquitination of PTEN modulates its effect on AKT activation. Cell Res. 2013;23(4):552–564. doi:10.1038/cr.2013.27
33. Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277(49):47928–47937. doi:10.1074/jbc.M207509200
34. Zhang B, Zeng M, Li B, et al. Arbutin attenuates LPS-induced acute kidney injury by inhibiting inflammation and apoptosis via the PI3K/Akt/Nrf2 pathway. Phytomedicine. 2021;82:153466. doi:10.1016/j.phymed.2021.153466
35. Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011;23(10):1515–1527. doi:10.1016/j.cellsig.2011.05.004
36. Park EJ, Dusabimana T, Je J, et al. Honokiol protects the kidney from renal ischemia and reperfusion injury by upregulating the glutathione biosynthetic enzymes. Biomedicines. 2020;8(9):352. doi:10.3390/biomedicines8090352
37. Bakrim S, Benkhaira N, Bourais I, et al. Health benefits and pharmacological properties of stigmasterol. antioxidants. 2022;11(10):1912. doi:10.3390/antiox11101912
38. Hua FZ, Ying J, Zhang J, et al. Naringenin pre-treatment inhibits neuroapoptosis and ameliorates cognitive impairment in rats exposed to isoflurane anesthesia by regulating the PI3/Akt/PTEN signalling pathway and suppressing NF-κB-mediated inflammation. Int J Mol Med. 2016;38(4):1271–1280. doi:10.3892/ijmm.2016.2715
39. Wang M, Mao Y, Wang B, et al. Quercetin improving lipid metabolism by regulating lipid metabolism pathway of ileum mucosa in broilers. Oxid Med Cell Longev. 2020;2020:8686248. doi:10.1155/2020/8686248
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.