Back to Journals » Journal of Pain Research » Volume 12
Salvia divinorum: from recreational hallucinogenic use to analgesic and anti-inflammatory action
Authors Coffeen U , Pellicer F
Received 6 October 2018
Accepted for publication 18 January 2019
Published 22 March 2019 Volume 2019:12 Pages 1069—1076
DOI https://doi.org/10.2147/JPR.S188619
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Ulises Coffeen, Francisco Pellicer
Research in Neurosciences, Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz, Mexico City, México
Abstract: Salvia divinorum is a herbal plant native to the southwest region of Mexico. Traditional preparations of this plant have been used in illness treatments that converge with inflammatory conditions and pain. Currently, S. divinorum extracts have become popular in several countries as a recreational drug due to its hallucinogenic effects. Its main active component is a diterpene named salvinorin A (SA), a potent naturally occurring hallucinogen with a great affinity to the κ opioid receptors and with allosteric modulation of cannabinoid type 1 receptors. Recent biochemical research has revealed the mechanism of action of the anti-inflammatory and analgesic effect of SA at the cellular and molecular level. Nevertheless, because of their short-lasting and hallucinogenic effect, the research has focused on discovering a new analogue of SA that is able to induce analgesia and reduce inflammation with a long-lasting effect but without the hallucinatory component. In this review, we explore the role of S. divinorum, SA and its analogues. We focus mainly on their analgesic and anti-inflammatory roles but also mention their psychoactive properties.
Keywords: Salvia divinorum, salvinorin A, pain, inflammation, psychoactivity
Introduction
Salvia divinorum (Lamiaceae) is a herbal plant native to the southwest region of Mexico. It was used for medical and religious purposes for centuries in the Mazatecan culture and has a hallucinatory component. Recently, S. divinorum extracts have been used in several countries as a recreational drug due to its hallucinogenic effects;1 its expanded use has been facilitated by its accessibility on the Internet.2 However, traditional preparations with smaller quantities have been used in illness treatments that converge with inflammatory conditions and pain, such as headaches, gastrointestinal (GI) problems, or rheumatism.3
Mazatecan people also use S. divinorum for healing multiples conditions, including insect bites, eczema, candidiasis, cystitis, and menstrual cramps, and even depression or alcohol addiction.4 These multiple therapeutic targets implicate a complex pharmacology and mechanism of action for this mystical plant.
In 1982, Ortega et al isolated a new neo-clerodane diterpene named salvinorin, which is the main active compound of S. divinorum.5 Two years later, Valdés et al isolated the components named divinorin A (a psychotropic terpenoid) and divinorin B, but later it was confirmed that both structures correspond to salvinorin A (SA) and B (SB), respectively, proposed by Ortega et al.6 Currently, phytochemical research has led to the isolation of several diterpenes, from SA to salvinorin F. As mention earlier, the main active diterpene of the plant is the SA, and it has been reported to be the most powerful naturally occurring hallucinogen.7,8
Pharmacological screening of a battery of 50 receptors, transporters, and ion channels showed that SA shows significant activity only with the κ opioid receptors (KORs), and importantly, SA shows no action at the 5-HT2A serotonin receptor, which is the main molecular target for the classical hallucinogens such as psilocybin and lysergic acid diethylamide (LSD).9 Also, it has been described that the role of SA in the cannabinoid system, through the modulation of the cannabinoid type 1 (CB1) receptors, maybe due to the cross-interaction between KOR and CB1 receptors.10,11 Moreover, the peripheral action of SA includes inhibition of leukotrienes and cytokines related with inflammatory processes.12,13
In this article, we review the role of S. divinorum, SA, and its analogues, focusing mainly on their analgesic and anti-inflammatory roles, as well as their psychoactive properties.
Psychoactivity
In a field research project performed between 1973 and 1983, Díaz described that the intake of an aqueous preparation of crushed leaves of S. divinorum produced short-lasting light-headedness, dysphoria, tactile and proprioceptive sensations, a sense of depersonalization, amplified sound perception, and increased visual and auditory imagery, with no actual hallucinations.14 However, currently, the consumption of leaf extracts of S. divinorum with increased SA concentrations, which are sold on Internet sites, produces a potent hallucinatory effect. The reported hallucinogenic dosage of salvinorin A in humans is 250–500 µg, and its potency is slightly inferior to the well-known synthetic hallucinogen, LSD.15
Experimental consumption of SA in healthy volunteers indicated experiences related to disruptions in vestibular and interoceptive signals (eg, change in spatial orientation), and also recurring experiences like revisiting childhood memories, cartoon-like imagery, and contact with entities.16 Chronic consumption produces subjective symptoms of withdrawal, mainly anxiety, irritability, and malaise; also, this pattern of use results in failure to fulfill major role obligations leading to exacerbation of social and interpersonal problems, which are criteria used to diagnose substance use disorder.17
Animal research reveals that even in less evolved species, SA exhibits rewarding effects. In a model of conditioned place preference (CPP) in zebrafish, SA induced a “trance-like” effect which was blocked by pretreatment with the KOR antagonist Nor-binaltorphimine and with the CB1 antagonist rimonabant.18 In rats, SA action depends on the strain, dosage, and administration route. In Wistar rats, SA has shown rewarding effects in CPP model and induces an increase of extracellular dopamine in nucleus accumbens (Nacc);19 however, in two different strains (Male Lister Hooded and Sprague Dawley), SA could not establish a self-administration behavior and was only capable of slightly modifying extracellular levels of dopamine in Nacc.20 In this regard, in our laboratory, we found that intraperitoneal administration of S. divinorum ethyl acetate extract in Wistar rats does not modify dopamine levels in Nacc, suggesting that S. divinorum extract does not induce addictive behavior.21
As mentioned earlier, studies in humans, non-human primates, and rodents have shown that the hallucinogenic effects produced by SA are associated with the activation of the KOR but not 5-HT2A receptor.22,23 Also, the minutes-long hallucinogenic and dissociative effects of recreational S. divinorum in humans have been described in detail.24 Recently, our group analyzed these effects in rats, by recording the changes in electrocorticogram (ECoG) activity in the anterior and posterior regions of the brain (associated with motor activity and somatosensory perception, respectively) following the administration of S. divinorum extract. We found that S. divinorum extract increased absolute power gradually in the anterior cortex, whereas it decreased absolute power in the posterior cortex at all frequency bands, from 10 minutes after the administration of S. divinorum,21 in a similar way to the decrease in spectral activity in the posterior region caused by inhalation of SA in humans.25
Hooker et al, through a small positron emission tomography (PET) system that measures brain metabolic changes using a radiotracer (18 FDG), revealed evidence that intraperitoneal administration of SA in rodents may activate brain circuits that mediate the effects of the drug on cognition, mood, fear and anxiety, and motor output, regions associated with the distribution of KOR, for example, the periaqueductal gray, bed nucleus of the stria terminalis, the cerebellar vermis, and the hypothalamus. Also, this effect was observed in the auditory, sensory, and frontal cortices.26 The same group showed that intravenous administration of SA in baboons crosses the blood–brain barrier reaching 3.3% of the injected dose within 40 seconds and clearing to half of the peak by 8 minutes, consistent with the distinctly rapid onset and recovery of hallucinations from smoked S. divinorum in humans. SA was mainly concentrated in the cerebellum and visual cortex, which may explain its behavioral and hallucinogenic effects when inhaled.27 Interestingly, Stiefel et al proposed that the consciousness-altering effects produce by SA are processed in part by the claustrum, a site with a high density of KOR.28
Inflammation
In Mazatec culture, S. divinorum is normally ingested by chewing fresh leaves or drinking an infusion for treating GI disorders like abdominal swelling, diarrhea, and intestinal spasms, illnesses that course with chronic inflammation. During the last decade, different studies have focused on proving the effect of S. divinorum and SA in inflammatory models.
For instance, using croton oil as an irritant that produces experimental chronic inflammation in the mouse small intestine, Capasso et al demonstrated that under physiological state SA exerts a weak effect on motility (independent effect from activation of KOR), but during the inflammatory state, SA inhibits motility at doses that were inactive in control animals through a KOR-dependent mechanism.29 The same group has shown an inhibitory effect of SA on ileitis-induced hypermotility, involving not only KOR but also CB1 receptors. KOR ligands play a role in the treatment of GI disorders, such as postoperative ileus, irritable bowel syndrome, and intestinal inflammation.30–32 Moreover, activation of CB receptors inhibits excitatory cholinergic neurotransmission in mouse gastric preparations.33 In this sense, SA inhibited contractions of the mouse colon, stomach, and ileum in vitro; prolonged colonic propulsion; and slowed upper GI transit in vivo by activating KOR, CB1, and interestingly by activating cannabinoid type 2 receptors (CB2).34 These findings suggest a cross-interaction between KOR and CB receptors in the regulation of GI tract under inflammatory conditions.
In vitro studies have shown that SA reduces the inducible nitric oxide synthase (iNOS, the enzyme which synthesizes NO in pathophysiological states) expression in lipopolysaccharide (LPS) insult in macrophages, but not cyclooxygenase (COX-2).12 SA also reduces the levels of pro-inflammatory cytokine TNF-α and restores the cytokine IL-10 production, which acts by limiting the inflammatory response in macrophages.12 Furthermore, SA reduces endotoxemia-induced GI hyper-motility through opioid and endocannabinoid pathways and also prevents epithelial barrier dysfunction via NOS-2 related pathway but not COX2.35
KOR and CB1 receptors are localized and overexpressed in the enteric nervous system and immune cells after peripheral inflammation, for example, in clinical disorders like IBD. In this regard, Fichna et al found that intraperitoneal, oral, and intracolonic administration of SA displayed anti-inflammatory activity in two models of experimental colitis as well as analgesia in mice with ongoing acute intestinal inflammation, effects mediated by KOR and CB1 receptors, showing the role of SA as a possible therapeutic agent in the treatment of IBD.36
Recently, new target of SA action has been identified, that is, by inhibiting leukotriene (LT) biosynthesis. LTs are potent inflammatory lipids mediators, synthesized from arachidonic acid. SA inhibits LTB4, which has been associated with increased chemotaxis, in a concentration-dependent manner . SA reduces LT production in isolated macrophages and in zymosan-induced peritonitis and carrageenan-induced pleurisy. These features open the possibility to use this naturally occurring compound for the treatment of LT-related diseases.13 Moreover, SA displays a significant control over the allergic inflammation, and its beneficial effect is also correlated with LTs inhibition.37
Analgesia
Pain is one of the most common causes of the medical and dental consultations. People suffering from long-lasting pain have their social and working activities affected. The development of adverse effects and the substantial costs that carry the current pain treatments are problems that drive to find new therapeutic alternatives. The biomedical research on S. divinorum, their metabolites, and semi-synthetic analogs in the last decade has shown their potential as an analgesic agent.
Peripheral administration of SA in mice induces antinociceptive effect in thermal (tail-flick) and chemo-nociceptive (acetic acid abdominal constriction) assays in a dose-dependent manner. Moreover, in the hot plate test, SA shows a dose–response effect diminishing nociceptive behavior. Also, similar to inflammation process, pretreatment with nor-BNI (KOR antagonist) reverses the analgesic effect.38 Further, following the same experimental line, using the hot plate test, in our laboratory, we coadministrated S. divinorum hydroponic extract (containing both salvinorin A and B) and anandamide (cannabinoid agonist), and we found a synergic effect at low doses of both compounds (10 and 30 µg/kg, respectively). This coadministration induced an analgesic effect in two of the main behavioral responses of this test, licking and escape, involved in the spinal and supraspinal nociceptive process (Figure 1).
Despite the evidence for the analgesic effect of S. divinorum in traditional use or in experimental models, their role in the central processing of pain has not been totally studied. On this subject, intrathecal administration of SA in mice increases the tail-flick latencies in a dose-dependent manner (13.9–23.1 nmol);39 furthermore, intracerebroventricular injection of different doses (1–30 µg) of SA and salvinorinyl-2-propionate (SA derived) produces analgesia only in wild-type mice but not in KOR knockout mice. The same group determined, by means of ligand binding experiments, that salvinorin A has a high affinity for κ1 receptor (Ki18,7±3.4) but not for κ2 opioid receptor (low-affinity KOR, result from δ–κ heterodimerization, Ki >10,000), demonstrating selectivity for this receptor subclass.40–43
The relief from chronic and neuropathic pain is a challenge for medical science. Current treatments produce tolerance and addiction and are also associated with a high financial cost. SA could be an alternative to traditional treatments, but has the limitation due to its low potency and a very short half-life.41 However, Guida et al showed evidence that the repeat administration of SA for almost 7 days was capable of reducing mechanical allodynia induced by formalin, reducing neuronal hyperexcitability and glial activation and subsequently contributing to the establishment of a series of dysfunctions associated with chronic pain, a mechanism involving KOR and CB1 receptors at spinal cord level.44
The development of neuropathic pain is associated with cortical nuclei belonging to the neuropathic pain matrix. One of these structures is the insular cortex (IC), which is related to cognitive processes such as memory or subjective responses such as suffering, a fundamental part of the human consciousness.45,46 The IC shows a moderate expression of KOR (although the IC is anatomically surrounded by the claustrum, it does not have the high KOR density that the claustrum has). However, we found in a recent experiment that the direct microinjection of SA in this nucleus in deep cortical layers (V and VI) is capable of inducing a powerful antinociceptive effect in a model of neuropathic pain in rats. This effect may be explained by the high affinity of this compound to KOR and also because of the allosteric modulation of the CB1 receptors.47
Analogues
The therapeutic use of SA has different issues including the administration route (eg, oral route is not viable because of its susceptibility to enzymatic degradation), the short-term effect due to the short binding duration (only minutes) and its high metabolic rate (salvinorin B, the major metabolite is inactive), and also because it is a substrate for P-glycoprotein efflux transporter.23,27,48 Therefore, the development of new derivates of S. divinorum that are capable of producing analgesic and anti-inflammatory effects but with a long-lasting duration is a current challenge.
Currently, there are about 600 semisynthetic SA analogues that involve the substitution at the C2 acetate group, only a change at C2, or a change to the furan ring. These structural modifications of SA increase its efficacy and duration of action (for a complete review, see Roach and Shenvi).49 Some of these analogues have been proved in acute, inflammatory, and neuropathic pain models (Table 1, Figure 2).
  | Table 1 Salvinorin A and analogues |
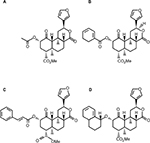  | Figure 2 Chemical structures of (A) salvinorin A, (B) herkinorin, (C) PR-38, and (D) β-THP SalB. Abbreviations: PR-38, 2-O-cinnamoylsalvinorin B; β-THP SalB, β-tetrahydropyran salvinorin B. |
One of the first analogues of SA is herkinorin, a µ opioid selective agonist that possesses antinociceptive properties. Herkinorin was tested in a model of acute and inflammatory pain in rats (formalin test). The drug was able to reduce the nociceptive behavior even after repeated administrations, suggesting minimal tolerance. Herkinorin activated µ opioid receptor (MOR) and KOR in vitro, without recruiting β arrestins or internalizing the receptor (β arrestin interactions is associated with receptor desensitization and morphine antinociceptive tolerance), so Herkinorin is useful for producing anti-nociception without inducing desensitization and resulting in drug tolerance.50
Another novel analogue 2-O-cinnamoylsalvinorin B (PR-38), which has been synthesized from SA in a simple two-stage process, displayed a good affinity for KOR and MOR with Ki values of 9.6 and 52 nM, respectively. Recently, in a complete research, Salaga et al showed that PR-38 is a strong regulator of intestinal motility, a mechanism mediated by MOR and KOR, and also PR-38 preserved the ability of SA to interact with CB1 receptor. Furthermore, it is able to regulate pain signaling in physiological and pathophysiological conditions via MOR but not KOR or CB1. Moreover, they showed that PR-38 is available orally, which implies a great potential for clinical use.51
β-Tetrahydropyran salvinorin B (β-THP SalB) has a large protecting group with the substitution of the tetrahydropyran at the C-2 position and has similar binding affinity and efficacy at the KOR compared to SA. β-THP SalB shows analgesic effects in the tail-withdrawal and formalin assays. It reduces edema and decreases neutrophil infiltration into inflamed tissue, and suppresses mechanical and cold allodynia in paclitaxel-induced neuropathic pain. Besides, β-THP SalB has a long-lasting effect, suggesting that it may have an improved metabolic profile and bioactivity in vivo compared to SA.52
Side effects
The prominent use of S. divinorum, SA, and its analogues in the chronic diseases that occur with inflammation and pain urge to consider the possible cytotoxicity after the prolonged use of these substances. However, currently there are only a few studies related to this issue.
A retrospective review of exposures to S. divinorum reported to the California Poison Control System for 10 years points out that intentional Salvia exposures resulted in a variety of neurologic, cardiovascular, and GI effects. Besides, the use of concomitant substances of abuse was associated with a high rate of complications and psychomotor disturbances. Nonetheless, this study has some limitations like the small sample size; the lack of laboratory tests or biological markers or specific physiological effects; therefore, the exposure to S. divinorum was confirmed only by history.53
In this sense, a study in rodents showed that acute administration of SA in rats and chronic administration in the mouse has no effects on cardiac conduction, temperature, or galvanic skin response. Also, a nonsignificant rise was seen in pulse pressure. Moreover, histological studies of spleen, blood, brain, liver, kidney, and bone marrow did not show any significant histological changes at any of the doses examined. This suggests that SA has relatively low toxicity.54 In vitro studies in different cell lines, including dopaminergic neurons, lung, kidney, liver, and intestine, showed that the administration of both S. divinorum and SA is associated with cytotoxic effects, in a dose- and time-dependent manner.55 Similarly, the same group showed that administration of 50 µM of SA is cytotoxic in different hepatic cell lines; besides, 10 µM of the compound also induced a significant decrease in the cellular viability of cells, in particular, after longer periods of incubation, suggesting that SA shows a low toxicological profile.56 Nevertheless, extensive in vivo and clinical studies are needed to clearly evaluate their toxicological effects.
Final remarks
Today, we are facing a crisis in relation to the use and abuse of opiates and related molecules. In fact, new molecules are not being developed as analgesics, fundamentally because of the long time taken by the processes to position these new agents until they are properly marketed. One solution in this regard is to look back at the ancestral ethnobotany and the repositioning of existing molecules for new clinical applications. In this sense, S. divinorum can be used as an alternative therapy for inflammatory and neuropathic pain, due in part to the presence of salvinorin A, a powerful KOR agonist and an allosteric modulator of CB1 receptors.
The experimental evidence supports the fact that S. divinorum, SA, and their analogues decrease the pain induced by neuropathy and inflammation. Moreover, the fact that S divinorum administration does not increase the release of dopamine in the Nacc suggests that salvinorins and their analogues can be a suitable therapeutic alternative without the risk of producing addiction.
Long-lasting pain has been among the major therapeutic challenges of the 21st century due to its disabling effects, especially with the growing population of the elderly. In this regard, finding new molecules or associations to decrease or alleviate pain is of utmost importance.
Acknowledgment
The authors would like to thank Orlando Jaimes, Karina Simón, and Bernardo Contreras for their academic and technical support.
Author contributions
Both authors contributed equally to the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Zawilska JB, Wojcieszak J. Salvia divinorum: from Mazatec medicinal and hallucinogenic plant to emerging recreational drug. Hum Psychopharmacol. 2013;28(5):403–412. | ||
Hernández-Bello R, García-Rodríguez RV, García-Sosa K, et al. Salvinorin A content in legal high products of Salvia divinorum sold in Mexico. Forensic Sci Int. 2015;249:197–201. | ||
Valdés LJ, Díaz JL, Paul AG. Ethnopharmacology of SKA María Pastora (Salvia divinorum, Epling and Játiva-M.). J Ethnopharmacol. 1983;7(3):287–312. | ||
Maqueda AE. The Use of Salvia divinorum from a Mazatec Perspective. Labate BC, Cavnar C (eds.) In: Plant Medicines, Healing and Psychedelic Science. Cham: Springer International Publishing; 2018:55–70. | ||
Ortega A, Blount JF, Manchand PS. Salvinorin, a new trans-neoclerodane diterpene from Salvia divinorum(Labiatae). J Chem Soc Perkin Trans 1. 1982;0:2505–2508. | ||
Valdes LJ, Butler WM, Hatfield GM, Paul AG, Koreeda M. Divinorin a, a psychotropic terpenoid, and divinorin B from the hallucinogenic Mexican MINT, Salvia divinorum. J Org Chem. 1984;49(24):4716–4720. | ||
Grundmann O, Phipps SM, Zadezensky I, Butterweck V. Salvia divinorum and salvinorin A: an update on pharmacology and analytical methodology. Planta Med. 2007;73(10):1039–1046. | ||
Listos J, Merska A, Fidecka S. Pharmacological activity of salvinorin A, the major component of Salvia divinorum. Pharmacol Rep. 2011;63(6):1305–1309. | ||
Roth BL, Baner K, Westkaemper R, et al. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci USA. 2002;99(18):11934–11939. | ||
Capasso R, Borrelli F, Cascio MG, et al. Inhibitory effect of salvinorin A, from Salvia divinorum, on ileitis-induced hypermotility: cross-talk between kappa-opioid and cannabinoid CB(1) receptors. Br J Pharmacol. 2008;155(5):681–689. | ||
Corchero J, Manzanares J, Fuentes JA. Cannabinoid/opioid crosstalk in the central nervous system. Crit Rev Neurobiol. 2004;16(1–2):159–172. | ||
Aviello G, Borrelli F, Guida F, et al. Ultrapotent effects of salvinorin A, a hallucinogenic compound from Salvia divinorum, on LPS-stimulated murine macrophages and its anti-inflammatory action in vivo. J Mol Med. 2011;89(9):891–902. | ||
Rossi A, Pace S, Tedesco F, et al. The hallucinogenic diterpene salvinorin A inhibits leukotriene synthesis in experimental models of inflammation. Pharmacol Res. 2016;106:64–71. | ||
Díaz JL. Salvia divinorum: enigma psicofarmacológico Y resquicio mente-cuerpo. Salud Mental. 2014;37(3):183–193. | ||
Siebert DJ. Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol. 1994;43(1):53–56. | ||
Johnson MW, Maclean KA, Reissig CJ, Prisinzano TE, Griffiths RR. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug Alcohol Depend. 2011;115(1–2):150–155. | ||
El-Khoury J, Baroud E. Case series: Salvia divinorum as a potential addictive hallucinogen. Am J Addict. 2018;27(3):163–165. | ||
Braida D, Limonta V, Pegorini S, et al. Hallucinatory and rewarding effect of salvinorin a in zebrafish: kappa-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology. 2007;190(4):441–448. | ||
Braida D, Limonta V, Capurro V, et al. Involvement of kappa-opioid and endocannabinoid system on salvinorin A-induced reward. Biol Psychiatry. 2008;63(3):286–292. | ||
Serra V, Fattore L, Scherma M, et al. Behavioural and neurochemical assessment of salvinorin a abuse potential in the rat. Psychopharmacology. 2015;232(1):91–100. | ||
Simón-Arceo K, González-Trujano ME, Coffeen U, et al. Neuropathic and inflammatory antinociceptive effects and electrocortical changes produced by Salvia divinorum in rats. J Ethnopharmacol. 2017;206:115–124. | ||
Roth BL, Baner K, Westkaemper R, et al. Salvinorin A: a potent naturally occurring nonnitrogenous opioid selective agonist. Proc Natl Acad Sci USA. 2002;99(18):11934–11939. | ||
Butelman ER, Kreek MJ. Salvinorin A, a kappa-opioid receptor agonist hallucinogen: pharmacology and potential template for novel pharmacotherapeutic agents in neuropsychiatric disorders. Front Pharmacol. 2015;6:190. | ||
Maqueda AE, Valle M, Addy PH, et al. Salvinorin-A induces intense dissociative effects, blocking external sensory perception and modulating interoception and sense of body ownership in humans. Int J Neuropsychopharmacol. 2015;18(12):pyv06505. | ||
Ranganathan M, Schnakenberg A, Skosnik PD, et al. Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist salvinorin A in humans. Biol Psychiatry. 2012;72(10):871–879. | ||
Hooker JM, Patel V, Kothari S, Schiffer WK. Metabolic changes in the rodent brain after acute administration of salvinorin A. Mol Imaging Biol. 2009;11(3):137–143. | ||
Hooker JM, Xu Y, Schiffer W, Shea C, Carter P, Fowler JS. Pharmacokinetics of the potent hallucinogen, salvinorin a in primates parallels the rapid onset and short duration of effects in humans. Neuroimage. 2008;41(3):1044–1050. | ||
Stiefel KM, Merrifield A, Holcombe AO. The claustrum’s proposed role in consciousness is supported by the effect and target localization of Salvia divinorum. Front Integr Neurosci. 2014;8:20. | ||
Capasso R, Borrelli F, Zjawiony J, et al. The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A reduce inflammation-induced hypermotility in mice. Neurogastroenterol Motil. 2008;20(2):142–148. | ||
Hicks GA, Dehaven-Hudkins DL, Camilleri M. Opiates in the control of gastrointestinal tract function: current knowledge and new avenues for research. Neurogastroenterol Motil. 2004;16(Suppl 2):67–70. | ||
Sengupta JN, Su X, Gebhart GF. Kappa, but not mu or delta, opioids attenuate responses to distention of afferent fibers innervating the rat colon. Gastroenterology. 1996;111(4):968–980. | ||
Jiménez N, Puig MM, Pol O. Antiexudative effects of opioids and expression of kappa- and delta-opioid receptors during intestinal inflammation in mice: involvement of nitric oxide. J Pharmacol Exp Ther. 2006;316(1):261–270. | ||
Mulè F, Amato A, Baldassano S, Serio R. Involvement of CB1 and CB2 receptors in the modulation of cholinergic neurotransmission in mouse gastric preparations. Pharmacol Res. 2007;56(3):185–192. | ||
Fichna J, Schicho R, Andrews CN, et al. Salvinorin A inhibits colonic transit and neurogenic ion transport in mice by activating kappa-opioid and cannabinoid receptors. Neurogastroenterol Motil. 2009;21(12):1326–e128. | ||
Fichna J, Dicay M, Hirota SA, et al. Differential effects of salvinorin A on endotoxin-induced hypermotility and neurogenic ion transport in mouse ileum. Neurogastroenterol Motil. 2011;23(6):583–e212. | ||
Fichna J, Dicay M, Lewellyn K, et al. Salvinorin A has antiinflammatory and antinociceptive effects in experimental models of colitis in mice mediated by KOR and CB1 receptors. Inflamm Bowel Dis. 2012;18(6):1137–1145. | ||
Rossi A, Caiazzo E, Bilancia R, et al. Salvinorin A inhibits airway hyperreactivity induced by ovalbumin sensitization. Front Pharmacol. 2016;7(190):525. | ||
Mccurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. Antinociceptive profile of salvinorin A, a structurally unique kappa opioid receptor agonist. Pharmacol Biochem Behav. 2006;83(1):109–113. | ||
John TF, French LG, Erlichman JS. The antinociceptive effect of salvinorin A in mice. Eur J Pharmacol. 2006;545(2–3):129–133. | ||
Ansonoff MA, Zhang J, Czyzyk T, et al. Antinociceptive and hypothermic effects of salvinorin a are abolished in a novel strain of kappa-opioid receptor-1 knockout mice. J Pharmacol Exp Ther. 2006;318(2):641–648. | ||
Zukin RS, Eghbali M, Olive D, Unterwald EM, Tempel A. Characterization and visualization of rat and guinea pig brain kappa opioid receptors: evidence for kappa 1 and kappa 2 opioid receptors. Proc Natl Acad Sci USA. 1988;85(11):4061–4065. | ||
Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399(6737):697–700. | ||
Chavkin C, Sud S, Jin W, et al. Salvinorin A, an active component of the hallucinogenic SAGE Salvia divinorum is a highly efficacious kappa-opioid receptor agonist: structural and functional considerations. J Pharmacol Exp Ther. 2004;308(3):1197–1203. | ||
Guida F, Luongo L, Aviello G, et al. Salvinorin A reduces mechanical allodynia and spinal neuronal hyperexcitability induced by peripheral formalin injection. Mol Pain. 2012;8:60. | ||
In.Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain. 2013;154(Suppl 1):S29–S43. | ||
Loeser JD. Pain and suffering. Clin J Pain. 2000;16(2 Suppl):S2–S6. | ||
Coffeen U, Canseco-Alba A, Simón-Arceo K, et al. Salvinorin A reduces neuropathic nociception in the insular cortex of the rat. Eur J Pain. 2018;22(2):311–318. | ||
Orton E, Liu R. Salvinorin A: a mini review of physical and chemical properties affecting its translation from research to clinical applications in humans. Transl Perioper Pain Med. 2014;1(1):9–11. | ||
Roach JJ, Shenvi RA. A review of salvinorin analogs and their kappa -opioid receptor activity. Bioorg Med Chem Lett. 2018;28(9):1436–1445. | ||
Lamb K, Tidgewell K, Simpson DS, Bohn LM, Prisinzano TE. Antinociceptive effects of herkinorin, a MOP receptor agonist derived from salvinorin A in the formalin test in rats: new concepts in mu opioid receptor pharmacology: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121(3):181–188. | ||
Sałaga M, Polepally PR, Sobczak M, et al. Novel orally available salvinorin A analog PR-38 inhibits gastrointestinal motility and reduces abdominal pain in mouse models mimicking irritable bowel syndrome. J Pharmacol Exp Ther. 2014;350(1):69–78. | ||
Paton KF, Kumar N, Crowley RS, Harper JL, Prisinzano TE, Kivell BM. The analgesic and anti-inflammatory effects of salvinorin A analogue β-tetrahydropyran salvinorin B in mice. Eur J Pain. 2017;21(6):1039–1050. | ||
Vohra R, Seefeld A, Cantrell FL, Clark RF. Salvia divinorum: exposures reported to a statewide poison Control system over 10 years. J Emerg Med. 2011;40(6):643–650. | ||
Mowry M, Mosher M, Briner W. Acute physiologic and chronic histologic changes in rats and mice exposed to the unique hallucinogen salvinorin A. J Psychoactive Drugs. 2003;35(3):379–382. | ||
Martinho A, Silva SM, Gallardo E. Cytotoxic effects of salvinorin A, a major constituent of Salvia divinorum. Med Chem. 2016;12(5):432–440. | ||
Cruz A, Gallardo E, Martinho A. In vitro effects of Salvinorin A on mRNA expression of liver metabolizing enzymes in hepatic cell lines. SciTz Med Clin Toxicol. 2017;1(1):1001. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

