Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Salusin-β Mediates High Glucose-Induced Inflammation and Apoptosis in Retinal Capillary Endothelial Cells via a ROS-Dependent Pathway in Diabetic Retinopathy
Authors Wang H, Zhang M, Zhou H, Cao L, Zhou J, Chen Q, Zhang X
Received 13 January 2021
Accepted for publication 22 April 2021
Published 21 May 2021 Volume 2021:14 Pages 2291—2308
DOI https://doi.org/10.2147/DMSO.S301157
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Hao Wang,1– 3 Meng Zhang,1,3 Hongli Zhou,1,3 Lang Cao,1,3 Jie Zhou,1,3 Qinyun Chen,1,3 Xuedong Zhang1,3
1Department of Ophthalmology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Ophthalmology, Fuling Center Hospital of Chongqing City, Chongqing, People’s Republic of China; 3Chongqing Key Laboratory of Ophthalmology, Chongqing Eye Institute, Chongqing, People’s Republic of China
Correspondence: Xuedong Zhang
Department of Ophthalmology, The First Affiliated Hospital of Chongqing Medical University, 1 Youyi Road, Chongqing, People’s Republic of China
Email [email protected]
Background: Diabetic retinopathy (DR) is characterized by retinal vascular endothelial cell death and vascular inflammation, which are microvascular complications of diabetes mellitus (DM). Salusin-β, a newly identified peptide, is closely associated with hypertension, atherosclerosis and diabetic cardiomyopathy. However, the exact role of salusin-β in high glucose (HG)-induced retinal capillary endothelial cell (REC) inflammation and apoptosis remains unclear.
Patients and Methods: A total of 60 patients with type 2 diabetes and 20 healthy controls were included in this study. Based on fundus fluorescein angiography findings, the diabetic patients were divided into three subgroups: diabetes without retinopathy (DWR), non-proliferative DR (NPDR) and proliferative DR (PDR). Serum salusin-β levels were measured by enzyme-linked immunosorbent assay. Human RECs (HRECs) were cultured in normal glucose (NG) and HG medium with or without salusin-β. Salusin-β expression was analysed by Western blotting and immunofluorescence staining. Expression of the pro-inflammatory cytokines MCP-1, IL-1β, TNF-α, and VCAM-1 was analysed by Western blotting. Reactive oxygen species (ROS) production was measured with 2′,7′-dichlorofluorescein diacetate (DCFH-DA). Cell apoptosis rates were determined by flow cytometry. The levels of p38, JNK, p-p38, and p-JNK and the apoptosis-related proteins cleaved caspase-3, Bax, and cl2 were analysed by Western blotting.
Results: Serum salusin-β levels were higher in diabetic patients than in healthy controls (p = 0.0027), especially in patients with NPDR and PDR (both p< 0.01). HG upregulated salusin-β expression in HRECs in a time-dependent manner. Salusin-β exacerbated inflammation and apoptosis, upregulated intracellular ROS production in HG-induced HRECs, and activated ROS-dependent JNK and p38 MAPK signalling, while knockdown of salusin-β suppressed these effects.
Conclusion: Our findings indicate that salusin-β can promote inflammation and apoptosis via ROS-dependent JNK and p38 MAPK signalling in HG-induced HRECs and could be a therapeutic target for DR.
Keywords: salusin-β, reactive oxygen species, inflammation, apoptosis, diabetic retinopathy
Introduction
Diabetic retinopathy (DR) is the leading cause of blindness in working-age adults worldwide and has gradually received attention.1 DR is one of the most common microvascular complications of diabetes mellitus (DM) and is primarily induced by long-term hyperglycaemia or factors such as hypertension and dyslipidaemia, which harm retinal capillaries.2 The early stage of DR is characterized by apoptosis and pro-inflammatory changes in retinal capillary endothelial cells (RECs), resulting in acellular capillaries and vascular permeability.3–6 Unfortunately, there is no effective treatment for the early stage of DR. Thus, a better understanding of DR pathogenesis and novel therapeutic approaches are urgently needed.
DR is a chronic, progressive low-grade inflammatory disease that leads to abnormal retinal structure and function. Interestingly, human RECs (HRECs) play a crucial role in antioxidation, regulating inflammatory factors, maintaining inner blood-retinal barriers, and mediating retinal neurotrophic supplementation.7,8 However, inflammation and oxidative stress induced by long-term hyperglycaemia can cause retinal microvascular endothelial cell apoptosis or dysfunction, induce blood-retinal barrier breakdown, and increase vascular permeability, all of which promote DR progression.9–11
Salusin-β, a newly identified multifunctional bioactive peptide containing 20 amino acid residues, is widely expressed and synthesized in blood vessels, bone marrow, endocrine glands, the brain, the liver and the kidney and plays a vital role in atherosclerosis, hypertension, and metabolic syndrome.12,13 Additionally, the serum levels of salusin-β are markedly elevated in patients with Behcet's disease, cerebrovascular disease, hypertension and coronary artery disease compared with those in healthy controls (HCs).14,15 Recent studies have demonstrated that salusin-β mediates multiple cellular functions, such as proliferation, apoptosis, oxidative stress and inflammation.16,17 Indeed, salusin-β knockdown reduced cell proliferation, apoptosis, oxidative stress, and inflammation in cisplatin- or LPS-induced renal tubular cells and mice with acute kidney injury; however, salusin-β overexpression reversed these effects.18 Additionally, another study indicated that salusin-β silencing reduced high glucose (HG)-induced apoptosis by upregulating Bcl-2 expression and downregulating Bax and caspase-3 expression in human umbilical vein endothelial cells.17 Moreover, other studies revealed that salusin-β blockade alleviated oxidative stress, inflammation, and cardiac dysfunction in diabetic rats.16
To date, studies have shown that HG is an inducer of salusin-β16,17 and that salusin-β is a contributor to apoptosis, oxidative stress and the inflammatory response, all of which are critically involved in the pathogenesis of DR. Thus, we hypothesized that salusin-β participates in the initiation and progression of DR. Therefore, in this study, we investigated whether serum levels of salusin-β in DR patients were different from those in HCs. Furthermore, we examined the potential roles and underlying mechanisms of salusin-β in HG-induced HRECs inflammation and apoptosis.
Patients and Methods
Participants
This clinical trial was approved by the Ethics Committee of Animal and Human Experimentation of Chongqing Medical University, and all patients signed informed consent forms. All experimental procedures complied with the guidelines of the Declaration of Helsinki. Five millilitres of peripheral venous blood was collected into coagulation tubes from 60 type 2 diabetes mellitus (T2DM) patients and 20 HCs between 7 am and 10 am after 10 h of fasting. Blood samples were centrifuged at 3000 × g for 5 min at 4°C, and the serum samples were then stored in sealed Eppendorf tubes at −80°C until analysis. Base on the international DR grading criteria,19 the sixty T2DM patients were grouped into the diabetes without retinopathy (DWR), non-proliferative DR (NPDR), and proliferative DR (PDR) groups (DWR, n= 20; NPDR, n= 20; PDR, n=20) by well-trained retinal specialists according to the results of fundus photography and fluorescein angiography. DM patients were diagnosed in accordance with the criteria of the American Diabetes Association.20 Individuals with autoimmune disease, haematological disease, cardiovascular disease, cerebrovascular disease, dialysis, active infection, cancer and other ocular disorders were excluded to avoid potential baseline activation. Participants in the HCs group were matched by age and sex. The anthropometric and biochemical characteristics of the participants are shown in Table 1.
 |
Table 1 Comparison of Clinical and Biochemical Data of Subjects |
Determination of Serum Salusin-β Levels
Serum salusin-β levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit purchased from USCN Life Science Inc. (Wuhan, Hubei, China) according to the manufacturer’s instructions. The detection range was from 24.69 to 2000 pg/mL. After the reaction, the absorbance values of the samples and standards were immediately determined in a microplate reader (Model 3550, Bio-Rad Laboratories, Hercules, CA, USA) at 450 nm.
Cell Culture and Transfection
HRECs were purchased from the Type Culture Collection of the Chinese Academy of Science. Cells were cultured in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% foetal bovine serum (Gibco), 100 U/mL streptomycin (HyClone, Logan, UT, USA) and 100 U/mL penicillin (HyClone) in a humidified atmosphere containing 5% CO2 at 37°C. A trypsin-EDTA solution was used to dissociate the cells from the flasks (Corning, Lowell, MA, USA) at confluence. Before treatment, cells were placed in serum-free medium for 12 h. To mimic the elevated glucose level in diabetes, HRECs were cultured in 5.5 mM glucose (NG) or 25 mM glucose (HG) medium for 48 h, and the former was used as a control for the indicated times. Recombinant adenoviruses harbouring a short hairpin RNA (shRNA) against salusin-β (Ad-salusin-shRNA) and scrambled shRNA were commercially constructed by Genomeditech Co. (Shanghai, China). The targeted sequence of salusin-β and the negative control sequence were reported previously17 (Table 2). To obtain salusin-β-knockdown cells, HRECs were subcultured in six-well plates under normal conditions to a confluence of approximately 70% and subjected to adenovirus-mediated transduction of shRNA against salusin-β or scrambled shRNA (MOI=100) following the manufacturer’s protocol. After 24 h of transfection, cells were collected and used for the following assays.
 |
Table 2 Sequences of Scrambled shRNA and Salusin-β-shRNA |
Western Blot Analysis
Cells were washed with precooled phosphate-buffered saline (PBS) and were then sonicated on ice in RIPA buffer (Beyotime Biotechnology, Shanghai, China) containing 1% PMSF. The total protein concentrations were determined with a BCA kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s protocol. Equal amounts of protein were loaded in each lane and separated on SDS-PAGE gels. After the proteins were transferred to PVDF membranes, the membranes were blocked with 5% skim milk powder for 2 h at room temperature. The membranes were then incubated first with specific primary antibodies overnight at 4°C and then with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000 dilution) for 1 h at 37°C. The immunoreactive bands were visualized with enhanced chemiluminescence reagents (4A Biotech Co., Beijing, China) in a Fusion Solo 6S image analysis system (Vilber Lourmat, Paris, France). The band intensities were quantified with Gel-Pro Analyser 4.0 (Media Cybernetics, Silver Spring, MD, USA). β-Actin was used as the loading control. Anti-MCP-1 (1:5000; cat. no. ab151538) and anti-VCAM1 (1:5000; cat. no. ab134047) antibodies were purchased from Abcam (Cambridge, UK). Anti-TNF-α (1:1000; cat. no. 3707), anti-IL-1β (1:10,000; cat. no. 12242), anti-JNK (1:1000; cat. no. 9252), anti-p-JNK (1:1000; cat. no. 4668), anti-p38 (1:1000; cat. no. 8690), anti-p-p38 (1:1000; cat. no. 4511) and anti-cleaved caspase-3 (1:1000; cat. no. 9664) antibodies were purchased from Cell Signaling Technology (Boston, MA, USA). Anti-Bax (1:6000; cat. no. 50599-2-lg) and Anti-Bcl2 (1:1000; cat. no. 26593-1-AP) antibodies were purchased from Proteintech Group (Chicago, IL, USA). The anti-salusin-β (1:260; cat. no. PAC026Hu01) antibody was purchased from USCN Life Science Inc. (Wuhan, Hubei, China). The anti-β-actin (1:10,000; cat. no. 700068) antibody was obtained from Zenbio (Chengdu, Sichuan, China).
Reactive Oxygen Species (ROS) Assay
A ROS assay kit from Beyotime Biotechnology (Shanghai, China) was used to measure intracellular ROS levels in treated HRECs. The reagent 2ʹ,7ʹ-dichlorofluorescein diacetate (DCFH-DA) is rapidly oxidized into fluorescent dichlorofluorescein (DCF) by intracellular ROS, and the amount of ROS can thus be quantified. In brief, HRECs were seeded in confocal dishes, starved for 12 h, and incubated for 48 h with normal glucose (NG), NG+ salusin-β, HG, and HG + salusin-β medium with or without pretreatment with the antioxidant N-acetyl-L-cysteine (NAC, 5 mM) (Sigma-Aldrich, St. Louis, MO, USA) for 6 h. After being washed twice with serum-free medium, the treated cells were incubated with 10 µM DCFH-DA for 20 min at 37°C in a light-protected, humidified chamber. Fluorescence signals were visualized with a laser scanning confocal microscope (Zeiss, Germany) at 488 nm with constant parameters. Image-Pro Plus software (Version 6.0, Media Cybernetics, Bethesda, MD, USA) was used to quantify the relative fluorescence intensity of DCF per cell in the scan area.
Flow Cytometry
Annexin V-FITC/propidium iodide (PI) double staining was used to examine the effect of salusin-β on HG-induced apoptosis of HRECs with or without NAC pretreatment. After treatment, cells were collected and washed three times with precooled PBS, resuspended in 1× binding buffer and stained with 5 µL of Annexin V-FITC and 5 µL of PI in the dark at room temperature according to the manufacturer’s instructions. Finally, the samples were analysed with a CytoFLEX flow cytometer (Beckman Coulter, India) within 1 h of staining.
Immunofluorescence Staining
After the indicated treatments, cells on slides were fixed with 4% paraformaldehyde (PFA) for 30 min and permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature. Cells were blocked with 10% goat serum for 30 min at 37°C in a humidified chamber and subsequently incubated with the anti-salusin-β primary antibody at 4°C overnight. After being washed three times with PBS, cells were incubated with goat anti-rabbit IgG DyLight 488 (1:250; cat. no. A23220, Abbkine Scientific, Redlands, CA, USA) for 1 h at 37°C in the dark. Images were acquired with a fluorescence microscope (Olympus IX71, Tokyo, Japan).
Statistical Analysis
Clinical data were collected and are presented as means ± standard deviations for normally distributed variables and as medians (interquartile ranges [IQRs]) for non-normally distributed variables. Independent Student’s t-test and one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test were used to compare normally distributed continuous variables between two groups and among multiple groups, respectively. Kruskal–Wallis and stepwise–stepdown tests were used to compare non-normally distributed continuous variables. The χ2-test was used to compare categorical variables, which are presented as percentages (%). Statistical analysis was performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA). A value of p<0.05 (two-tailed) was considered to indicate statistical significance. At least three individual experiments were performed.
Results
DR Patients Exhibit Elevated Levels of Circulating Salusin-β
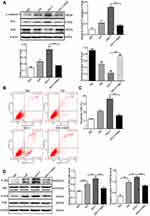
To examine whether long-term hyperglycaemia can increase the expression of salusin-β, we first measured the serum levels of salusin-β in patients. Our results show that the serum levels of salusin-β were significantly increased in T2DM patients compared with HCs (p<0.01) (Figure 1A). Furthermore, T2DM patients were separated into the DWR, NPDR, and PDR subgroups. Subgroup analysis showed that the serum levels of salusin-β in the NPDR and PDR groups were significantly increased compared to those in the HC group (both p<0.01), while no significant differences were found between the DWR and HC groups or the NPDR and PDR groups (Figure 1B). Although no significant difference was found between the DWR and HC groups (p = 0.285), the serum salusin-β levels in the DWR group were higher than those in the HC group.
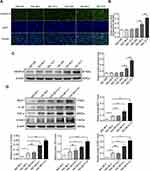
HG Increases Salusin-β Expression in HRECs
After treatment with HG (25 mM) for 24 h, 48 h, and 72 h, the expression of salusin-β was analysed by immunofluorescence and Western blotting. The expression levels of salusin-β in the HG groups treated for 48 h and 72 h were significantly increased compared with those in the corresponding NG groups (Figure 2A–C). Moreover, the protein level of salusin-β in the HG group treated for 72 h was much higher than that in the HG group treated for 48 h (Figure 2A–C). Additionally, no significant differences were found between the HG group treated for 24 h and any NG group (Figure 2A–C). These results show that HG can increase the expression of salusin-β in HRECs in a time-dependent manner. Thus, the time point of 48 h was selected for HG treatment in subsequent experiments.
Salusin-β Promotes the Expression of Inflammatory Factors in HG-Induced HRECs in a Concentration-Dependent Manner
After treatment with increasing doses of salusin-β (0.1 nM, 1 nM, and 10 nM) for 48 h, the expression levels of the inflammatory factors MCP-1, IL-1β, TNF-α, and VCAM-1 in HG-induced HRECs were measured by Western blotting (Figure 2D). The expression levels of MCP-1, IL-1β, TNF-α, and VCAM-1 in the HG group were significantly increased compared with those in the NG group (p<0.05, p<0.05, p<0.05, and p<0.01, respectively) after treatment for 48 h. In addition, compared with those in the HG group, the levels of the pro-inflammatory cytokines MCP-1, IL-1β, TNF-α, and VCAM-1 were significantly increased in both the HG + 1 nM salusin-β (HG 48 h +1) group (p<0.001, p<0.01, p<0.01, and p<0.0001, respectively) and the HG + 10 nM salusin-β (HG 48 h +10) group (all p<0.0001) (Figure 2D). However, no significant difference was found between the HG 48 h and HG 48 h+0.1 salusin-β groups. These results indicate that HG induces inflammation and that combined treatment of HG and salusin-β induces a more robust inflammatory response in HRECs than HG alone. Therefore, a concentration of 1 nM salusin-β was selected for subsequent experiments.
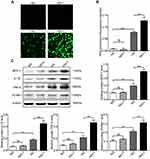
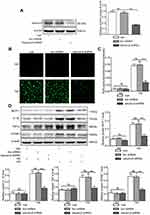
High Glucose Increases the Production of ROS and Salusin-β Further Increases ROS Production in HG-Induced HRECs
After cells were cultured in NG and HG with or without 1 nM salusin-β for 48 h, intracellular ROS production was analysed by DCF fluorescence. The level of ROS production in the HG group was significantly higher than that in the NG group, as shown in Figure 3A and B (p<0.0001). Compared with that in the HG group, the ROS production level in the HG+1 group was obviously increased (p<0.001) (Figure 3A and B). However, no significant difference was found between the NG and NG +1 groups (Figure 3A and B). These results suggest that HG stimulates the production of ROS; moreover, the combination of HG and salusin-β causes greater ROS production in HRECs than does HG alone. A 1 nM concentration of salusin-β was not sufficient to increase the production of ROS in the NG group; a higher concentration of salusin-β may induce changes in ROS production in the NG group.
Salusin-β Increases Inflammation and Apoptosis in HG-Induced HRECs
After cells were cultured with NG, NG + 1 nM salusin-β, HG, and HG + 1 nM salusin-β for 48 h, the expression levels of MCP-1, IL-1β, TNF-α, VCAM-1, cleaved caspase-3, Bax, and Bcl2 were measured by Western blotting, and the apoptosis rates were determined by flow cytometry. The expression levels of inflammatory cytokines were measured and compared with those in the NG group, revealing that the expression levels of MCP-1, IL-1β, TNF-α, and VCAM-1 in the HG group were significantly elevated (p<0.01, p<0.01, p<0.01, and p<0.001, respectively) (Figure 3C). The levels of MCP-1, IL-1β, TNF-α, and VCAM-1 were significantly higher in the HG + 1 group than in the HG group (p<0.0001, p<0.0001, p<0.001, and p<0.01, respectively), although no significant differences in these levels were found between the NG and NG+1 groups (Figure 3C). Subsequently, apoptosis-related proteins were analysed. The expression levels of cleaved caspase-3 and Bax were significantly increased but that of Bcl2 was decreased in the HG group compared with the NG group (p<0.05, p<0.01, and p<0.001, respectively) (Figure 4A). These changes were accompanied by an increased apoptosis rate in the HG group (p<0.01) (Figure 4B and C). Compared with the HG group, the HG+1 group showed significantly increased expression of cleaved caspase-3 and Bax (p<0.01 and p<0.0001, respectively), decreased expression of Bcl2 (p<0.01) (Figure 4A) and an increased apoptosis rate (p<0.001) (Figure 4B and C). These results show that 1 nM salusin-β exacerbates inflammation and apoptosis in HG-induced HRECs. Although the concentration of 1 nM salusin-β was not sufficient to increase the expression of inflammatory factors and apoptosis in the NG group, a higher concentration of salusin-β may induce such increases.
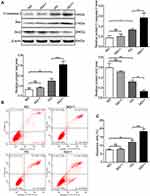
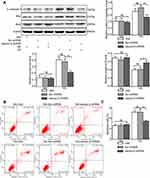
NAC Attenuates the Expression of Inflammatory Factors and Apoptosis Stimulated by Salusin-β in HG-Induced HRECs
To verify the role of ROS in the salusin-β-mediated induction of inflammatory factor expression and apoptosis, HRECs were cultured for 48 h in NG, HG, and HG+1 nM salusin-β with or without NAC (5 mM) pretreatment. As shown in Figure 5A and B, the effect of the ROS scavenger, NAC, was confirmed in the HG+1+NAC group. The expression levels of MCP-1, IL-1β, TNF-α, and VCAM-1 in the HG+1+NAC group were significantly reduced compared with those in the HG+1 group (all p<0.0001) (Figure 5C). Moreover, the expression levels of cleaved caspase-3 and Bax were significantly decreased and that of Bcl2 was increased in the HG+1+NAC group compared with the HG+1 group (all p<0.0001) (Figure 6A), accompanied by a significantly decreased apoptosis rate in the HG+1+NAC group (p<0.0001) (Figure 6B and C). These results showed that NAC alleviates inflammation and apoptosis activated by salusin-β in HG-induced HRECs.
Salusin-β Activates and NAC Inhibits the JNK and P38 MAPK Pathways
To further examine the mechanism underlying the effects of salusin-β on HG-induced HRECs, the protein levels of p38, JNK, p-p38, and p-JNK were analysed by Western blotting. The ratios of p-JNK/JNK and p-p38/p38 were significantly increased in the HG group compared with the NG group (both p<0.0001) (Figure 6D). Moreover, the p-JNK/JNK and p-p38/p38 ratios were significantly increased in the HG+1 group compared with the HG group (both p<0.001) (Figure 6D) but were significantly decreased in the HG+1+NAC group compared with the HG+1 group (both p<0.0001) (Figure 6D). These results suggest that HG activates the JNK and p38 MAPK pathways and that 1 nM salusin-β enhances the activation of both pathways. These results also suggest that NAC inhibits the JNK and p38 MAPK pathways, which were activated by 1 nM salusin-β.
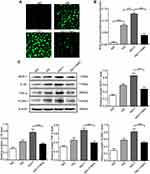
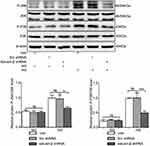
Salusin-β Knockdown Suppresses Oxidative Stress in HG-Induced HRECs
Adenoviral vectors expressing shRNA against salusin-β were used to knock down salusin-β expression. The effectiveness of salusin-β knockdown in HRECs was confirmed at the protein level (Figure 7A). After knockdown of salusin-β, HRECs were cultured in NG and HG medium for 48 h. DCFH-DA fluorescent dye was used to measure intracellular ROS levels in treated HRECs. As shown in Figure 7B and C, HG resulted in overproduction of ROS in HRECs, but this effect was abrogated by salusin-β knockdown.
Salusin-β Knockdown Attenuates Inflammation and Apoptosis in HG-Treated HRECs
The protein expression levels of inflammatory factors—ie, MCP-1, IL-1β, TNF-α, and VCAM-1—were increased in HG-induced HRECs, and salusin-β knockdown obviously reduced the increases in the expression levels of these inflammatory factors mediated by HG in HRECs (p<0.0001, p<0.0001, p<0.001, and p<0.001, respectively) (Figure 7D). The increased levels of apoptosis-related proteins, including cleaved caspase-3 and Bax, resulting from HG treatment were reduced by salusin-β deficiency (p<0.01 and p<0.001, respectively), and the level of Bcl2 was increased (p<0.05) (Figure 8A). Consistent with the above results, the flow cytometry data further confirmed that salusin-β knockdown significantly attenuated HG-induced apoptosis in HRECs (p<0.001) (Figure 8B and C).
Salusin-β Knockdown Inhibits the JNK and P38 MAPK Pathways Activated by HG in HRECs
After knockdown of salusin-β with shRNA, the p-JNK/JNK and p-p38/p38 ratios in the HG group were significantly reduced in HG-treated HRECs compared with NG-treated HRECs (p<0.01 and p<0.0001, respectively) (Figure 9). Collectively, the above data suggest that salusin-β promotes HG-induced inflammation and apoptosis in HRECs at least partially through the ROS-dependent JNK and p38 MAPK pathways.
Discussion
In the present study, we investigated whether salusin-β modulates HG-induced inflammation and apoptosis in HRECs and explored the underlying mechanisms. Our study indicated that the serum salusin-β levels were significantly increased not only in the total cohort of DM patients but also in patients in the DR subgroups. Additionally, HG induced an inflammatory response, apoptosis, and intracellular ROS production accompanied by overexpression of salusin-β in HRECs, and exogenous salusin-β enhanced these effects at least partly in a glucose concentration-dependent manner. Moreover, salusin-β knockdown attenuated oxidative stress, inflammation and apoptosis in HG-induced HRECs and inhibited the phosphorylation of JNK and p38, which were upregulated by HG. These results suggest that salusin-β knockdown exerts protective effects against HG-induced inflammation and apoptosis in HRECs.
Previous studies indicated that salusin-β, an endogenous vasoactive peptide first identified by Shichiri in 2003, is widely expressed in human, rat, and mouse tissues.21–23 The protein expression level of salusin-β was increased in the myocardium of diabetic rats,16 and the serum level of salusin-β was increased in diabetic patients.14 HG stimulates salusin-β expression in multiple types of cells, such as cardiomyocytes and human umbilical vein endothelial cells.16,17 Consistent with these previous findings, our results showed that serum salusin-β levels were increased not only in the total cohort of DM patients but also in the DR patient subgroups. We also found that HG significantly increased the expression of salusin-β in HRECs in a time-dependent manner. These results indicate that hyperglycaemia and high glucose may stimulate salusin-β expression and that salusin-β may play a role in DR progression.
In the past few decades, studies have shown that oxidative stress plays a key role in the pathogenesis of DR and that excessive ROS accumulation in and around retinal blood vessels causes mitochondrial defects, apoptosis, inflammation, and structural and functional changes (such as microcirculatory abnormalities and neurodegeneration) in the context of DR.24,25 Previous studies have indicated that ROS mediates several biological processes in which salusin-β is involved. For example, salusin-β promotes the foam cell formation and monocyte adhesion by increasing the production of ROS in atherosclerosis.26,27 Salusin-β stimulates the migration of VSMCs and intimal hyperplasia after vascular injury through an oxidative stress-related pathway and stimulates the production of intracellular ROS in human umbilical vein endothelial cells.28–31 However, whether ROS participate in the effects of salusin-β on HG-induced HRECs in DR remains unknown. In our study, we found that the combination of HG and exogenous salusin-β increased the production of intracellular ROS more than HG alone, while silencing salusin-β suppressed HG-induced ROS production in HRECs. These results indicate that HG-induced salusin-β contributes to the excessive production of ROS in HRECs treated with HG.
Retinal endothelial cell death and vascular inflammation are the characteristic features of DR32,33 and are caused mainly by sustained hyperglycaemia.34 Previous studies indicated that salusin-βplays a key role in mediating cell death and vascular inflammation concomitant with upregulation of ROS production in mice.35 Additionally, salusin-β blockade significantly decreased pro-inflammatory cytokine expression, macrophage infiltration, and pulmonary vascular remodelling via the ROS/NF-κB signalling pathway in a rodent model of pulmonary arterial hypertension.36 Salusin-β contributed to apoptosis of renal tubular epithelial cells in acute kidney injury mice via activation of the PKC/ROS/DNA damage/p53 apoptotic pathway.18 Chronic blockade of salusin-β in the paraventricular nucleus attenuated hypertension and hypothalamic inflammation through the ROS/NF-κB signalling pathway in spontaneously hypertensive rats.37 Consistent with these findings, our results showed that salusin-β induced by HG increased intracellular ROS production and subsequently upregulated the expression of the pro-inflammatory cytokines IL-1β, MCP-1, TNF-α, and VCAM-1 and increased the levels of apoptosis-associated proteins to induce an inflammatory response and apoptosis. Moreover, these effects were suppressed by shRNA-mediated silencing of salusin-β in HRECs treated with HG. Furthermore, to prove that salusin-β induces inflammation and apoptosis through increasedROS, we pretreated HRECs with NAC, a ROS scavenger, before incubation with salusin-β to eliminate ROS. We finally found that the increased inflammation and apoptosis induced by salusin-β were mitigated by NAC. These results suggest that salusin-β is responsible for the HG-induced inflammation and apoptosis mediated by ROS production.
The JNK and P38 MAPK signalling pathways can be activated by numerous cellular stresses, such as oxidative, hypoxic and genotoxic stresses, and they regulate various extracellularly stimulated processes, including apoptosis, inflammation, innate immunity, and responses to various neuropeptides.38,39 A previous study reported that shRNA-mediated knockdown of JNK and p38 MAPK attenuated inflammation and apoptosis in mice with diabetic cardiomyopathy.40 The JNK and p38 MAPK signalling pathways are pro-apoptotic and pro-inflammatory pathways activated by excess intracellular ROS and have been proven to participate in the development of DR in several studies.41,42 In this study, we discovered that the levels of p-JNK and p-p38 were increased in HG-induced HRECs, consistent with the results of previous studies.43–45 Combined treatment with exogenous salusin-β and HG resulted in higher levels of p-JNK and p-p38 than treatment with HG alone, whereas both knockdown salusin-β and NAC pretreatment reduced these levels. These results demonstrate that salusin-β induced by HG can activate the JNK and p38 MAPK signalling pathways in a ROS-dependent manner. Overall, we demonstrated that salusin-β contributes to the progression of DR by activating the ROS-dependent JNK and p38 MAPK signalling pathways and promoting inflammation and apoptosis in HG-induced HRECs (Please see Figure 10 for the detailed information.). The limitations of our study include the limited numbers of patients and HCs. Moreover, this was primarily a cell-based study; thus, our findings might not reflect the results in vivo. Therefore, studies with a larger patient cohort will be useful to support our findings, and a DR animal model is needed to further explore the mechanisms. Although many recent studies have addressed the role of salusin-β in different diseases, its biotoxicity and effects on normal physiological processes are still unclear. Therefore, much remains to be done before these findings can be clinically translated.
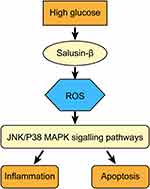 |
Figure 10 Salusin-β mediates high glucose-induced inflammation and apoptosis in retinal capillary endothelial cells via a ROS-dependent pathway. |
Conclusions
In conclusion, our results indicate that salusin-β can increase HG-induced inflammation and apoptosis by activating the ROS-dependent JNK and p38 MAPK signalling pathways in HRECs and participates in the progress of DR. Our study will pave the way for the discovery of a new therapeutic target for DR.
Acknowledgment
This study was sponsored by the National Natural Science Foundation of China [grant number 81870673 and 81371043].
Disclosure
The authors report no conflicts of interest for this work.
References
1. Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55(9):2401–2411. doi:10.2337/db05-1635
2. Tan GS, Cheung N, Simó R, Cheung GC, Wong TY. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017;5(2):143–155. doi:10.1016/S2213-8587(16)30052-3
3. Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97(12):2883–2890. doi:10.1172/JCI118746
4. Cunha-Vaz J, Bernardes R. Nonproliferative retinopathy in diabetes type 2. Initial stages and characterization of phenotypes. Prog Retin Eye Res. 2005;24(3):355–377. doi:10.1016/j.preteyeres.2004.07.004
5. Hammes HP, Lin J, Renner O, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51(10):3107–3112. doi:10.2337/diabetes.51.10.3107
6. Engerman RL. Pathogenesis of diabetic retinopathy. Diabetes. 1989;38(10):1203–1206. doi:10.2337/diab.38.10.1203
7. Boynton GE, Stem MS, Kwark L, Jackson GR, Farsiu S, Gardner TW. Multimodal characterization of proliferative diabetic retinopathy reveals alterations in outer retinal function and structure. Ophthalmology. 2015;122(5):957–967. doi:10.1016/j.ophtha.2014.12.001
8. García de la Torre N, Fernández-Durango R, Gómez R, et al. Expression of angiogenic MicroRNAs in endothelial progenitor cells from type 1 diabetic patients with and without diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56(6):4090–4098. doi:10.1167/iovs.15-16498
9. Savage SR, McCollum GW, Yang R, Penn JS. RNA-seq identifies a role for the PPARβ/δ inverse agonist GSK0660 in the regulation of TNFα-induced cytokine signaling in retinal endothelial cells. Mol Vis. 2015;21:568–576.
10. Pincelli Netto M, Lima VC, Pacheco MA, Unonius N, Gracitelli CP, Prata TS. Macular inner retinal layer thinning in diabetic patients without retinopathy measured by spectral domain optical coherence tomography. Med Hypothesis Discov Innov Ophthalmol. 2018;7(3):133–139.
11. Wang W, Zhang Y, Jin W, Xing Y, Yang A. Catechin weakens diabetic retinopathy by inhibiting the expression of NF-κB signaling pathway-mediated inflammatory factors. Ann Clin Lab Sci. 2018;48(5):594–600.
12. Shichiri M, Ishimaru S, Ota T, Nishikawa T, Isogai T, Hirata Y. Salusins: newly identified bioactive peptides with hemodynamic and mitogenic activities. Nat Med. 2003;9(9):1166–1172. doi:10.1038/nm913
13. Kolakowska U, Olanski W, Wasilewska A. Salusins in hypertension and related cardiovascular diseases. Curr Drug Metab. 2016;17(8):827–833. doi:10.2174/1389200217666160629113527
14. Fujimoto K, Hayashi A, Kamata Y, et al. Circulating levels of human salusin-β, a potent hemodynamic and atherogenesis regulator. PLoS One. 2013;8(10):e76714. doi:10.1371/journal.pone.0076714
15. Erden I, Demir B, Uçak H, Cicek D, Dertlioğlu SB, Aydin S. Serum salusin-α and salusin-β levels in patients with Behcet’s disease. Eur J Dermatol. 2014;24(5):577–582. doi:10.1684/ejd.2014.2397
16. Zhao M-X, Zhou B, Ling L. Salusin-β contributes to oxidative stress and inflammation in diabetic cardiomyopathy. Cell Death Dis. 2017;8(3):e2690. doi:10.1038/cddis.2017.106
17. Zhu X, Zhou Y, Cai W, Sun H, Qiu L. Salusin-β mediates high glucose-induced endothelial injury via disruption of AMPK signaling pathway. Biochem Biophys Res Commun. 2017;491(2):515–521. doi:10.1016/j.bbrc.2017.06.126
18. Lu QB, Du Q, Wang HP, Tang ZH, Wang YB, Sun HJ. Salusin-β mediates tubular cell apoptosis in acute kidney injury: involvement of the PKC/ROS signaling pathway. Redox Biol. 2020;30:101411. doi:10.1016/j.redox.2019.101411
19. American Academy of Ophthalmology Retina/Vitreous Panel. Preferred practice pattern guidelines. Diabetic retinopathy SF, CA(American academy of ophthalmology); 2016. Available from: http://www.aao.org/ppp.
20. Prevention, IV, and Delay of Type. Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi:10.2337/dc11-S011
21. Takenoya AF, Hori T, Kageyama H, et al. Coexistence of salusin and vasopressin in the rat hypothalamo-hypophyseal system. Neurosci Lett. 2005;385(2):110–113. doi:10.1016/j.neulet.2005.05.061
22. Suzuki N, Shichiri M, Tateno T, Sato K, Hirata Y. Distinct systemic distribution of salusin-α and salusin-β in the rat. Peptides. 2011;32(4):805–810. doi:10.1016/j.peptides.2010.12.012
23. Sato K, Koyama T, Tateno T, Hirata Y, Shichiri M. Presence of immunoreactive salusin-α in human serum and urine. Peptides. 2006;27(11):2561–2566. doi:10.1016/j.peptides.2006.06.005
24. Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;101799.
25. Cheng Y, Yu X, Zhang J, et al. Pancreatic kallikrein protects against diabetic retinopathy in KK Cg-A(y)/J and high-fat diet/streptozotocin-induced mouse models of type 2 diabetes. Diabetologia. 2019;62(6):1074–1086. doi:10.1007/s00125-019-4838-9
26. Sun HJ, Zhao MX, Liu TY, et al. Salusin-β induces foam cell formation and monocyte adhesion in human vascular smooth muscle cells via miR155/NOX2/NFκB pathway. Sci Rep. 2016;6(1):23596. doi:10.1038/srep23596
27. Sun HJ, Zhao MX, Ren XS, et al. Salusin-β promotes vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via ROS/NFκB/MMP-9 pathway. Antioxid Redox Signal. 2016;24(18):1045–1057. doi:10.1089/ars.2015.6475
28. Aneja A, Tang WHW, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121(9):748–757. doi:10.1016/j.amjmed.2008.03.046
29. Esfahani M, Saidijam M, Najafi R, Goodarzi MT, Movahedian A. The effect of salusin-β on expression of pro- and anti-inflammatory cytokines in human umbilical vein endothelial cells (HUVECs). ARYA Atheroscler. 2018;14(1):1–10. doi:10.22122/arya.v14i1.1602
30. Sag CM, Schnelle M, Zhang JQ, et al. Distinct regulatory effects of myeloid cell and endothelial cell NAPDH oxidase 2 on blood pressure. Circulation. 2017;135(22):2163. doi:10.1161/CIRCULATIONAHA.116.023877
31. Yin YL, Zhou ZH, Liu WW, Chang Q, Sun GQ, Dai YL. Vascular endothelial cells senescence is associated with NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation via reactive oxygen species (ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int J Biochem Cell B. 2017;84:22–34. doi:10.1016/j.biocel.2017.01.001
32. Talahalli R, Zarini S, Sheibani N, Murphy RC, Gubitosi-Klug RA. Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(3):1699–1708. doi:10.1167/iovs.09-3557
33. Huysman E, Mathieu C. Diabetes and peripheral vascular disease. Acta Chir Belg. 2009;109(5):587–594. doi:10.1080/00015458.2009.11680493
34. Roy S, Kern TS, Song B, Stuebe C. Mechanistic insights into pathological changes in the diabetic retina implications for targeting diabetic retinopathy. Am J Pathol. 2017;187(1):9–19. doi:10.1016/j.ajpath.2016.08.022
35. Xu T, Zhang Z, Liu T, et al. Salusin-β contributes to vascular inflammation associated with pulmonary arterial hypertension in rats. J Thorac Cardiovasc Surg. 2016;152(4):1177–1187. doi:10.1016/j.jtcvs.2016.05.056
36. Zhang L, Yu J, Ye M, Zhao H. Upregulation of CKIP-1 inhibits high-glucose induced inflammation and oxidative stress in HRECs and attenuates diabetic retinopathy by modulating Nrf2/ARE signaling pathway: an in vitro Study. Cell Biosci. 2019;9(1):67. doi:10.1186/s13578-019-0331-x
37. Li HB, Qin DN, Cheng K, et al. Central blockade of salusin β attenuates hypertension and hypothalamic inflammation in spontaneously hypertensive rats. Sci Rep. 2015;5(1):11162. doi:10.1038/srep11162
38. Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. 2015;89(6):867–882. doi:10.1007/s00204-015-1472-2
39. Peluso I, Yarla NS, Ambra R, Pastore G, Perry G. MAPK signalling pathway in cancers: olive products as cancer preventive and therapeutic agents. Semin Cancer Biol. 2019;56:185–195. doi:10.1016/j.semcancer.2017.09.002
40. Zuo G, Ren X, Qian X, et al. Inhibition of JNK and p38 MAPK-mediated inflammation and apoptosis by ivabradine improves cardiac function in streptozotocin-induced diabetic cardiomyopathy. J Cell Physiol. 2019;234(2):1925–1936. doi:10.1002/jcp.27070
41. Xie MS, Zheng YZ, Huang LB, Xu GX. Infliximab relieves blood retinal barrier breakdown through the p38 MAPK pathway in a diabetic rat model. Int J Ophthalmol-Chi. 2017;10(12):1824–1829.
42. Li Y, Bai YJ, Jiang YR, et al. Apelin-13 is an early promoter of cytoskeleton and tight junction in diabetic macular edema via PI-3K/Akt and MAPK/Erk signaling pathways. Biomed Res Int. 2018;2018.
43. Liu JL, Bhuvanagiri S, Qu XH. The protective effects of lycopus lucidus turcz in diabetic retinopathy and its possible mechanisms. Artif Cell Nanomed B. 2019;47(1):2900–2908. doi:10.1080/21691401.2019.1640230
44. El-Remessy AB, Rajesh M, Mukhopadhyay P, et al. Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia. 2011;54(6):1567–1578. doi:10.1007/s00125-011-2061-4
45. Ali TK, Al-Gayyar MM, Matragoon S, et al. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 2011;54(3):657–668. doi:10.1007/s00125-010-1935-1
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.