Back to Journals » Journal of Multidisciplinary Healthcare » Volume 13
Salivary Cortisol, Subjective Stress and Quality of Sleep Among Female Healthcare Professionals
Authors Bani-Issa W , Radwan H , Al Marzooq F, Al Awar S, Al-Shujairi AM, Samsudin AR, Khasawneh W , Albluwi N
Received 31 August 2019
Accepted for publication 8 January 2020
Published 5 February 2020 Volume 2020:13 Pages 125—140
DOI https://doi.org/10.2147/JMDH.S229396
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Wegdan Bani-Issa,1 Hadia Radwan,2 Farah Al Marzooq,3 Shamsa Al Awar,4 Arwa M Al-Shujairi,5 Ab Rani Samsudin,6 Wafa Khasawneh,7 Najla Albluwi5
1Sharjah Institute for Medical Research, College of Health Sciences, Department of Nursing, University of Sharjah, Sharjah, United Arab Emirates; 2College of Health Sciences, Clinical Nutrition and Dietetics, University of Sharjah, Sharjah, United Arab Emirates; 3Sharjah Institute for Medical Research, University of Sharjah, Sharjah, United Arab Emirates; 4Department of Obstetrics and Gynaecology, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates; 5Research Institute of Medical and Health Sciences, University of Sharjah, Sharjah, United Arab Emirates; 6College of Dental Medicine, Oral and Craniofacial Health Sciences, University of Sharjah, Sharjah, United Arab Emirates; 7California State University, Long Beach, CA, USA
Correspondence: Wegdan Bani-Issa
Department of Nursing, College of Health Sciences, University of Sharjah, P.O. Box No. 27272, Sharjah, United Arab Emirates
Tel +971 06 505 7522
Fax +9716 505 7502
Email [email protected]
Background: Stress is globally recognised as a risk factor impacting workers’ health and workplace safety. Women healthcare professionals are at risk for considerable stress given the demanding nature of their jobs and current working conditions. This study assessed levels of stress among women healthcare professionals using measures of their cortisol levels, subjective stress and quality of sleep.
Participants and Methods: This study used a cross-sectional design. Data were collected from 335 apparently healthy adult women healthcare professionals working in the United Arab Emirates. Participants provided morning and bedtime saliva samples for analysis of their cortisol levels. The Perceived Stress Scale, Stress Symptoms Scale, Brief Coping Scale and Pittsburgh Sleep Quality Index were used to assess perceived stress level, symptoms of stress, stress-coping strategies and sleep quality, respectively.
Results: In total, 121 (36.15%) women had impaired morning cortisol levels (below the normal range of 0.094– 1.551 μg/dL) and 48 (14.3%) had impaired bedtime cortisol levels (above 0.359 μg/dL). Around 57% of women reported moderate levels of perceived stress, with the most frequently reported stress symptoms being heart rate and back/neck pain. Poor sleep quality was reported by around 60% of participating women. No significant association was found between cortisol and psychosocial measurements of stress or sleep quality. However, night shift and longer shift duration (more than 8 hrs) were significantly associated with impaired morning and bedtime cortisol levels (P ≥ 0.05). Impaired cortisol levels were strongly dependent on using adaptive coping strategies such as active coping, acceptance and seeking emotional support (P ≥ 0.05).
Conclusion: Evaluating cortisol levels and subjective stress could help to identify groups with impaired response to stress and elevated cortisol levels. Our findings support the need to examine shift work patterns and stress coping strategies in women healthcare professionals to promote their health and productivity and maintain workplace safety.
Keywords: coping strategies, cortisol, female healthcare professionals, nurses, sleep quality, subjective stress
Background
Mental health is an important indicator of good health. The World Health Organization (WHO) recommended mental health as a global development priority.1 Although mental health problems affect women and men equally, numerous studies have shown that women are 40% more likely than men to develop mental illness.2 Various social factors place women at greater risk for poor mental health compared with men, including the stress of juggling many roles and maintaining a work–life balance.3
Cortisol is a hormone released by the adrenal gland as part of the flight or fight impulse in response to fear or stress. Cortisol is an adaptation hormone that plays a major role in several bodily functions, including mental health (stress and depression)4 and recollection of emotional events.5 Cortisol release is influenced by the circadian rhythm, coping mechanisms, lifestyle factors and daily stressors.5 In situations of chronic psychological stress and disrupted sleep-wake cycles, the adrenal glands secrete an abnormal amount of cortisol in an abnormal rhythm.6
Stress in the workplace is globally acknowledged as a risk factor impacting workers’ health and safety. A longitudinal study involving 110 Swedish women aged 47–53 years reported that negative work environments significantly contributed to high biological stress, as confirmed by elevated morning cortisol levels.7 Women working in the healthcare sector are at greater risk for stress, attributable to the demanding nature of their job, an environment of constant (and often rapid) change and hospital working conditions. The WHO noted that
a healthy workplace is one in which workers and managers collaborate to use a continual improvement process to protect and promote the health, safety and well-being of all workers and the sustainability of workplace.8
Women are at the frontline of many healthcare professions, including physicians, nurses, other healthcare providers and administrative and ancillary staff. Around 67% of healthcare providers and social sector staff are women.9 Recent studies have explored work stress among female healthcare professionals in many countries. A study in India reported female physicians exhibited high levels of stress compared with their male counterparts.10 A comparative cross-sectional descriptive study involving 57 Brazilian nurses working morning, afternoon and evening shifts in emergency departments and medical surgical wards found decreased salivary cortisol levels on days off compared with workdays.11 A study conducted in Spain showed that female nurses (n = 98) working in public hospitals reported higher levels of perceived stress and higher levels of plasma-extracted cortisol than male nurses (n = 98).12 Finally, a 1-year German longitudinal study involving 70 physicians (mean age 30 years, 40 women) suggested that longer shift hours were associated with changes in the diurnal cortisol pattern.13
Studies of stress among female healthcare professionals that measure levels of cortisol are scarce. Most women healthcare workers in the United Arab Emirates (UAE) are expatriates, and may face stressors related to from being away from home, financial problems, work-related stress, relationship problems, societal problems and personal health-related stress.14 Therefore, measuring stress by cortisol levels may offer a reflection of their overall stress levels that could be used to make recommendations to healthcare policy makers regarding integrating strategies to overcome stress among women healthcare professionals in the UAE.
This study aimed to evaluate morning and bedtime cortisol levels in female healthcare professionals working in UAE hospitals, and compare sociodemographic characteristics, subjective stress and quality of sleep between participants with normal and impaired cortisol levels (morning and bedtime). Salivary collection allows for non-invasive, timed measurement of free cortisol. This is stable for several days before processing, allowing for a valid assessment of the hypothalamus-pituitary-adrenal (HPA) axis in the free-living state.
Aims and Objectives
The overall aim of this study was to develop specific recommendations for UAE stakeholders and decision makers to alleviate stress levels among women healthcare professionals. Based on a representative sample of women healthcare professionals working in the UAE healthcare sector, two specific objectives were: a) to estimate levels of morning and bedtime cortisol among women healthcare professionals, and b) to compare sociodemographic characteristics, subjective stress measures (perceived stress, stress symptoms and stress coping strategies) and quality of sleep between participants with impaired morning and bedtime cortisol levels and those with normal cortisol levels.
Methods
Study Design
This study used a cross-sectional quantitative design. Data were collected using self-report questionnaires covering stress and sleep quality. Morning and bedtime unstimulated saliva samples were also collected. Saliva was collected between October 2016 and December 2017.
Study Setting and Participants
Currently, no data are available on the prevalence of stress among women healthcare professionals in the UAE; however, the literature suggests 60% of working women experience some level of stress.15 To achieve results at a significance level of 0.05, 95% confidence interval and a 3% margin of error, 369 participants were required. To compensate for non-respondents, 469 women healthcare professionals were randomly selected using a cluster sampling approach.
Participants were recruited from government and non-government hospitals from across the seven UAE emirates. Women working either day or night shifts were invited to participate. Professional groups included were nurses, physicians, dentists, pharmacists and allied health professionals (e.g. physiotherapists and radiographers). Eligible participants were those aged ≥20 years who were not pregnant and did not have major life events stressors that impacted their stress level within the last year. Participants who were using therapies that may affect sleep patterns (e.g. diuretics, continuous positive airway pressure) were excluded from this study.
Salivary Cortisol: Collection and Measurement
Two unstimulated saliva samples (morning and bedtime) using the passive drooling method were collected from each participant to examine the diurnal variations of hormonal secretion affected by the circadian rhythm.16 Saliva sampling is a quick, non-invasive and reliable method to measure biologically active, unbound plasma levels of cortisol in adults.16,17 The passive drool technique is considered the gold standard when collecting saliva samples for biological testing as it allows researchers to store saliva samples for further analysis.16
Volunteer participants who met the study inclusion criteria were given comprehensive written and oral instructions on how to collect reliable and adequate saliva samples. To enhance the validity, rigour and integrity of the salivary sampling, the researchers used a unified standardised protocol as per the manufacturing manual for sample collection, transportation, storage and analysis.16 First, to minimise circadian effect on cortisol levels, saliva samples were collected from participants at approximately the same time. Morning samples were collected between 7:00 and 8:00 and bedtime samples were collected between 19:00 and 20:00.16 Samples were collected during shift hours when participants were on shift (morning samples from day shift participants and night samples when participants were on night shifts), or during the subsequent off day after the participants’ shift (i.e. the first morning for night shift participants who had already provided bedtime samples during their shift, or the first night for day shift participants who had provided morning samples during their shift).
Second, instructions regarding the collection and storing procedures for salivary samples were provided at recruitment to maintain sample purity and avoid repetition of samples.16 It was emphasised that no eating, drinking, smoking, chewing gum and teeth brushing were allowed for 10–15 mins before giving a sample. Participants were also instructed to rinse their mouth with water at least 10 mins before giving a sample and if possible, to wait another 10 mins after rinsing to avoid sample dilution. Participants’ collected their saliva samples in 5 mL sterile plastic containers.
Participants were instructed to quickly freeze their sample in their regular household freezers (usually below −20°C) if collected at home, and take the frozen sample to their workplace. To facilitate sample collection, the research team assigned a person at each data collection site to collect and store samples in designated deep freezers at the data collection sites, and notify the research team to collect the samples in a timely manner. Samples were then transferred and stored in a specialised deep freezer at −20°C at the Sharjah Institute for Medical and Health Sciences Research until further processing. Because the weather was hot for most of the time during the data collection period, portable freezers were used to transport samples. Furthermore, all samples were examined for purity and suitability, and were analysed by the same laboratory scientist who is an expert in saliva analysis for cortisol.
Samples were processed using the expanded range high sensitivity salivary cortisol enzyme immunoassay kit (kit 1–3102, Salimetrics, USA), according to the manufacturer’s instructions. Absorbance values were used to calculate the concentrations using a standard curve by four-parameter logistic fit (4PL) model.16,17
Data Collection from Questionnaires
Information was collected on participants’ demographic and socioeconomic characteristics, medical history and lifestyles. This information included age, Emirate/city, nationality, ethnicity, years of work in the UAE, level of education, marital status, number of children, occupation, working shift (day vs night), shift hours, family income, smoking, exercise status and level of happiness. Participants’ body weight and height were measured by trained research assistants, and the body mass index (BMI) was calculated. BMI categories were defined according to the WHO: BMI <18.5 kg/m2 indicated underweight, 18.5–24.9 kg/m2 indicated normal range, 25–29.9 kg/m2 indicated overweight and ≥30 kg/m2 indicated obesity.
Next, each participant was asked to complete four measures. All measures used in this study were standardised, validated and had previously been used in similar research. Three measures covered stress levels and one measure assessed sleep quality. The 14-item Perceived Stress Scale (PSS) has been used to assess perceived stress in the general population with good internal reliability (Cronbach’s alpha >0.70).18 An example item from this scale is “In the last month, how often have you been upset because of something that happened unexpectedly?” Responses are on a 4-point Likert-type scale from 0 = never to 4 = very often. The PSS total score was divided into two categories: scores below 27 were considered to reflect low to moderate stress levels, and scores over 27 were considered to reflect high levels of stress.19,20
Participants’ symptoms of stress were assessed using the 41-item Stress Symptoms Scale (SSS), which rates symptoms of stress experienced in the previous 2 weeks using a 4-point Likert scale from 0 = never to 4 = very often.21 The total scores were grouped into two categories: less than the average rating (≤40) and more than the average rating (>40).21,22 Examples of symptoms measured included rapid breathing, presence of low back pain and headaches. The SSS has been used to measure symptoms related to job stress in nurses, and was reported to have adequate psychometric properties.22
Coping strategies were assessed with the 28-item Brief Coping Scale (BCS). This tool uses a 4-point Likert-type scale to assess a range of coping responses among adults, including poor or maladaptive coping strategies (denial, self-blame, behavioural disengagement, venting, substance abuse) and adaptive coping strategies (use of instrumental [tangible] support, use of emotional support, religion, planning, self-distraction, humour, positive reframing, acceptance, active coping).23,24 The BCS has reasonably good reliability (total Cronbach’s alpha of 0.50–0.90).24 A response of “3” on the 4-point Likert scale for an item was considered as “yes” for using that strategy to cope with stress.
The Pittsburgh Sleep Quality Index (PSQI) was used to evaluate participants’ perceived sleep quality and pattern.25 The PSQI is a widely used self-report questionnaire that measures the overall quality of sleep over the last month. Responses are on a 4-point Likert-type scale from 0 = none during the last month to 3 = three or more times during the last month. The scale differentiates “poor” from “good” sleep by measuring seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication and daytime dysfunction. The PSQI items are assessed on a scale from zero to 21, with a score of zero representing no problem and 21 indicating severe problems in relation to all assessment items. In general, a score of 5 or higher represents poor sleep quality.24 The PSQI has been widely used to assess sleep quality, and has good psychometric properties and a completion time of 5 mins.25,26 A total PSQI score greater than 5 produced a diagnostic sensitivity of 89.6% and specificity of 86.5% (kappa = 0.75, p ≤ 0.001) in distinguishing good and poor sleepers.26
Statistical Analysis
Descriptive statistical analysis was performed using SPSS software version 22 and SAS software version 9.4. Means, standard deviations, frequencies and percentages were used to describe levels of morning and bedtime cortisol and all other study variables. We used t-tests and chi-square tests to compare differences in continuous and categorical variables, respectively. We stratified the sample by their cortisol levels into a group with an impaired response to stress (participants with out of range values) and a group with a normal response to stress (normal cortisol levels) for both morning and bedtime cortisol. The groups were compared for statistical differences with regard to their sociodemographic characteristics and PSS, SSS, BCS and PSQI scores using Pearson’s chi-square tests. Further, we performed analyses for individual BCS and PSQI domains by cortisol categories.
Results for morning and bedtime cortisol levels were displayed separately to allow comparison and correlation with other study variables. Cortisol levels were described as follows. Morning salivary levels: normal range (0.094–1.551 µg/dL), below normal range (<0.094 µg/dL) and above normal range (>1.551 µg/dL). Bedtime salivary levels: normal range (not determined to 0.359 µg/dL) and above normal range (>0.359 µg/dL).27 All tests were two-tailed and the level of significance was set at p ≤ 0.05.
Results
Participants’ Characteristics and Cortisol Levels
This study included 335 women healthcare professionals, representing a 71.4% response rate. A major reason for refusal to participate in this study was a lack of interest or time. Twenty-five participants were excluded because of missing data or their saliva samples were not suitable for analysis (either inadequate quantity or they were stained).
Participants were from six different Emirates, with close to half from Sharjah (n = 159, 47.5%). However, 85% of participants were of non-Emirati, with the largest group being Indian (n = 124, 37.0%), followed by Filipino (n = 60, 17.9%) and other Arab and non-Arab minorities. Participants had spent an average of 16.8 years (standard deviation [SD] = 11.7 years) in the UAE. The average age of the study population was 36.8 years (SD = 7.8 years), with most (72.2%) participants aged below 42 years; 293 (87.5%) were of reproductive age.
The most common qualification was a bachelor’s degree (n = 300, 89.6%). A total of 227 (67.8%) participants were married and 66.0% had children (average of two children). Close to 80% of participants were average-income families, and only 105 (31.3%) participants had hired help at home. About half of the participants worked night shift (n = 163, 48.66%) and 75 (22.4%) had long shift duration (more than 8 hrs per shift) during the last month. Very few participants were smokers (2.7%), and walking was the regular physical activity of 49 (14.6%) participants.
Just under half of the participants had a BMI that was normal/underweight (n = 155, 46.3%), and 180 (53.73%) were either overweight or obese. In addition, 73.7% of the participants reported not taking any medication and more than half (n = 206, 61.5%) considered themselves as generally happy (Table 1).
 |
Table 1 Distribution of General Profile Variables by Cortisol Levels (N= 335) |
Table 2 shows the results of participants’ morning and bedtime salivary cortisol levels. Morning cortisol levels ranged from 0.2265–3.007 µg/dL, with a mean of 0.26 µg/dL (SD = 0.34 µg/dL). The majority (n = 214, 63.9%) of participants had morning cortisol levels in the normal range, and 121 (36.1%) had impaired (below average) levels. Bedtime cortisol levels ranged from 0.1033–0.1552 µg/dL, with a mean of 0.13 µg/dL (SD = 0.24 µg/dL). Only 48 (14.3%) participants had abnormally high bedtime cortisol levels, and the remainder (85.7%) had bedtime cortisol levels in the normal range (Table 2). There was a significant correlation between morning and bedtime cortisol levels (r = 0.196, p = 0.001).
 |
Table 2 Morning and Bedtime Salivary Cortisol Levels in Participants (N= 335) |
There were no significant differences in participants’ demographic characteristics between the normal and impaired morning cortisol groups. However, working night shifts (χ21 [n = 335] = 4.69, p = 0.03) and longer shift duration (more than 8 hrs) (χ21 [n = 335] = 3.55, p = 0.045) were significantly different between the normal and impaired morning cortisol groups (Table 1). Similarly, working night shift and longer shift duration were significantly associated with bedtime cortisol level (χ21 [n = 335] = 4.55, p = 0.03 and χ21 [n = 335] = 14.7, p = 0.001, respectively) (Table 1). This suggested that impaired cortisol levels were associated with working night shift and longer shift duration (more than 8 hrs) in our participants.
Psychological Measures, Sleep Quality to Cortisol Levels
PSS and Cortisol
Participants’ PSS scores ranged from 9–42, with a mean of 25.7 (SD = 6.06), suggesting overall moderate stress levels; 153 (45.7%) participants had high levels of stress (score >27). More than half of the participants reported being upset “fairly often” about unexpected events (57.0%), nervous and stressed out (51.6%) and anxious about to-do lists (46.8%), as well as often feeling angry and overwhelmed when tasks accumulated (46.3%). Conversely, participants reported successfully coping with daily problems, irritations and changes, handling personal problems, and being in control of their lives and the way they spent their time.
With regards to perceived stress, neither morning nor bedtime cortisol levels significantly differed across PSS categories. (Figure 1: Distribution of morning and bedtime cortisol levels across Perceived Stress Scale categories: low 0–26 vs high >27 stress). Even when participants were clustered into impaired and normal groups by morning cortisol levels, no significant associations were found between morning cortisol levels and PSS scores (p > 0.05). Similarly, no significant associations were observed between PSS scores and bedtime cortisol levels (p > 0.05) (Table 3: Distribution of Perceived Stress Scale, Stress Symptoms Scale, Brief Coping Scale and Pittsburgh Sleep Quality Index scores, by morning and bedtime cortisol). This suggested that both morning and bedtime cortisol levels were independent of perceived stress level.
 | 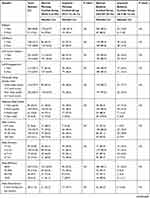 |  |
Table 3 Distribution of Perceived Stress Scale, Stress Symptoms Scale, Brief Coping Scale, Pittsburgh Sleep Quality Index by Morning and Bedtime Cortisol (N=335) |
 |
Figure 1 Distribution of morning and bedtime salivary cortisol according to Perceived Stress Scale (PSS). |
SSS and Cortisol
Participants’ mean SSS score was 50.5 (SD = 27.0). Almost half of the participants had low stress symptoms (score <40). High frequencies were observed for experiences of stress symptoms concerning items related to vital signs (heart rate: 56%, rapid pulse: 51%) and back/neck pain (56%) symptoms.
Salivary cortisol levels were not significantly correlated with SSS scores (p > 0.05): morning cortisol, r = 0.30 (p = 0.56) and bedtime cortisol, r = 0.15 (p = 0.16). (See Figure 2: Distribution of morning and bedtime salivary cortisol across the SSS). No associations were observed between morning cortisol categories and SSS score (p > 0.50). Similarly, a higher SSS score was independent of bedtime cortisol levels, and no significant associations were seen between the two variables (p > 0.05). These results suggested that both morning and bedtime cortisol levels were independent of SSS score (Table 3).
 |
Figure 2 Distribution of morning and bedtime salivary cortisol according to Stress Symptoms Scale (SSS). |
BCS and Cortisol
To identify coping mechanisms among participants, we analysed the 14 BCS subscales. Average scores per subscale ranged from 2.3 (SD = 0.9) for substance abuse to 6.4 (SD = 1.6) for religion as a coping strategy. The four most frequently used adaptive coping strategies were emotional support, active coping, acceptance and planning. Among the maladaptive coping strategies, the least used coping style was substance abuse (5.1%) followed by denial (25.6%) (Table 3).
Examining all 14 coping styles revealed that the morning and bedtime cortisol levels (impaired vs normal) were significantly positively associated with the active coping, acceptance and seeking emotional support coping styles (p ≤ 0.05) (Table 3).
PSQI and Cortisol
Close to two-thirds of the participating women (60.3%) reported poor sleep quality. Most participants also reported poor sleep components. Only 85 (25.4%) participants reported very good subjective sleep quality; 54 (16.1%) reported being in bed for more than 60 mins before falling asleep (sleep latency) and 35 (10.4%) reported 7 or more sleep hours per night (Table 3).
Morning and bedtime cortisol levels were not significantly correlated with quality of sleep (r = 0.26, p = 0.57 for morning cortisol; r = 0.013, p = 0.92 for bedtime cortisol) (See Figure 3. Distribution of morning and bedtime cortisol by Pittsburgh Sleep Quality Index scores).
 |
Figure 3 Distribution of morning and bedtime salivary cortisol according to Pittsburgh Sleep Quality Index (PSQI). |
Participants with normal and impaired morning and bedtime cortisol levels did not differ from participants with normal cortisol levels in terms of overall sleep quality or any of the seven components (subjective sleep quality, sleep duration, sleep efficiency, disturbance, sleep medication, daytime dysfunction and latency; p > 0.05) (Table 3). This suggested there were independent associations between levels of morning and bedtime cortisol and quality of sleep in our participants.
Discussion
This study highlighted an important subject that is generally neglected in healthcare systems worldwide. We evaluated the mental health of women healthcare professionals in the UAE using an objective and reliable tool (salivary cortisol levels) to measure stress, as well as subjective psychological measures of stress and sleep quality.28–30 Importantly, we found that a considerable number of women healthcare professionals had an impaired response to stress and demonstrated abnormal cortisol levels, particularly those working night shift and with longer shift duration. Chronic stress has been linked to the onset of mental health problems and overall poorer physical status in working women,31–33 potentially leading to poor physical and social health, and suboptimal performance at work.34 The literature provides strong evidence that disrupted chronobiological rhythms among female workers35,36 exacerbate work-related stress,37 especially among human service professionals.36
As expected, we found morning salivary cortisol levels were higher compared with bedtime levels, which was consistent with natural physiology,38 with a strong correlation between those measurements. The proportion of women with impaired cortisol is a relevant observation given the implication of cortisol in regulating neuroendocrine function, physical, emotional and cognitive health, and burnout as well as response to stress.39
Previous studies found that a hypoactive HPA axis was associated with higher ratings of anxiety among women,29 which can be attributed to “flatter” cortisol diurnal and nocturnal slopes.40,41 However, given the cross-sectional design, the present study could not validate this relationship. It is crucial to emphasise that cortisol must be examined at different times during the day over a period of time to accurately predict the impact of accumulative stress on human body.42
We expected that impaired cortisol levels (both morning and bedtime) would be related to general sociodemographic or personal variables, because the majority of participants were expatriates and away from their homes. It is known that expatriate women face financial, social, cultural and emotional challenges associated with living in different culture and being away from their own home countries.43 However, our results suggested that stress mostly emanated from the workplace rather than from participants’ personal lives, particularly working night shift and longer shift duration (more than 8 hrs). This corresponds with the nature of the unpredictable work environment, work load and staff shortages, especially as most participants were nurses.44–46 A Korean study showed that nurses working night shift exhibited higher cortisol levels than nurses working regular hours.47 Long working shift hours among nurses were also associated with chronic stress and fatigue, which disturbed cortisol levels leading to diminished physical, mental and cognitive abilities, increased work place errors and absenteeism.47
As the workplace environment is a potential source of stress, it is recommended that stressors at work are identified and strategies to create a healthy work environment are integrated, especially for nurses. For example, night shift workers may need 3–4 days to adjust their circadian rhythms of cortisol secretions.48,49 All nurses, from clinical staff to executives, should also be trained to identify those with impaired stress coping and adopt interventions to reduce the risks to patient safety.49
Policy makers and workplace managers must consider shift patterns in the UAE healthcare system. This will contribute to higher professional and personal satisfaction, as well as to an enhanced sense of achievement, and alleviate burnout syndrome.44 Healthcare reform should include the mental health of healthcare professionals (both men and women), as data are also scarce on mental health among male healthcare professionals. The connections between mental health and productivity at work, malpractice and medical incidents should also be examined in further longitudinal research. This recommendation is pertinent to our study given that some healthcare institutions had started to apply a 12-hrs shift system, which may strongly impact nurses’ stress and cortisol levels. Further comparative studies are needed in this domain.
Importantly, a considerable number of participants in our study reported high levels of perceived stress and its manifestations, such as increased heart rate and back and neck pain; these findings were consistent with prior research.50,51 Several studies from different parts of the world have reported greater levels of perceived stress and associated manifestations among female healthcare professionals, especially doctors and nurses, leading to deteriorated mental and physical health and diminished productivity at work over time.50–52
The lack of a significant association between stress and its physical manifestations in this study could partially be explained by the use of positive adaptive coping strategies, including acceptance, emotional support, emotion-focused and active coping (described as problem-focused coping).23,24 Such positive coping styles may play a key role in buffering the impact of stress on cortisol levels.23,24 Seeking emotional support may play a critical role in safeguarding against the impact of psychological stress, and greater tolerance of stress may emerge related to HPA axis activity.53 A coping style characterised by greater use of social support systems was found to be inversely correlated with cortisol levels in women.53,54 Further, an active coping strategy of trying to be resilient and in control of stress-provoking situations might have augmented the release of cortisol in our participants.
A cross-sectional study involving consultant physicians (n = 582) found that more frequent use of an adaptive coping style was associated with lower stress levels among female physicians.55 It is important to note that our participants had been in the UAE for a long period of time (mean: 16 years), and it is possible that they became acculturated and immersed in the UAE culture and learned to identify and positively cope with sources of stress. Participants were also more likely to live with their own families, who could perhaps be a source of emotional support for them. However, more longitudinal studies are needed to clarify the nature of the correlation between stress and cortisol circadian rhythm in expatriates compared with Emirati women healthcare professionals, as sources, levels of stress and coping styles may differ across ethnic groups.54
Importantly, the availability of a supportive environment that attracts and supports women who relocate with their families to live and work in the UAE could be an additional reason for our participants’ adaptive coping with stress and explain the less fluctuation in cortisol levels.56 The UAE government has highlighted the happiness of working women (regardless of nationality) as national developmental goal in its 2021 national agenda, including efforts to support them relocate smoothly with their families into the UAE culture.
Acceptance, active coping and emotional support as adaptive coping strategies appeared to mediate the correlation between stress and cortisol levels in participants with normal cortisol. Therefore, it is critical to direct specific attention to coping strategies among those with impaired cortisol levels. Strengthening their coping resources at work and guiding them to adopt emotion- and problem-focused strategies are critical to assist them to cope with daily stressors.57 Because of the cultural diversity of women healthcare professionals in the UAE healthcare system, there is a need to individualise stress management coping techniques to target and consider all potential mediators of stress across different groups of women.57
Although 60% of participants reported poor subjective sleep quality, morning and bedtime salivary cortisol levels were not significantly correlated with quality of sleep. A previous study investigated the effects of sleep quality and quantity on cortisol response to acute stress among 73 younger adults.58 That study found that sleep duration did not affect cortisol stress responses, whereas sleep quality might. In addition, contrary to their male counterparts, women’s stress responses were less dependent on their self-reported sleep quality.58 Those findings were consistent with results from the present study. A 5-year cohort study from the United Kingdom involving 3314 working participants found that those reporting short sleep duration on three occasions had higher morning and diurnal cortisol levels than other participants.59 Further studies are needed to evaluate melatonin levels to better predict physiological parameters of stress and sleep.
To conclude, we found a considerable number of women healthcare professionals with impaired cortisol levels. The main sources of stress for our participants were the workplace environment, which was demonstrated by the correlation between shift work and number of hours per shift and cortisol levels. To assist the UAE government to achieve the vision of creating a happier workplace environment, it is critical that health policy- and decision-makers consider shift patterns for women healthcare providers to prevent the impact of chronic stress on cortisol activity, increase workplace productivity and enhance patient safety. Teaching women healthcare providers positive coping styles may also assist in buffering the impact of shift related stress on women’s health.
Limitations
This study relied on participants’ self-reported information, which might be associated with bias, especially in terms of different interpretations of questions and their relevance to the study. Data gathering would be strengthened by further studies following a longitudinal design, whereby salivary cortisol levels could be assessed over time and across different stress statuses of participants. This would support a better understanding of the evolution of stress responses, and facilitate the design of strategies to more effectively alleviate stress among healthcare professionals. We did not examine shift history, which could be a limitation of this study. Further, our participants were mainly expatriate women, and more research is needed to investigate the sources of stress for this group in more depth and integrate strategies to foster positive coping to eliminate the impact of chronic stress on health and wellbeing. A larger sample of women healthcare professionals may also reveal different findings, as the small sample size was a limitation of our study.
Conclusion and Recommendations
The findings reported in this study emphasise the involvement of physical and physiological factors in cortisol levels and stress. Measuring plasma (or salivary) cortisol levels could supplement demographic and psychopathological data and help identify groups with impaired response to stress. The interaction between sleep, stress and cortisol warrants further investigation; however, evidence on the detrimental effects of workplace stress on long-term health outcomes and employees’ quality of life is strong. This study also showed that cortisol (reflecting stress) was at lower levels with improved coping mechanisms, highlighting that coping strategies are crucial for healthcare professionals.
Abbreviations
HPA, hypothalamus-pituitary-adrenal; BMI, body mass index; PSS, Perceived Stress Scale; SSS, Stress Symptoms Scale; BCS, Brief Coping Scale; PSQI, Pittsburgh Sleep Quality Index.
Ethics Statement
This study was approved by the Research Ethics Committee at the Ministry of Health and Promotion/UAE (MOHAP/DXB/SU BC/No 6 I 2O1 7) and Research Ethics Committee at the principle investigator’s institution (REC/15/11/P007). This study was conducted in accordance with the Declaration of Helsinki.
Informed written consent was obtained from participants before enrolment in this study. Participants who provided informed consent were also informed that the collected data may be published in a peer-reviewed journal and presented in scientific conferences. Permission was given by study participants for the research team to collect saliva samples that included cortisol analysis and melatonin analysis (melatonin results to be discussed in a separate paper). All participants who provided saliva samples and accompanying information were de-identified by assigning arbitrary numbers to ensure their anonymity.
Data Sharing Statement
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank the study participants and investigators. They would also like to acknowledge data management, biostatistical analysis and scientific writing support provided by KBP-Biomak, a contract research organisation. Special thanks to the research assistants from the nursing department for their assistance in data collection namely: Massoma H. Qambar, Arwa S. Mohamed Hamid, Anood M. Ebrahim Aashoor Alteneiji, Raqya M. Musaed Al-Husaini and Mariam A. Hussein Shahdad.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Mental Health (Out of the Shadows: Making Mental Health a Global Development Priority) [Internet]. World Health Organization; 2018. Available from: http://www.who.int/mental_health/en/.
2. Freeman D, Freeman J. The Stressed Sex: Uncovering the Truth About Men, Women, and Mental Health.
3. Dietrich A, Ormel J, Buitelaar JK, Verhulst FC, Hoekstra PJ, Hartman CA. Cortisol in the morning and dimensions of anxiety, depression, and aggression in children from a general population and clinic-referred cohort: an integrated analysis. The TRAILS study. Psychoneuroendocrinology. 2013;38(8):1281–1298. doi:10.1016/j.psyneuen.2012.11.013.
4. Antypa D, Vuilleumier P, Rimmele U. Suppressing cortisol at encoding reduces the emotional enhancement in subjective sense of recollection. Neurobiol Learn Mem. 2018;155:86–91. doi:10.1016/j.nlm.2018.06.012
5. Wu JY, Hsu SC, Ku SC, Ho CC, Yu CJ, Yang PC. Adrenal insufficiency in prolonged critical illness. Crit Care. 2008;12(3):R65. doi:10.1186/cc6895
6. Whitaker MJ, Debono M, Huatan H, Merke DP, Arlt W, Ross RJ. An oral multiparticulate, modified‐release, hydrocortisone replacement therapy that provides physiological cortisol exposure. J Clin Endocr. 2014;80(4):554–561. doi:10.1111/cen.12316
7. Evolahti A, Hultcrantz M, Collins A. Women’s work stress and cortisol levels: a longitudinal study of the association between the psychosocial work environment and serum cortisol. J Psychosom Res. 2006;61(5):645–652. doi:10.1016/j.jpsychores.2006.07.022
8. Burton J. Who Healthy Workplace Framework and Model. Geneva, Switzerland: World Health Organisation; 2010:12.
9. Buchan J, Dhillon IS, Campbell J. Health Employment and Economic Growth: An Evidence Base. Geneva: World Health Organization;2017. Available from: https://www.who.int/hrh/resources/WHO-HLC-Report_web.pdf.
10. Passey S, Sandhu JS, Shenoy S. Stress among Indian doctors: a gender variation. Int J Indian Psychol. 2015;3(1):40–48.
11. Rocha MC, Martino MM, Grassi-Kassisse DM, Souza AL. Stress among nurses: an examination of salivary cortisol levels on work and day off. Revista Da Escola De Enfermagem Da USP. 2013;47(5):1187–1194. doi:10.1590/S0080-623420130000500025
12. Del Pilar Sánchez-López M, Saavedra AI, Dresch V, Limiñana-Gras RM. Conformity to traditional gender norms in a feminized occupation: the influence on health behaviors. Health. 2014;6(20):2775. doi:10.4236/health.2014.620317
13. Li J, Bidlingmaier M, Petru R, et al. Impact of shift work on the diurnal cortisol rhythm: a one-year longitudinal study in junior physicians. J Occup Med Toxicol. 2018;3(1):23. doi:10.1186/s12995-018-0204-y
14. Expats life for women in the United Arab Emirates. Available from: https://www.cadogantate.com/en/moving-services/news/expat-life-women-uae.
15. Parashar M, Singh M, Kishore J, Pathak R, Panda M. Prevalence and correlates of stress among working women of a tertiary health centre in Delhi, India. INJMS. 2017;8(2):77–81. doi:10.4103/mjdrdypu.MJDRDYPU_240_17
16. Salimetrics LLC, SalivaBio LLC. Saliva collection and handling advice. 2011. Available from: http://www.salimetrics.com.
17. Cox KL, Devanarayan V, Kriauciunas A, et al. Eli Lilly & Company and the National Center for Advancing Translational Sciences. 2004. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22553884.
18. Cohen S, Janicki‐Deverts DENISE. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009 1. J Appl Soc Psychol. 2012;42(6):1320–1334. doi:10.1111/j.1559-1816.2012.00900.x
19. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;385–396. doi:10.2307/2136404
20. Baik SH, Fox RS, Mills SD, et al. Reliability and validity of the perceived stress scale-10 in hispanic Americans with English or Spanish language preference. J Health Psychol. 2019;24(5):628–639. doi:10.1177/1359105316684938
21. Elkin A. Stress Management for Dummies. John Wiley & Sons; 2013.
22. Al Hosis KF, Mersal FA, Keshk LI. Effects of job stress on health of Saudi nurses working in ministry of health hospitals in Qassim region in KSA. Life Sci J. 2013;10(1):1036–1044.
23. Carver CS. You want to measure coping but your protocol’ too long: consider the brief cope. Int J Behav Med. 1997;4(1):92. doi:10.1207/s15327558ijbm0401_6
24. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56(2):267. doi:10.1037/0022-3514.56.2.267
25. Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
26. de la Vega R, Tomé-Pires C, Solé E, et al. The Pittsburgh Sleep Quality Index: validity and factor structure in young people. Psychol Assess. 2015;27(4). doi:10.1037/pas0000128
27. Aardal E, Holm AC. Cortisol in saliva-reference ranges and relation to cortisol in serum. Clin Chem Lab Med. 1995;33(12):927–932. doi:10.1515/cclm.1995.33.12.927
28. Herrera AY, Hodis HN, Mack WJ, Mather M. Estradiol therapy after menopause mitigates effects of stress on cortisol and working memory. J Clin Endocrinol Metab. 2017;102(12):4457–4466. doi:10.1210/jc.2017-00825
29. Lu Q, Pan F, Ren L, Xiao J, Tao F. Sex differences in the association between internalizing symptoms and hair cortisol level among 10-12 year-old adolescents in China. PLoS One. 2018;13(3):e0192901. doi:10.1371/journal.pone.0192901
30. Thoma MV, Mewes R, Nater UM. Preliminary evidence: the stress-reducing effect of listening to water sounds depends on somatic complaints: a randomized trial. Medicine. 2018;97(8):e9851. doi:10.1097/MD.0000000000009851
31. Wright KP, Drake AL, Frey DJ, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. doi:10.1016/j.bbi.2015.01.004
32. Knight EL, Christian CB, Morales PJ, Harbaugh WT, Mayr U, Mehta PH. Exogenous testosterone enhances cortisol and affective responses to social-evaluative stress in dominant men. Psychoneuroendocrinology. 2017;85:151–157. doi:10.1016/j.psyneuen.2017.08.014
33. Milrad SF, Hall DL, Jutagir DR, et al. Depression, evening salivary cortisol and inflammation in chronic fatigue syndrome: A psychoneuroendocrinological structural regression model. Int J Psychophysiol. 2018;131:124–130. doi:10.1016/j.ijpsycho.2017.09.009
34. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374. doi:10.1038/nrendo.2009.106
35. Ramin C, Devore EE, Wang W, Pierre-Paul J, Wegrzyn LR, Schernhammer ES. Night shift work at specific age ranges and chronic disease risk factors. Occup Environ Med. 2015;72(2):100–107. doi:10.1136/oemed-2014-102292
36. Nea FM, Kearney J, Livingstone MB, Pourshahidi LK, Corish CA. Dietary and lifestyle habits and the associated health risks in shift workers. Nutr Res Rev. 2015;28(2):143–166. doi:10.1017/S095442241500013X
37. Özdemir PG, Selvi Y, Özkol H, et al. The influence of shift work on cognitive functions and oxidative stress. Psychiatry Res. 2013;210(3):1219–1225. doi:10.1016/j.psychres.2013.09.022
38. Fiorentino L, Saxbe D, Alessi CA, Woods DL, Martin JL. Diurnal cortisol and functional outcomes in post-acute rehabilitation patients. J Gerontol a Biol Sci Med Sci. 2012;67(6):677–682. doi:10.1093/gerona/glr230
39. Stawski RS, Almeida DM, Lachman ME, Tun PA, Rosnick CB, Seeman T. Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: timing is everything. J Gerontol B-Psychol. 2011;66(suppl_1):i71–i81. doi:10.1093/geronb/gbq094
40. Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25. doi:10.1037/0033-2909.133.1.25
41. González-Cabrera J, Fernández-Prada M, Iribar-Ibabe C, Peinado JM. Acute and chronic stress increase salivary cortisol: a study in the real-life setting of a national examination undertaken by medical graduates. Stress. 2014;17(2):149–156. doi:10.3109/10253890.2013.876405
42. Torres L, Mata-Greve F, Harkins A. A preliminary investigation of acculturative stress and diurnal cortisol among Latina women. J Lat Psychol. 2018;6(2):149. doi:10.1037/lat0000088
43. Doki S, Sasahara S, Matsuzaki I. Stress of working abroad: a systematic review. Int Arch Occ Env Hea. 2018;91(7):767–784. doi:10.1007/s00420-018-1333-4
44. Baba M, Ohkura M, Koga K, Nishiuchi K, Matsuse R, Inoue T. Analysis of salivary cortisol levels to determine the association between depression level and differences in circadian rhythms of shift-working nurses. J Occup Health. 2015;57(3):237–244. doi:10.1539/joh.14-0079-OA
45. Botha E, Gwin T, Purpora C. The effectiveness of mindfulness based programs in reducing stress experienced by nurses in adult hospital settings: a systematic review of quantitative evidence protocol. JBI Database Syst Rev Implement Rep. 2015;13(10):21–29. doi:10.11124/jbisrir-2015-2380
46. Niu SF, Chung MH, Chu H, et al. Differences in cortisol profiles and circadian adjustment time between nurses working night shifts and regular day shifts: a prospective longitudinal study. Int J Nurs Stud. 2015;52(7):1193–1201. doi:10.1016/j.ijnurstu.2015.04.001
47. Minhee S. An exploratory study on occupational stress and anxiety through salivary cortisol and self-report scale in korean nurses on shift and regular work. J Korean Biol Nurs Sci. 2017;19(3):206–213. doi:10.7586/jkbns.2017.19.3.206
48. Bracci M, Ciarapica V, Copertaro A, et al. Peripheral skin temperature and circadian biological clock in shift nurses after a day off. Int J Mol Sci. 2016;17(5):623. doi:10.3390/ijms17050623
49. Cockerham M, Kang DH, Howe R, Weimer S, Boss L, Kamat SR. Stress and cortisol as predictors of fatigue in medical/surgical nurses and nurse leaders: a biobehavioral approach. Nurse Educ Pract. 2017;8(5):76. doi:10.5430/jnep.v8n5p76
50. Cheung T, Yip P. Depression, anxiety and symptoms of stress among Hong Kong nurses: a cross-sectional study. J Environ Res Public Health. 2015;12(9):11072–11100. doi:10.3390/ijerph120911072
51. Alexandrova-Karamanova A, Todorova I, Montgomery A. Burnout and health behaviors in health professionals from seven European countries. Int Arch Occ Env Hea. 2016;89(7):1059–1075. doi:10.1007/s00420-016-1143-5
52. Sadhanandan HD, Amaravathi M. Effect of stress outcomes on job performance of women doctors. Management. 2019;6(3):36–42.
53. Sladek MR, Doane LD, Jewell SL, et al. Social support coping style predicts women’s cortisol in the laboratory and daily life: the moderating role of social attentional biases. Anxiety Stress Coping. 2017;30(1):66–81. doi:10.1080/10615806.2016.1181754
54. García FE, Barraza-Peña CG, Wlodarczyk A, et al. Psychometric properties of the Brief-COPE for the evaluation of coping strategies in the Chilean population. Psicologia. 2018;31(1):22.
55. Alosaimi FD, Alawad HS, Alamri AK. Stress and coping among consultant physicians working in Saudi Arabia. Ann Saudi Med. 2018;38(3):214–224. doi:10.5144/0256-4947.2018.214
56. The United Arab Emirate government. National Agenda 2021. Available from: https://www.vision2021.ae/en/national-agenda-2021.
57. Vinothkumar M, Arathi A, Joseph M, et al. Coping, perceived stress, and job satisfaction among medical interns: the mediating effect of mindfulness. Ind Psychiatry J. 2016;25(2):195. doi:10.4103/ipj.ipj_98_14
58. Bassett SM, Lupis SB, Gianferante D, Rohleder N, Wolf JM. Sleep quality but not sleep quantity effects on cortisol responses to acute psychosocial stress. Stress. 2015;18(6):638–644. doi:10.3109/10253890.2015.1087503
59. Abell JG, Shipley MJ, Ferrie JE, Kivimäki M, Kumari M. Recurrent short sleep, chronic insomnia symptoms and salivary cortisol: a 10-year follow-up in the Whitehall II study. Psychoneuroendocrinology. 2016;68:91–99. doi:10.1016/j.psyneuen.2016.02.021
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
