Back to Journals » OncoTargets and Therapy » Volume 10
Salinomycin repressed the epithelial–mesenchymal transition of epithelial ovarian cancer cells via downregulating Wnt/β-catenin pathway
Authors Li R, Dong T, Hu C, Lu J, Dai J, Liu P
Received 3 November 2016
Accepted for publication 23 January 2017
Published 28 February 2017 Volume 2017:10 Pages 1317—1325
DOI https://doi.org/10.2147/OTT.S126463
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Rui Li,* Taotao Dong,* Chen Hu, Jingjing Lu, Jun Dai, Peishu Liu
Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Jinan, Shandong, People’s Republic of China
*These authors contributed equally to this work
Abstract: Epithelial ovarian cancer (EOC) is the leading cause of death among all gynecological malignancies. Most patients are diagnosed in the advanced stage and have distant metastasis ultimately. Salinomycin has been demonstrated to reduce invasive capacity of multiple tumor cells. The objective of this study was to investigate the effects of salinomycin on EOC cells. The cell counting kit 8 (CCK-8) and Boyden chamber assays showed that salinomycin could effectively reduce the abilities of proliferation, migration and invasion in EOC cells. The western blot assay showed that salinomycin could increase the expression of epithelial markers (E-cadherin and Keratin) while decrease the expression of mesenchymal markers (N-cadherin and vimentin) in a dose-dependent manner. These results were ascertained by reverse transcription polymerase chain reaction (RT-PCR). Besides, salinomycin could downregulate the expression of proteins associated with the Wnt/β-catenin pathway and repress the nuclear translocation of β-catenin. It was also shown that salinomycin could reverse the aberrant activation of the canonical Wnt pathway induced by GSK-3β inhibitor (SB216763). Our results revealed that salinomycin could inhibit the proliferation, migration and invasion in EOC cells. In addition, the inhibitive effect of salinomycin on the invasive ability was mediated by repressing the epithelial–mesenchymal transition (EMT) program, which may be achieved through its inhibition of the Wnt/β-catenin pathway.
Keywords: salinomycin, epithelial–mesenchymal transition, epithelial ovarian cancer, Wnt/β-catenin pathway
Introduction
Epithelial ovarian cancer (EOC) is the leading cause of death among all gynecological malignancies.1 Most patients were diagnosed at the advanced stages of the disease (International Federation of Gynecology and Obstetrics [FIGO] stage III/IV) due to the lack of significant signs or symptoms in early stages.2 The primary management of such entity remains cytoreductive surgery accompanied with platinum-based cytotoxic chemotherapy. Despite advances in treatment during the last few decades, the prognosis of women with EOC has not substantially improved since most patients suffer from distant metastasis ultimately.3
Epithelial–mesenchymal transition (EMT) is a cell-biology program, which was originally defined as the formation of mesenchymal cells from the epithelia during embryonic development.4 It is a physiological phenotypic transition by which epithelial cells shed intercellular connections and migrate to other locations in the body.5 It is generally characterized with the downregulation of epithelial markers, including E-cadherin and keratin, as well as the upregulation of mesenchymal markers, including N-cadherin and vimentin. Aside from its crucial role in the formation of multiple tissues and organs, it can also actively promote carcinoma progression.6 EMT has been shown to confer several cellular traits to neoplastic cells by which they acquire abilities to invade, disseminate and subsequently form distant metastatic colonies.7 The EMT program in cancer cells seems to be induced by various signals from the nearby microenvironment. Compelling evidence suggested that signaling pathways, such as TGF-β pathway, Notch pathway, Wnt pathway and other pathways, could trigger the activation of EMT by inducing the expression of EMT-inducing transcription factors (EMT-TFs).8
Three Wnt pathways have been characterized, including the canonical Wnt/β-catenin signaling pathway, the noncanonical planar cell polarity (Wnt/PCP) pathway and the Wnt/Ca2+ pathway.9 The dysregulation of these pathways, especially the canonical one, participates in the development of many cancers, including lung cancer and colon cancer.10–12 As a pivotal molecule in the canonical Wnt signaling pathway, β-catenin is primarily degraded by ubiquitination mediated by GSK-3β, whose kinase activity is inhibited by Ser9 phosphorylation.13 The activation of the Wnt/β-catenin pathway will cause a cascade of events, including the β-catenin destruction complex degradation, β-catenin accumulation and nuclear translocation, activation of downstream target genes (such as Axin2 and CCND1), as well as the expression of EMT-TFs (such as Slug and Snail).14
Salinomycin, a monocarboxylic polyether antibiotic, was originally used to eradicate bacteria, fungi and parasites in poultry. Gupta et al15 employed a novel method to identify agents that could target breast cancer stem cells (CSCs) from ~16,000 compounds. It was shown that salinomycin could kill breast CSCs more effectively than the conventional antitumor drug paclitaxel. The abilities of salinomycin to eradicate CSCs, influence the EMT and suppress the invasion capacity in several types of tumors have been proven in recent years.16–18 However, the effects of salinomycin on EOC cells are unclear. The main purpose of this study was to investigate its effects on EMT in EOC cells and clarify the underlying mechanism.
Materials and methods
Cell lines and culture conditions
The human EOC cell lines, A2780 and SK-OV-3, were purchased from the Cell Bank of the Chinese Academy of Sciences in Shanghai, China. The A2780 cells were cultured in Roswell Park Memorial Institute-1640 medium supplemented with 10% fetal bovine serum, 2.0 g/L NaHCO3, 100 U/mL penicillin and 100 μg/mL streptomycin. The SK-OV-3 cells were cultured in McCoy’s 5a medium supplemented with 10% fetal bovine serum, 2.2 g/L NaHCO3, 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were cultured at 37°C in 5% CO2 and 95% air.
Drugs and antibodies
Salinomycin and GSK-3β inhibitor (SB216763) were purchased from Sigma Aldrich (St Louis, MO, USA). Respective stock solutions (400 μM and 50 nM) were prepared with dimethyl sulfoxide (DMSO) and stored at −20°C. The working concentration was diluted in the corresponding medium, and the DMSO’s final concentration was under 0.1% (v/v). Control groups were treated with an equal volume of DMSO. The antibodies E-cadherin, N-cadherin, vimentin, keratin, GSK-3β, β-catenin and GAPDH (Cell Signaling Technology, MA, USA), phosphorylated GSK-3β-ser9 (p-GSK-3β-ser9; Santa Cruz Biotechnology, TX, USA) and Slug (Abcam, Cambridge, UK) were used for Western blot assay. The antibody β-catenin (Santa Cruz) was used for immunofluorescence assay.
Cell viability assay
The A2780 and SK-OV-3 cells were plated in a 96-well plate at a concentration of 3,500 cells/well and 2,500 cells/well, respectively. Cells were incubated overnight to adhere. Then, the medium was replaced with fresh medium, and salinomycin was added into the medium at a range of doses (0, 2, 4, 8 and 16 μM). After incubating for 24, 48, 72 h, cell viability was measured by CCK-8 (Dojindo, Kumamoto, Japan). A total of 10 μL CCK-8 solution was added into each well, and the absorbance was read at 490 nm using a microplate reader after incubating for 2 h.
Migration and invasion assay
The migration and invasion assays were performed with 24-well Boyden chambers (8 μm pore size; Corning Costar, Cambridge, MA, USA). For the invasion assay, the chambers were precoated with Matrigel (100 μL, 1:6 dilution in serum free medium; BD Biosciences, San Jose, CA, USA). After treatment with salinomycin for 48 h, A2780 cells and SK-OV-3 cells were digested and suspended in the serum-free medium. Then, 3×104 SK-OV-3 cells or 8×104 A2780 cells were plated into the upper chamber, and medium with 10% serum was added into the lower chamber as a chemoattractant. After incubating for 24 h, noninvading cells were removed. The cells that invaded to the lower surface of chambers were fixed in 3.7% methanol for 5 min and stained with Giemsa for 30 min. The stained cells were photographed using the Olympus IX51 inverted microscope and counted in five individual fields. For the migration assay, the procedures were the same except that the chambers were not precoated with Matrigel.
Western blot assay
Following treatment with different drugs for respective concentration and time, cell lysate was prepared in radio-immunoprecipitation assay lysis buffer (Beyotime, Jiangsu, China) with 1% phenylmethanesulfonyl fluoride (Thermo Fisher Scientific Inc., Waltham, MA, USA). The proteins were then separated on a 10% polyacrylamide gel and transferred to a pure nitrocellulose blotting membrane. The membranes were blocked with 5% nonfat milk for 1 h and incubated with the appropriate primary antibodies (anti-E-cadherin, anti-N-cadherin, anti-vimentin, anti-keratin, anti-GSK-3β, anti-p-GSK-3β-ser9, anti-β-catenin, anti-Slug and anti-GAPDH) overnight at 4°C. Then, the membranes were washed and incubated with the respective secondary antibodies. The bands were detected using chemiluminescent substrate (Thermo Fisher Scientific Inc.) and quantified by ImageJ software. The GAPDH band served as control.
Quantitative real-time reverse transcription polymerase chain reaction (real-time RT-PCR)
The total RNA of cells cultured with drugs for 24 h was extracted, and the concentration was detected using Nanophotometer Pearl (Implen Company, München, Germany). Then, the RNA (500 ng/10 μL reaction system) was transcripted into cDNA using reverse transcription kit (Takara, Shiga, Japan). PCR reaction was performed on Roche LightCyclerR480 with SYBR-green (Toyobo, Osaka, Japan) in a 10 μL reaction system, and GAPDH was used as the control. Primers used in this study are listed in Table 1.
  | Table 1 Primers used for real-time RT-PCR |
Immunofluorescence assay
A2780 and SK-OV-3 cells were plated in 24-well plates and incubated overnight to adhere. Cells cultured with DMSO vehicle or salinomycin were fixed with cold methyl alcohol for 15 min and then permeabilized in 0.5% Triton X-100 (Solarbio, Beijing, China) for 10 min. After blocking with 5% bovine serum albumin (BSA) for 30 min at room temperature, EOC cells were incubated with primary antibody (anti-β-catenin) at 4°C overnight. After washing with phosphate buffer saline three times, the cells were incubated with anti-rabbit secondary antibody (GeneCopoeia, CA, USA) at 37°C for 1 h. Then, cells were washed and incubated with 4′,6-Diamidino-2-phenylindole dihydrochloride (Solarbio, Beijing, China) for 3 min at room temperature for nuclear staining. Imaging was performed using the Olympus IX51 inverted microscope.
Statistical analysis
All the experiments were performed at least in triplicate. The data were analyzed with Student’s t-test and expressed as mean ± standard deviation (SD). Statistical significance was defined as P<0.05. All the statistical analyses were performed using GraphPad Prism Version 5.01.
Results
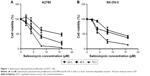
Salinomycin inhibited the proliferation of human EOC cells
To test the effect of salinomycin on the proliferation of human EOC cells, we treated the ovarian cell lines, A2780 and SK-OV-3 cells, with a range of doses of salinomycin (0, 2, 4, 8 and 16 μM) for 24, 48 and 72 h. We found that salinomycin significantly inhibited the proliferation of A2780 cells in a dose- and time-dependent manner (Figure 1A, half maximal inhibitory concentration [IC50]24h: 13.8 μM, IC5048h: 6.888 μM and IC5072h: 4.382 μM). Similar results were also found in the SK-OV-3 cell lines (IC5024h: 12.7 μM, IC5048h: 9.869 μM and IC5072h: 5.022 μM), as shown in Figure 1B.
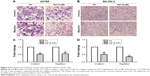
Salinomycin inhibited the capacity of migration and invasion of ovarian cancer cells
The effect of salinomycin on the invasion capacity of A2780 and SK-OV-3 cells was measured with Boyden chambers coated with Matrigel after treatment with respective concentrations of salinomycin for 48 h. We found that the number of cells invading through the membrane was significantly decreased compared with the control group (Figure 2A and C). Then, we used the Boyden chambers without Matrigel to detect the effect of salinomycin on the cell migration capacity. As we supposed, the migration ability was also decreased after salinomycin treatment (Figure 2B and D). All these results suggested that salinomycin could effectively inhibit the migration and invasion abilities of EOC cells.
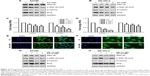
Salinomycin altered the expression of EMT markers of EOC cells
It has been demonstrated that EMT could increase the migration and invasion abilities in most cancers. Therefore, we examined the EMT-associated markers (E-cadherin, keratin, N-cadherin and vimentin) to evaluate whether salinomycin could affect EMT in ovarian cancer cells. The epithelial markers (E-cadherin and keratin) were upregulated after the treatment with salinomycin for 48 h in both A2780 and SK-OV-3 cells. Meanwhile, the mesenchymal markers (N-cadherin and vimentin) were downregulated (Figure 3A–D). Then, we examined the expression of corresponding mRNA by RT-PCR, and similar results were obtained, which verified that salinomycin could induce the expression of epithelial markers and inhibit the expression of mesenchymal ones (Figure 3E and F).
Salinomycin inhibited the Wnt/β-catenin pathway in EOC cells
To examine the effect of salinomycin on the Wnt/β-catenin pathway, the expression of downstream effectors of the pathway, the p-GSK-3β-ser9 (the inactivated GSK-3β), β-catenin and Slug in EOC cells treated with salinomycin was assessed. As the Western blot results showed, decreased expression of the three effectors was observed in both A2780 and SK-OV-3 cells (Figure 4A and B). In addition, the mRNA expression levels of Wnt target genes, such as Axin2 and CCND1, were detected by RT-PCR. Consistent with our expectation, the expression of these two genes was decreased following salinomycin treatment in both A2780 and SK-OV-3 cells (Figure 4C and D). Using the immunofluorescence assay, we found that β-catenin was preferentially accumulated in the cytoplasm in the salinomycin-treated group compared with the control group (Figure 4E and F). GSK-3β is known as a key negative regulator of canonical Wnt/β-catenin signaling. To further certify that salinomycin could inhibit the Wnt/β-catenin pathway, we investigated whether salinomycin could reverse the activation of the Wnt pathway induced by the GSK-3β inhibitor (SB216763). After being incubated with SB216763 for 6 h, the EOC cells were treated with respective doses of salinomycin. As we expected, the p-GSK-3β-ser9, β-catenin and Slug were all downregulated compared to the group incubated with SB216763, indicating that salinomycin could reverse the activation of the pathway by SB216763 (Figure 4G and H). Taken together, our findings indicated that salinomycin could block the Wnt/β-catenin pathway in EOC cells.
Discussion
Ovarian cancer is the most lethal disease in gynecological cancers. The main reason is that most patients are diagnosed in advanced stage presenting with ascitic fluid and distant metastasis. Even with modern management, the rate of relapse and metastasis is still high.1 Metastasis is a complicated process, beginning with cancer cells invading locally through surrounding extracellular matrix and stromal cell layers.19 There has been an increasing awareness that the EMT program gives rise to the dissemination of carcinoma cells from primary epithelial tumors. Passing through EMT, the carcinoma cells acquire several cell biological traits that are needed to generate macroscopic metastases.20 These cells shed their epithelial phenotypes, including intercellular adhesion and polarity, and obtain the mesenchymal traits, such as invasiveness and motility.7 Considering the key role that the EMT program plays in steps of the invasion-metastasis cascade in various cancer cells, inhibition of EMT may be an attractive cancer therapy that could improve the outcome of malignant diseases.
In a recent study, Gupta et al15 searched for chemicals that could eradicate CSCs in breast cancer cell lines. Among the 16,000 compounds tested, salinomycin was identified as the most selective inhibitor for breast CSCs. However, the mechanisms accounting for the specific toxicity of salinomycin to breast CSCs remain unclear. Mani et al21 demonstrated that EMT may account for the formation of CSCs, which provided an interpretation of the relation between EMT and CSCs. These findings suggested that EMT may be a vital pathway through which salinomycin could affect CSCs.22 Subsequent publications demonstrated that salinomycin could repress the abilities of proliferation, invasion and EMT in several cancer cells.16–18,22–24 Our study found that salinomycin could also suppress the invasion and migration ability of EOC cells. Our study then demonstrated that salinomycin could suppress the EMT program of EOC cells, which may account for its inhibitory effects on the invasion and migration of EOC cells.
Then, we detected the candidate pathway by which salinomycin could affect the EMT of EOC cells. The canonical Wnt pathway plays an important role in the initiation and progression of tumors,10,12,25,26 and its dysregulation leads to the induction of EMT and enhancement of invasion ability.11,26–28 The Wnt/β-catenin signaling pathway has recently emerged as a critical player in the progression of EOC cells.14 Rask et al29 found that there was a significant increase in the expression of GSK-3β and β-catenin in ovarian adenocarcinomas compared with normal ovarian tissue and benign adenomas. Ford et al30 confirmed that the Wnt gatekeeper could modulate EMT and migration capacity in serous ovarian cancer cells. Our results demonstrated that salinomycin could block the Wnt/β-catenin signaling in EOC cells, as indicated by suppression of the Wnt pathway components, repression of β-catenin translocating to nucleus and the reversion of the aberrant Wnt signaling activation induced by SB216763. Furthermore, salinomycin was found to target LRP6, an essential Wnt coreceptor for Wnt/β-catenin signaling, and the inhibition of LRP6 led to the inhibition of Wnt/β-catenin signaling, suggesting a mechanism as to how salinomycin inhibited the canonical Wnt signaling pathway.31,32 Signaling pathways manipulating the EMT program seem more likely to depend on modulating the expression of EMT-TFs, which directly bind to and repress the activity of the E-cadherin promoter.7 Slug, also known as Snail2, is one of the EMT-TFs.20 Its positive role in the EMT is well understood, and its elevated expression has been documented in many invasive tumors.33–35 Owing to the relationship between β-catenin and Slug recently found in modulating the EMT program in cells,36,37 the canonical Wnt signaling could positively affect Slug expression via increasing β-catenin levels and in turn activate the EMT program. The inhibitive effect of salinomycin on EMT in EOC cells may be achieved by the suppression of Slug, which was mediated by the complicated interaction between salinomycin and the canonical Wnt pathway.
CCND1 and Axin2 are well known as target genes of the Wnt/β-catenin pathway, especially Axin2, which has been reported to indicate the Wnt pathway activity.14,38,39 The expressions of these two genes could reflect the status of the β-catenin-dependent Wnt pathway. In a recent study, elevated expression of Axin2 was reported in all patients with serous ovarian cancer,40 suggesting that aberrant Wnt signaling may be more widespread in EOC than previously thought. Besides, Wu et al12 have certified that overexpression of Axin2 could stimulate invasion of colon cancer cells. Ford et al30 supported the link between Axin2 and EMT in EOC cells. In our study, the downregulation of Axin2 was detected in consistent with suppression of EMT, indicating the role of Wnt/β-catenin signaling in the inhibitive effect of salinomycin on EMT.
All these findings suggested that the inhibitory effect of salinomycin on the EMT of EOC cells may be mediated by suppression of the canonical Wnt pathway. Consistent with our results, recent findings indicated that the Wnt/β-catenin pathway might be a way by which salinomycin could suppress the EMT program in hepatic cancer cells.22,24
Conclusion
Our studies verified that salinomycin could suppress the proliferation, invasion and migration capacity of EOC cells, and the latter two kinds of effects might be mediated by repressing the EMT program. In addition, our studies also found that the aberrant activation of Wnt/β-catenin pathway did contribute to EMT in EOC cells and proved again that salinomycin could suppress this pathway, providing insight into a possible mechanism whereby salinomycin could affect EMT in EOC cells. These results suggest that salinomycin might be considered as a promising metastasis-targeted therapy in EOC cells.
Acknowledgment
This study was supported by grants from the National Natural Science Foundation of China (81302268).
Disclosure
The authors report no conflicts of interest in this work.
References
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. | ||
Conteduca V, Kopf B, Burgio SL, Bianchi E, Amadori D, De Giorgi U. The emerging role of anti-angiogenic therapy in ovarian cancer (review). Int J Oncol. 2014;44(5):1417–1424. | ||
Vaughan S, Coward JI, Bast RJ, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11(10):719–725. | ||
Thiery JP. Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. | ||
Radisky DC, LaBarge MA. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008;2(6):511–512. | ||
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. | ||
Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19(11):1438–1449. | ||
Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–829. | ||
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. | ||
Chen C, Zhao M, Tian A, Zhang X, Yao Z, Ma X. Aberrant activation of Wnt/beta-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget. 2015;6(19):17570–17583. | ||
He W, He S, Wang Z, et al. Astrocyte elevated gene-1(AEG-1) induces epithelial-mesenchymal transition in lung cancer through activating Wnt/β-catenin signaling. BMC Cancer. 2015;15(1):107. | ||
Wu ZQ, Brabletz T, Fearon E, et al. Canonical Wnt suppressor, Axin2, promotes colon carcinoma oncogenic activity. Proc Natl Acad Sci U S A. 2012;109(28):11312–11317. | ||
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. | ||
Arend RC, Londono-Joshi AI, Straughn JJ, Buchsbaum DJ. The Wnt/beta-catenin pathway in ovarian cancer: a review. Gynecol Oncol. 2013;131(3):772–779. | ||
Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–659. | ||
Kuo SZ, Blair KJ, Rahimy E, et al. Salinomycin induces cell death and differentiation in head and neck squamous cell carcinoma stem cells despite activation of epithelial-mesenchymal transition and Akt. BMC Cancer. 2012;12:556. | ||
Dong T, Zhou H, Wang L, Feng B, Lv B, Zheng M. Salinomycin selectively targets ‘CD133+’ cell subpopulations and decreases malignant traits in colorectal cancer lines. Ann Surg Oncol. 2011;18(6):1797–1804. | ||
Kusunoki S, Kato K, Tabu K, et al. The inhibitory effect of salinomycin on the proliferation, migration and invasion of human endometrial cancer stem-like cells. Gynecol Oncol. 2013;129(3):598–605. | ||
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. | ||
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. | ||
Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. | ||
Zhou Y, Liang C, Xue F, et al. Salinomycin decreases doxorubicin resistance in hepatocellular carcinoma cells by inhibiting the beta-catenin/TCF complex association via FOXO3a activation. Oncotarget. 2015;6(12):10350–10365. | ||
Koo KH, Kim H, Bae Y, et al. Salinomycin induces cell death via inactivation of Stat3 and downregulation of Skp2. Cell Death Dis. 2013;4(6):e693. | ||
Wang F, Dai W, Wang Y, et al. The synergistic in vitro and in vivo antitumor effect of combination therapy with salinomycin and 5-fluorouracil against hepatocellular carcinoma. PLoS One. 2014;9(5):e97414. | ||
Gavilan E, Giraldez S, Sanchez-Aguayo I, Romero F, Ruano D, Daza P. Breast cancer cell line MCF7 escapes from G1/S arrest induced by proteasome inhibition through a GSK-3beta dependent mechanism. Sci Rep. 2015;5:10027. | ||
Guo J, Fu Z, Wei J, Lu W, Feng J, Zhang S. PRRX1 promotes epithelial-mesenchymal transition through the Wnt/beta-catenin pathway in gastric cancer. Med Oncol. 2015;32(1):393. | ||
Su H, Jin X, Zhang X, et al. FH535 increases the radiosensitivity and reverses epithelial-to-mesenchymal transition of radioresistant esophageal cancer cell line KYSE-150R. J Transl Med. 2015;13:104. | ||
Chen D, Li W, Liu S, et al. Interleukin-23 promotes the epithelial-mesenchymal transition of oesophageal carcinoma cells via the Wnt/beta-catenin pathway. Sci Rep. 2015;5:8604. | ||
Rask K, Nilsson A, Brannstrom M, et al. Wnt-signalling pathway in ovarian epithelial tumours: increased expression of beta-catenin and GSK3beta. Br J Cancer. 2003;89(7):1298–1304. | ||
Ford CE, Jary E, Ma SS, Nixdorf S, Heinzelmann-Schwarz VA, Ward RL. The Wnt gatekeeper SFRP4 modulates EMT, cell migration and downstream Wnt signalling in serous ovarian cancer cells. PLoS One. 2013;8(1):e54362. | ||
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A. 2011;108(32):13253–13257. | ||
Lu W, Li Y. Salinomycin suppresses LRP6 expression and inhibits both Wnt/beta-catenin and mTORC1 signaling in breast and prostate cancer cells. J Cell Biochem. 2014;115(10):1799–1807. | ||
Guo W, Keckesova Z, Donaher JL, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148(5):1015–1028. | ||
Wu B, Wei J, Hu Z, et al. Slug silencing inhibited perineural invasion through regulation of EMMPRIN expression in human salivary adenoid cystic carcinoma. Tumour Biol. 2016;37(2):2161–2169. | ||
Jung DE, Kim JM, Kim C, Song SY. Embigin is overexpressed in pancreatic ductal adenocarcinoma and regulates cell motility through epithelial to mesenchymal transition via the TGF-β pathway. Mol Carcinog. 2016;55(5):633–645. | ||
Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’Ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163(4):847–857. | ||
Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG, Weiss SJ. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic breast cancer 1, early onset (BRCA1) repression. Proc Natl Acad Sci U S A. 2012;109(41):16654–16659. | ||
Lustig B, Jerchow B, Sachs M, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22(4):1184–1193. | ||
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. | ||
Schmid S, Bieber M, Zhang F, et al. Wnt and hedgehog gene pathway expression in serous ovarian cancer. Int J Gynecol Cancer. 2011;21(6):975–980. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.