Back to Journals » Journal of Pain Research » Volume 17
Safety, Efficacy, and Durability of Outcomes: Results from SECURE: A Single Arm, Multicenter, Prospective, Clinical Study on a Minimally Invasive Posterior Sacroiliac Fusion Allograft Implant
Authors Calodney A , Azeem N , Buchanan P , Skaribas I, Antony A, Kim C , Girardi G, Vu C, Bovinet C, Vogel R, Li S , Jassal N, Josephson Y, Lubenow T , Lam CM , Deer TR
Received 6 January 2024
Accepted for publication 7 March 2024
Published 20 March 2024 Volume 2024:17 Pages 1209—1222
DOI https://doi.org/10.2147/JPR.S458334
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Krishnan Chakravarthy
Aaron Calodney,1 Nomen Azeem,2 Patrick Buchanan,3 Ioannis Skaribas,4 Ajay Antony,5 Christopher Kim,6 George Girardi,7 Chau Vu,8 Christopher Bovinet,9 Rainer Vogel,10 Sean Li,11 Navdeep Jassal,12 Youssef Josephson,13 Timothy Lubenow,14 Christopher M Lam,15 Timothy R Deer6
1Precision Spine Care, Tyler, TX, USA; 2Florida Spine and Pain Specialists, Tampa, FL, USA; 3Spanish Hills Interventional Pain Specialists, Camarillo, CA, USA; 4Expert Pain P.A, Houston, TX, USA; 5The Orthopaedic Institute, Gainesville, FL, USA; 6The Spine and Nerve Center, Charleston, WV, USA; 7Front Range Pain Medicine, Fort Collins, CO, USA; 8Evolve Restoration Center, Santa Rosa, CA, USA; 9The Spine Center of Southeast Georgia, Brunswick, GA, USA; 10Comprehensive and Interventional Pain Management, Henderson, NV, USA; 11Premier Pain Centers, Shrewsbury, NJ, USA; 12Excel Pain and Spine, Lakeland, FL, USA; 13The Pain Management Center, Voorhees Township, NJ, USA; 14Rush University Medical Center, Chicago, IL, USA; 15University of Kansas Medical Center, Kansas City, KS, USA
Correspondence: Aaron Calodney, Precision Spine Care, Tyler, TX, USA, Email [email protected]
Introduction: Research suggests that sacroiliac joint (SIJ) dysfunction is responsible for 15% to 30% of reported low back pain cases. Recently, there has been an increasing interest in SIJ fusion using minimally invasive surgery (MIS) due to safety. Initially, devices designed for MIS were intended for lateral approaches. A minimally invasive sacroiliac fusion implant for use with a posterior approach has been developed and is regulated for clinical use under the regulatory framework required for human cells, tissues, or cellular or tissue-based products (HCT/Ps).
Methods: A multi-center, prospective, single-arm study was launched after initial studies provided preliminary data to support safety, efficacy, and durability of this minimally invasive sacroiliac posterior fusion LinQ allograft implant (NCT04423120). Preliminary results were reported previously. Final results for the full participant cohort are presented here.
Results: One-hundred and fifty-nine (159) participants were enrolled across 16 investigational sites in the US between January 2020 and March 2022. One-hundred and twenty-two (122) participants were implanted. At the 1-month follow-up, 82 participants satisfied all criteria for the composite responder endpoint, representing 73.2% of the study cohort. These results stayed consistent across the remaining study timepoints with 66.0%, 74.4%, and 73.5% of participants classified as responders at the 3-, 6- and 12-month follow-up visits, respectively. VAS scores were significantly reduced (p < 0.0001) and ODI scores were significantly improved (p < 0.0001). All domains of the PROMIS-29 were also significantly improved (all p’s < 0.0001). Only one procedure-related serious AE was reported in the study.
Conclusion: These results suggest that the posterior approach LinQ Implant System is a safe and effective treatment for sacroiliac joint dysfunction at 12 months, with results that are favorable compared to outcomes reported for an FDA-cleared lateral approach.
Keywords: sacroiliac joint disease, sacroiliac fusion, single point posterior fusion, back pain, minimally invasive spine surgery, sacroiliitis
Introduction
Low back pain continues to be an escalating cause of disability on both a national and international scale. The Lancet published a study in 2017 that examined the incidence, prevalence, and years lived with disability for 328 diseases in 195 countries. This study found that low back pain accounted for over 57 million years of living with disability, representing an 18% increase from 2006 to 2016 in the countries surveyed.1 Research suggests that sacroiliac joint (SIJ) dysfunction is responsible for 15% to 30% of reported low back pain cases.2–4 Traditional SIJ dysfunction treatments involve medications, therapy, injections, and ablations.3 In cases where these methods fail, surgical fixation is considered.
Recently, there has been an increasing interest in SIJ fusion using minimally invasive surgery (MIS), due to its lower associated complication rates, including estimated blood loss (EBL), operative time, and length of stay (LOS) compared to open fusion.5 A retrospective study comparing open surgery and MIS for SIJ dysfunction at a single institution found statistically significant reductions in EBL (681 mL v 41 mL), operative time (128 minutes v 68 mins), and LOS (3.3 days v 2 days) in the MIS group. Moreover, a systematic review analyzing 16 peer-reviewed articles, including 430 participants, evaluated the clinical effectiveness of open and MIS approaches for SIJ fusion.6 Patient satisfaction rates inferred from pain, function, and quality of life measures ranged from 18% to 100% (mean 54%) for open surgery and 56% to 100% (mean 84%) for MIS patients.
Initially, devices designed for MIS were intended for lateral approaches. A prospective, multi-center, randomized control study comparing MIS triangular implants to conservative management for SIJ dysfunction demonstrated statistically significant improvements in pain scores and Oswestry Disability Index (ODI) scores for the operative fixation group.7 Whang et al conducted a 5-year study on the MIS lateral approach with triangular implants, which reported a 54-point reduction in pain and a 26-point decrease in ODI. However, lateral approaches are associated with substantial postoperative restrictions due to muscle dissection and have a risk of serious neurovascular injury.8
A minimally invasive sacroiliac fusion allograft implant for use with a posterior approach has been developed (LinQ Implant System, PainTEQ, Tampa, FL) and is regulated for clinical use under the regulatory framework required for human cells, tissues, or cellular or tissue-based products (HCT/Ps). HCT/Ps are governed by the regulations set out in 21 CFR 1271. These regulations encompass requirements such as establishment registration, donor eligibility (which includes donor screening and testing), adherence to Current Good Tissue Practice (CGTP), additional regulatory mandates (like reporting adverse reactions, HCT/P deviations, and labelling), as well as stipulations about exemptions and alternative procedures. For allogeneic products, the regulatory requirements are more comprehensive compared to autologous products. However, the main focus of the FDA regulations is to mitigate the risk of communicable disease transmission via HCT/Ps. Thus, supplementary clinical evidence is needed to support the safety, efficacy, and durability of the bone allograft implant in conjunction with FDA regulation.
An initial multi-center, retrospective study examined clinical outcomes following use of this minimally invasive sacroiliac posterior fusion implant for the management of chronic pain resulting from SIJ dysfunction.9 This evaluation included 50 participants with outcomes at an average follow-up time of 612.2 days (1.7 years). In this evaluation, mean numeric rating scale (NRS) scores were reduced by 3.9 points from a baseline score of 7.0 to a score of 3.1 and no major adverse events or complications were reported for any of the participants.
A multi-center, prospective, single-arm study was launched after these initial studies provided preliminary data to support safety, efficacy, and durability of this minimally invasive sacroiliac posterior fusion implant in a larger patient cohort (NCT04423120). Specifically, the study was designed to supplement the existing prospective data,7,10 which is primarily limited to investigations of a single system using a lateral approach. The study design and methods have been reported elsewhere.11 Eligibility criteria mirrored those used in the investigations of the lateral approach7,10,12 to allow comparison of results to other previous published studies on sacroiliac fusion. Preliminary results from 69 participants enrolled in that trial have been published11 and showed that 68.1% of participants (47/69) were responders to the therapy, defined by meeting the composite criteria for the primary endpoint (> 20 mm reduction in VAS from baseline to 6 months in the absence of SAE, neurological worsening, or reintervention). The average reduction in visual analogue scale (VAS) score was 34.9 mm from a baseline value of 74.6 mm and 52.2% (36/69) of participants achieved 50% or greater pain relief. Pain-related disability was also improved in study participants, reflected by a mean improvement of 17.7 points on the ODI and over half of all participants (39/69; 56.5%) reporting a clinically significant improvement, defined as a 15-point or greater improvement in score. Final results for the full participant cohort are presented here.
Methods
Study Design and Objectives
This prospective, multi-center, single arm study was designed to evaluate the safety and efficacy of utilizing a minimally invasive sacroiliac posterior fusion allograft implant (LinQ Fusion Implant (Figure 1)); (PainTEQ, Tampa, FL) for management of chronic, low back pain associated with sacroiliac disease.
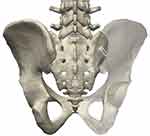 |
Figure 1 LinQ Allograft Implant is depicted within the right sacroiliac joint. |
Ethical Considerations
All study-specific documents, including the protocol, proposed participant information and informed consent form, were submitted for review and approved by the Institutional Review Boards (IRBs) in compliance with local legal requirements. The trial was carried out following the ethical principles of the International Conference on Harmonization (ICH) Harmonized Tripartite Guideline on the Structure and Content of Clinical Trial Reports on Good Clinical Practice, the current version of the Declaration of Helsinki and following all other requirements of local laws. All subjects were informed about the study’s purpose and procedures and provided written informed consent before participating.
Project Management, Protocol and Report Medical Writing, Statistics, Regulatory, Data Management, Source Verification, and Monitoring activities of the study were carried out by the Contract Research Organization (CRO), Pacific Research Institute, (Santa Rosa, CA), and PainTEQ (Tampa, FL). Electronic Data Capture (EDC) was performed by third-party vendor Celeri. The study was registered at clinicaltrials.gov (NCT04423120).
Trial Population
Participants aged 21 to 70 years with low back pain for greater than 6 months despite conservative care including therapy and medications were considered for this study if they tested positive for three out of four physical exam maneuvers for SIJ dysfunction, including FABER test, Gaenslen test, Stork/Gilett test, and Yeoman’s test. Further, eligible participants with an Oswestry Disability Index (ODI) score of at least 30 and a visual analog scale (VAS) score of at least 50 mm for low back and or buttock pain were selected. After clinical diagnosis of SIJ dysfunction was made, participants underwent local only SIJ injection to further confirm the diagnosis. Participants with a NRS or VAS improvement of at least 50% within 30 to 60 minutes of the injection were eligible for the study.
Participants were excluded if other conditions significantly contributed to their pain (eg, lumbar disc disease, lumbar disc herniation, lumbar spondylolisthesis, lumbar stenosis, lumbar facet disease, lumbar radiculopathy, or lumbar vertebral body fractures). Further, any participant receiving steroid exposure whether from SIJ injection or neuraxial injection within 30 days of the diagnostic injection or an SIJ radiofrequency ablation (RFA) within 6 months of enrollment were also excluded. Participants with a history of recent pelvic injury within a year of enrollment, sacral surgery, prior SIJ surgery with other devices or techniques, endometriosis, coccydynia, coccygectomy, pudendal neuralgia, active intrathecal pump therapy for pain, current systemic infection, local infection, or history of medications decreasing bone quality or soft tissue healing were also excluded from this study.
Study Procedures
Study methods and results for the primary endpoint analysis at the 6-month follow-up have been previously reported11 for a subset of participants. Briefly, eligible participants received SIJ fusion using the posterior approach and the LinQ Fusion Implant as described previously.11 After the procedure, participants were seen post-operatively (7–14 days) and at 1, 3, 6, and 12 months. The results presented here include all visits across the complete participant cohort.
Outcome Measures
The primary endpoint was a composite measure of binary success (responder rate) at 6-months. A participant was considered successful (responder) if they met the following criteria:
- VAS score (0–100 mm) for SIJ pain reduced by at least 20 mm from baseline.
- Absence of implant-related serious adverse events (SAEs).
- Absence of neurologic worsening related to the lumbosacral nerve roots.
- Absence of surgical reintervention (removal, revision reoperation or supplemental fixation) for SIJ pain.
Secondary endpoints included mean change from baseline for the following patient-reported outcomes at 6-months: VAS score for SIJ pain, ODI scores, and PROMIS-29 v2.1 scores. The PROMIS-29 is a validated, disease agnostic instrument which includes assessment of domains for pain interference, sleep disturbance, fatigue, anxiety, depression, ability to participate in social roles and activities, and physical function. The recall period for this patient-reported outcome (PRO) is seven days and higher scores indicate greater disability.
Safety Outcomes
Adverse events (AE) were monitored continuously and recorded at all study visits. Serious adverse events (SAEs), mortality resulting from implantation of the implant, and events associated with recovery and rehabilitation were tracked, as were all implant and procedure-related complications. AE rates were calculated by dividing the number of events in a specific category by the total number of implants (N=117).
Statistical Methods
Data imputation was only performed for VAS scores for pain intensity using the last observation carried forward (LOCF) method for pain intensity. This analysis was performed to determine the impact of attrition on study endpoints. All other analyses are completed case, meaning only available data was analyzed. All statistical analyses were performed and validated in August 2023 using MedCalc Statistical Software version 19.2.6 (MedCalc Software by Ostend, Belgium; https://www.medcalc.org; 2020). All statistical tests were two-sided with a significance level of 5%, unless otherwise specified. The following outcomes were analyzed using a repeated measures analysis of variance (RMANOVA): SIJ pain intensity VAS, ODI, and PROMIS-29 domain scores. All other data is reported descriptively as appropriate (eg, means, proportions, etc.).
Results
Participant Demographics
Participant disposition can be found in Table 1. One-hundred and fifty-nine (159) participants were enrolled across 16 investigational sites in the US between January 2020 and March 2022. One-hundred and twenty-two (122) participants were implanted; however, 5 participants were found to be ineligible for study participation after implant and their data has been excluded from analyses. Of the 117 participants with available data, 112 completed the 1-month visit, 100 completed the 3-month visit, 92 completed the 6-month visit, and 84 completed the 12-month follow-up visit. Seventy-three (73) participants withdrew from the study. Reasons for study withdrawal are shown in Table 2. Eight (8) were considered screen failures, not meeting study eligibility, 29 withdrew after consent but prior to implant, and 36 withdrew after implant. Specific reasons are detailed in Table 2.
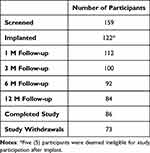 |
Table 1 Participant Disposition |
 |
Table 2 Study Withdrawal Reasons (N = 73) |
Participant demographics can be found in Table 3. The mean (SD) age across the 117 participants was 59.0 (9.8) and 70.1% were female. Average (SD) pain duration was 5.8 (7.0) years and baseline VAS and ODI scores were 76.2 (13.1) and 52.4 (12.4), respectively.
 |
Table 3 Participant Demographics and Baseline Characteristics (N = 117) |
Table 4. provides an overview of specifics related to the surgical procedure. The implant was placed predominately unilaterally (93.2%), equally on both sides (50.4% for right side placement). Mean (SD) fluoroscopy time during the procedure was 1.6 (1.8) minutes and most cases (74.4%) were performed under monitored anesthesia care (MAC) rather than general anesthesia.
 |
Table 4 Surgical Characteristics (N = 117) |
Efficacy
At the 1-month follow-up, 82 participants satisfied all criteria for the composite responder endpoint (had at least a 20 mm improvement in VAS score for SIJ pain in the absence of implant-related SAE, neurologic worsening related to the lumbosacral nerve roots, or surgical reintervention), representing 73.2% of the study cohort. These results stayed consistent across the remaining study timepoints with 66.0%, 74.4%, and 73.5% of participants classified as responders at the 3-, 6- and 12-month follow-up visits, respectively (Table 5).
 |
Table 5 VAS Outcomes |
Mean VAS scores for SIJ pain intensity were significantly reduced by 39.1, 35.4, 39.5, and 43.3 mm from a baseline score of 76.2 mm at 1-, 3-, 6- and 12-months, respectively (Table 5). RMANOVA for the completed case data set and imputed data set revealed similar effects for VAS scores for SIJ pain intensity. All timepoints were significantly reduced from baseline regardless of the data set (all ps <0.0001), suggesting that attrition had a minor impact on the results. In addition, 57.1% of participants achieved 50% or greater pain relief at 1 month, 52.0% achieved the same at 3 months, 58.9% had 50% or greater pain relief at 6 months and 61.4% achieved the same at 12 months. Up to 34.9% of participants continued to achieve 80% or greater pain relief at 12 months (Figure 2).
 |
Figure 2 Tornado plot of VAS score reduction from baseline pain at 12 months. Each bar represents one participant’s change in VAS with a positive number signifying a decrease in raw score. |
RMANOVA of ODI scores showed significant improvements of 20.2, 21.5, 19.8, and 25.3 points at 1-, 3-, 6- and 12-months follow-up from a baseline score of 52.4 (all ps <0.0001). At 1 month, 60.7% of participants had a clinically significant improvement in ODI score, defined as a 15 point or greater improvement and this rate had improved to 68.7% at 12 months follow-up (Table 6). In terms of change in ODI category (Table 7), most participants (82.1%) were categorized as severely disabled, crippled or bed-bound at baseline. At 12 months, only 24.1% of participants remained in these categories and 41.0% were categorized as minimal disability, suggesting a major shift in pain-related disability following treatment.
 |
Table 6 ODI Outcomes |
 |
Table 7 ODI Categories |
Table 8 displays PROMIS-29 domain scores and Table 9 shows the percentage of participants experiencing less than the general population for each domain of the PROMIS-29 with a normative T-Score. RMANOVAs for each domain of the PROMIS-29 revealed significant improvements from baseline across all timepoints (all ps <0.001), with the greatest improvement occurring for pain interference. In addition, the proportion of participants experiencing less than the general population for each domain of the PROMIS-29 with normative a T-score improved across all PROMIS-29 domains and timepoints. For example, at baseline 22.2% of participants experienced less anxiety than the general population. However, by 12 months, 56.0% of participants experienced less anxiety than the general population. The greatest improvement in this measure of PROMIS-29 occurred for ability to participant in social roles and activities. At baseline, 96.6% of participant experienced less ability; however this had improved at 12 months to 53.6% with less ability to participate, a 43% improvement.
 |
Table 8 PROMIS-29 Domain Scores |
 |
Table 9 Participants Experiencing Less Than the General Population for Each Domain of the PROMIS-29 with Normative T-Score |
Safety
All AEs which have occurred during the study are presented in Table 10. Only five (5) AEs have been reported across 117 implants, yielding an overall AE rate of 4.3%. Observed AEs included anesthesia aspiration (n = 1), increased SIJ pain (n = 1), participant fall (n = 1), hypokalemia (n = 1), and a mortality (n = 1). This mortality was related to a different procedure that took place months later after the implant and was associated with another medical practice, which occurred outside the trial and was determined by the investigator to be unrelated to the trial. Of these AEs, four events (3.4%) were categorized as serious adverse events (SAEs); however, only one of these serious adverse events was related to the procedure. This procedure-related SAE involved anesthesia aspiration after initiation of monitored anesthesia care (MAC) but prior to any activities related to the implant procedure.
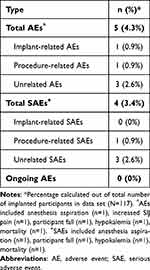 |
Table 10 Adverse Events (N = 117) |
Discussion
We present results from the full participant cohort in the SECURE study, which evaluated use of a posterior, single point fixation strategy, to build upon 6-month interim results previously presented.11 This prospectively acquired data set is the largest and first of its kind for any posterior approach and addresses the paucity of data for 12-month prospective outcomes. The primary endpoint for this trial was a composite measure of binary success at 6-months. As previously reported, 68.1% (47/69) of participants achieved the primary endpoint of at least a 20 mm improvement in VAS score for SIJ pain from baseline in the absence of any implant-related serious adverse event (SAE), neurologic worsening related to the lumbosacral nerve roots or surgical reintervention (removal, revision reoperation or supplemental fixation) for SIJ pain. When this analysis was repeated at 12 months, the number of participants achieving this endpoint increased to 73.5% (61/83), as did overall mean improvement in VAS score for SIJ pain (34.9-point improvement at 6 months compared to 43.3-point improvement at 12 months). In terms of clinically significant improvements, 61.4% (51/83) of participants experienced a 50% or greater improvement in VAS score for SIJ pain and 68.7% (57/83) had a 15 point or greater improvement in ODI scores at 12 months. These improvements seen at 12 months may reflect the occurrence of more solid fusion and arthrodesis over time resulting from posterior SIJ fusion, although future studies that assess radiographic confirmation of fusion would support this. The observed improvements may be indicative of sustained reparative augmentative processes occurring in the periarticular musculature and soft tissue connective structures that surround the treated joint. Previously these structures may have been subject to overuse and compensatory biomechanical adaptations due to the joint’s deficient mechanical function prior to use of the LinQ implant.
The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), a leading authority for recommendations related to improving the design, execution, and interpretation of clinical trials in pain management, suggest assessment of five core domains including, physical functioning, emotional functioning, participant ratings of improvement and satisfaction with treatment, symptoms and adverse events and participant disposition to adequately gauge treatment response in addition to pain intensity.13 This approach not only allows a comprehensive evaluation of treatment effect, but it also addresses the complex nature of pain assessment in which all these domains are interrelated and dependent. In other words, measurement of change in pain intensity only provides part of the story in terms of treatment effect in pain management clinical trials because pain is mediated by these other factors. In the current trial, all domains of the PROMIS-29 were significantly improved from baseline to 12 months (all ps <0.001), a finding that is in line with what has been observed for other trials of SIJ fusion.10,14,15 In terms of safety of the implant, no implant-related AEs were reported during the study. Further, no participants had an overnight hospital stay and most participants had the procedure performed under MAC anesthesia. These findings will be further discussed in the context of similar trials below.
These data add to the growing body of literature substantiating use of a posterior approach to SIJ fusion for the management of pain and disability associated with SIJ pain and provide a framework for comparison to the benchmark lateral approach with FDA-cleared implants. Specifically, the SECURE study was designed to closely align with that of the Investigation of Sacroiliac Fusion Treatment (INSITE; NCT01681004) and iFuse Implant System Minimally Invasive Arthrodesis (iMIA; NCT01741025) trials for comparison purposes to pivotal data for the iFuse Implant System (SI-BONE, Inc., Santa Clara, CA). Table 11 provides an overview of the relevant trial design aspects across the SECURE, iMIA12,14 and INSITE10,15 trials. For example, all three trials had virtually identical eligibility criteria, endpoints and visit schedules. The SECURE and INSITE trials shared the same primary endpoint and there was significant overlap in collection and presentation of the outcomes across all trials. Although it is not possible to directly compare the data for the LinQ and iFuse Implant Systems in a rigorous way such as when data is collected as part of a randomized, controlled trial with respective comparator groups, some generalizations can be made from a descriptive comparison of the study outcomes. Table 11 provides a high-level outcomes comparison across the three trials. One of the most notable baseline characteristic differences across the study cohorts is the older participant population observed in the SECURE study (59.0 on average compared to 49.4 on average in the iMIA trial). The impact of this difference on the interpretation of treatment effect between the trials is likely minimal. If anything, this finding suggests a disadvantage for the SECURE trial given that older participants often have additional age-related comorbidities which can confound the treatment effect. All other baseline characteristics including gender, BMI, pain duration, baseline VAS and ODI scores were comparable across all trials. In terms of surgical characteristics, the most notable differences were seen for fluoroscopy time, length of hospital stay and anesthesia type. This is attributed to the nature of the LinQ procedure, which does not require any drilling. In all cases, better outcomes were seen in the SECURE trial with fluoroscopy time being almost a minute less, on average, than that reported in the other trials, no reports of any overnight hospital stay after implant with the LinQ System, and use of MAC over general anesthesia. Notably all participants in the iMIA and INSITE trials were implanted under general anesthesia.10,12,14,15
 |
Table 11 SECURE, iMIA and INSITE Study Data Comparison at 12 Months |
VAS scores for SIJ pain and ODI scores were significantly improved across the SECURE, iMIA and INSITE trials and mean improvements for these two measures were also comparable across the three trials. For example, mean average VAS scores were improved by 43.3 points in the SECURE trial compared to 41.6- and 54.2-point improvements in the iMIA and INSITE trials, respectively. ODI scores were improved between approximately 25 and 30 points on average and approximately 70% of participants achieved a clinically meaningful change in ODI score in all trials at 12 months. We predict future outcomes to improve based on significant refinement to the surgical technique. The technique used in the SECURE trial represents a much-improved method by including use of pre-operative imaging for implant of the LinQ allograft. Recent data supports this conclusion. For example, Bovinet et al,16 showed an 85.6% average reduction in numerical rating scale (NRS) scores and that 98.6% of patients reported 50% or greater pain relief across 208 patients and 275 total LinQ implants.
Although there are similarities across the 3 trials in terms of efficacy of the therapy, the safety profile differs substantially. For example, 179 AEs were reported in the INSITE trial and of these 179 AEs, 18 were categorized as implant and/or procedure-related AEs, and 2 were implant-related serious adverse events. In comparison, only 5 AEs have been reported in the SECURE trial and of these, only one was categorized as procedure related. No implant-related SAEs have been observed in this trial.
This trial and the current analysis were not without limitations. Primarily, the lack of a control group and the partial cohort in this analysis are limitations to the generalizability of these results. In addition, features of the therapy prohibit blinding, so a traditional randomized-controlled trial was not possible. Twenty-four implanted participants were lost to follow-up within the course of this study. Patient outreach and in-person follow-up was very likely impacted by the COVID pandemic, beginning in March of 2020, with limitations on patient retention due to mandatory lockdown across all study sites. Despite these limitations, the research was independently conducted with minimal oversight or direction from the study Sponsor to limit bias from that perspective. Continued patient access to this proven therapy is reliant on the publication of sufficient clinical evidence.
Conclusions
The results suggest that use of the LinQ System is safe, effective and durable in the management of chronic, low back pain associated with sacroiliac disease compared to a similar benchmark system. In addition, the LinQ System may be superior to this system in terms of safety as evidenced by less fluoroscopy time, less time in the hospital and a lower incidence of implant/procedure-related AEs. These findings suggest that the posterior approach may be associated with an improved safety profile over the traditional lateral approach and help to address criticism of interventionalists to safely and appropriately diagnose and treat SIJ dysfunction by a posterior fusion strategy. Further, the data support safe and effective treatment of this patient population with this specific approach by interventionalists when proper patient selection is performed, along with proper procedural technique.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the study sponsor and authors on reasonable request.
Compliance with Ethics Guidelines
This prospective, multi-center, single arm study was conducted in accordance with current Good Clinical Practices and International Council for Harmonization guidelines and in compliance with the obligations and requirements of clinical investigators set forth by local government agencies. The study protocol (NCT04423120) and all investigational sites received Institutional Review Board approval prior to study initiation and all participants provided written informed consent prior to the performance of any study procedures. IRBs that provided approval for the study protocol and all study sites were as follows: WCG (WIRB-Copernicus-Group) Institutional Review Board, Kansas University Medical Center Institutional Review Board, Rush University Medical Center Institutional Review Board, University of Colorado School of Medicine Institutional Review Board.
Medical Writing and/or Editorial Assistance
The authors would like to thank Stephanie Washburn, PhD, and Kristina Davis, PhD, for their editorial assistance, material preparation, and data analysis and validation.
Acknowledgments
The number of individuals involved in the successful design and execution of a clinical trial far exceed the space provided here for individual acknowledgement. The authors would like to thank everyone involved in the execution of this clinical trial but would like to especially recognize Dawood Sayed, M.D. and Jason Pope, M.D. for their contributions to this study. The authors would like to thank all study participants for their involvement in the SECURE trial.
Author Contributions
All authors made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors reviewed previous versions of the manuscript. All authors read and approved the final manuscript, and agreed upon the Journal for submission.
Funding
This study was funded by PainTEQ. All fees related to the preparation and publication of this manuscript have been paid for by PainTEQ.
Disclosure
Dr Calodney reports being a consultant for Medtronic, Companion Spine, PainTeq, and Vertos, was a speaker for Relievant, and got research support from Medtronic, Nevro, Stryker, Boston Scientific, Spine Biopharma, Biorestorative, Vivex, Saluda, DiscGenics, outside the submitted work. Dr. Azeem serves on the advisory board for PainTEQ, Vertos, Spinal Simplicity, Vivex, Ethos labs and is a consultant/speaker for Abbott, Medtronic, Boston Scientific; he has received research support from PainTEQ, Vivex, Abbott, Medtronic, Ethos Labs. Dr. Buchanan serves as a consultant and Principal Investigator for Abbott and PainTEQ. Dr. Lubenow serves as a consultant for Abbott, Medtronic, Boston Scientific, PainTEQ, and Nevro and has received research support from Abbott and PainTEQ. Dr. Li serves as a consultant for Abbott, Avanos, Averitas Pharm, Biotronik, Boston Scientific, Nalu Medical, Nevro, PainTEQ, Saluda Medical, SPR Therapeutics, Vertos Medical, and is on the Speaker’s Bureau for Scilex Pharm, and has Stock Options in Nalu Medical. He has received research support from Avanos, Biotronik, Boston Scientific, SGX Medical, Nalu Medical, PainTEQ, Saluda Medical, and SPR Therapeutics. Dr. Deer serves as a consultant for Abbott, Medtronic, Cornorloc, PainTEQ, Spinal Simplicity, SPR, Saluda; he has received research support from Abbott, Vertiflex (BSI), PainTEQ, Vertos, and Saluda. Dr. Josephson serves as a consultant for PainTEQ, Boston Scientific, NALU, Bioness, Aurora, and Medtronic and has received research support from Aurora, and PainTEQ. Dr. Vu serves as a consultant for PainTEQ and Saluda and receives research support from Abbott, Flowonix, Saluda, Aurora, PainTEQ, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, and Thermaquil. Dr. Girardi serves as a consultant for Abbott and PainTEQ. Dr. Kim serves as a consultant for Boston Scientific, Vertos, PainTEQ, and Abbott and has received research support from Abbott and PainTEQ. Dr. Vogel previously served as a consultant for PainTEQ. Dr. Skaribas previously served as a consultant for PainTEQ. Dr. Antony serves as a consultant/speaker for Boston Scientific, Abbott, Nalu, PainTEQ, Saluda, Vertos; he has received research support from ViaDISC, Abbott, Boston scientific, PainTEQ, Saluda, and SPR. Dr. Bovinet serves as a consultant for PainTEQ, Boston Scientific, Vertos, Nevro, and Spinal Simplicity and has received research support from PainTEQ, Nevro, Boston Scientific. Dr. Jassal has received research support from AIS, Flowonix, Boston Scientific, PainTEQ, and Vertos. The authors report no other conflicts of interest in this work.
References
1. Disease, G.B.D., I. Injury, and C. Prevalence. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259.
2. Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255–265. doi:10.5435/00124635-200407000-00006
3. Vanelderen P, Szadek K, Cohen SP, et al. 13. Sacroiliac joint pain. Pain Pract. 2010;10(5):470–478. doi:10.1111/j.1533-2500.2010.00394.x
4. Sembrano JN, Polly DW Jr. How often is low back pain not coming from the back? Spine. 2009;34(1):E27–E32. doi:10.1097/BRS.0b013e31818b8882
5. Ledonio CG, Polly DW, Swiontkowski MF. Minimally invasive versus open sacroiliac joint fusion: are they similarly safe and effective? Clin Orthop Relat Res. 2014;472(6):1831–1838. doi:10.1007/s11999-014-3499-8
6. Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59–66. doi:10.3171/2014.10.SPINE14516
7. Dengler J, Kools D, Pflugmacher R, et al. Randomized trial of sacroiliac joint arthrodesis compared with conservative management for chronic low back pain attributed to the sacroiliac joint. J Bone Joint Surg Am. 2019;101(5):400–411. doi:10.2106/JBJS.18.00022
8. Whang PG, Darr E, Meyer SC, et al. Long-term prospective clinical and radiographic outcomes after minimally invasive lateral transiliac sacroiliac joint fusion using triangular titanium implants. Med Devices. 2019;12:411–422. doi:10.2147/MDER.S219862
9. Sayed D, Balter K, Pyles S, et al. A multicenter retrospective analysis of the long-term efficacy and safety of a novel posterior sacroiliac fusion device. J Pain Res. 2021;14:3251–3258. doi:10.2147/JPR.S326827
10. Polly DW, Cher DJ, Wine KD, et al. INSITE 1 -year result: randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium, implants vs. nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77:674–691. doi:10.1227/NEU.0000000000000988
11. Calodney AK, Azeem N, Buchanan P, et al. Six month interim outcomes from SECURE: a Single arm, Multicenter, Prospective, Clinical Study on a novel minimally invasive posterior sacroiliac fusion device. Expert Rev Med Devices. 2022;19(5):451–461. doi:10.1080/17434440.2022.2090244
12. Dengler J, Kools D, Pflugmacher R, et al. iMIA 1 year results: 1 year results of a randomized controlled trial of conservative management vs. minimally invasive surgical treatment for sacroiliac joint pain. Pain Physician. 2017;20:537–550. doi:10.36076/ppj.20.5.537
13. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi:10.1016/j.pain.2004.09.012
14. Sturesson B, Kools D, Pflugmacher R, et al. iMIA 6 month results. Six month outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion with triangular titanium implants vs. conservative management. Eur Spine J. 2017;26(3):708–719. doi:10.1007/s00586-016-4599-9
15. Whang PG, Cher D, Polly D, et al. Sacroiliac joint fusion using triangular titanium implants vs non-surgical management: six-month outcomes from a prospective randomized controlled trial. Int J Spine Surg. 2015;9:6. doi:10.14444/2006
16. Bovinet C. A single-physician case series-evaluating the efficacy of a novel minimally invasive posterior sacroiliac joint fusion system with a single posterior intra-articular allograft implant.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
